ABSTRACT
Immunotherapy with short term infusion (STI) of monoclonal anti-GD2 antibody (mAb) ch14.18 (4 × 25 mg/m2/d; 8–20 h) in combination with cytokines and 13-cis retinoic acid (RA) prolonged survival in high-risk neuroblastoma (NB) patients. Here, we investigated long-term infusion (LTI) of ch14.18 produced in Chinese hamster ovary cells (ch14.18/CHO; 10 × 10 mg/m2; 24 h) in combination with subcutaneous (s.c.) interleukin-2 (IL-2) in a single center program and report clinical response, toxicity and survival. Fifty-three high-risk NB patients received up to 6 cycles of 100 mg/m2 ch14.18/CHO (d8–17) as LTI combined with 6 × 106 IU/m2 s.c. IL-2 (d1–5; 8–12) and 160 mg/m2 oral RA (d19–32). Pain toxicity was documented with validated pain scores and intravenous (i.v.) morphine usage. Response was assessed in 37/53 evaluable patients following International Neuroblastoma Risk Group criteria. Progression-free (PFS) and overall survival (OS) was analyzed by the Kaplan-Meier method and compared to a matched historical control group from the database of AIEOP, the “Italian Pediatric Ematology and Oncology Association”. LTI of ch14.18/CHO showed acceptable toxicity profile indicated by low pain scores, reduced i.v. morphine usage and low frequency of Grade ≥3 adverse events that allowed outpatient treatment. We observed a best response rate of 40.5% (15/37; 5 CR, 10 PR), 4-year (4 y) PFS of 33.1% (observation 0.1- 4.9 y, mean: 2.2 y) and a 4 y OS of 47.7% (observation 0.27 – 5.20 y, mean: 3.6 y). Survival of the entire cohort (53/53) and the relapsed patients (29/53) was significantly improved compared to historical controls. LTI of ch14.18/CHO thus shows an acceptable toxicity profile, objective clinical responses and a strong signal of clinical efficacy in NB patients.
Introduction
Treatment with antibodies (Ab) directed against disialoganglioside GD2 has emerged as an important option for patients with neuroblastoma (NB).Citation1 Human/mouse chimeric anti-GD2 Ab ch14.18 was evaluated in patients with high-risk NB for consolidation after high dose chemotherapy followed by autologous hematopoietic stem cell rescue aloneCitation2 and in combination with cytokines.Citation3 In Europe, ch14.18 was re-cloned in Chinese hamster ovary (CHO) cellsCitation4 and made available within clinical trials of SIOPEN, the International Society of Paediatric Oncology Europe Neuroblastoma. Following the re-cloning procedure, ch14.18/CHO was first evaluated for safety in a Phase 1 study,Citation5 which confirmed the tolerability and showed activity at a dosing regimen of 20 mg/m2 given by 8 hour (h) infusions on 5 consecutive days (d) (cumulative dose: 100 mg/m2 per cycle).
One major obstacle associated with anti-GD2 Ab therapy is the induction of neuropathic pain,Citation6–8 which is an on-target side effect not observed with other human/mouse chimeric monoclonal Ab. In animal models, which approximate the pain associated with anti-GD2 Ab in humans in terms of timing and quality, anti-GD2-specific biding to Aδ and C pain fibers results in decreased mechanical stimulus thresholds.Citation9 Therefore, clinical use of anti-GD2 Ab therapy requires heavy co-administration of analgesic drugs, including intravenous (i.v.) morphine, to make this treatment tolerable. The majority of current treatment modalities with anti-GD2 Ab ch14.18 involve the application of a cumulative dose of 100 mg/m2/cycle as short term infusion (STI) (8–20 h infusions on 4–5 subsequent days).Citation2,3,5,10 Clinical observation indicates that if patients experience intense pain despite analgesic therapy, a decrease in speed of Ab infusion improves this toxicity. Therefore, we hypothesized that significant prolongation of the time of Ab infusion would improve tolerability of that treatment without impairing clinical activity and efficacy in high risk NB patients. For this purpose, we tested a new treatment regimen consisting of ch14.18/CHO at 100 mg/m2/cycle given as 10 d continuous long-term infusion (LTI) in combination with IL-2 and 13-cis retinoic acid (RA). We evaluated toxicity, objective clinical response and survival of treated patients.
Results
Evaluation of toxicity
The primary aim of this investigation was to evaluate pain as expected on target toxicity and the overall toxicity profile during LTI of anti-GD2 Ab ch14.18/CHO in combination with IL-2 (). For this purpose, we analyzed pain with validated assessment scores and determined the use of i.v. morphine in 49/53 evaluable patients within cycles over time (, ), as well as the total amount of i.v. morphine use per treatment cycle (). Low pain scores () and decreasing i.v. morphine usage () within cycles and from cycle to cycle indicate that the LTI is well tolerated and allows treatment in the outpatient setting: during cycle 1, 34/49 patients (69.4%) received part of the continuous ch14.18/CHO infusion as outpatients and this percentage increased to >90% in subsequent cycles.
Figure 1. Treatment schematic, pain assessment and intravenous morphine usage during LTI of ch14.18/CHO. A) Ch14.18/CHO was administered by LTI of 100 mg/m2 (d8–17) (horizontal bar) with 6 × 106 IU/m2 s.c. IL-2 (d1–5; 8–12) (black arrows) and p.o. isotretinoin (160 mg/m2/day d22–35). Pain toxicity of anti-GD2 antibody ch14.18/CHO was evaluated by systematic assessments of pain scores and intravenous morphine usage of 49/53 evaluable patients as described in the “Patients and Methods” section. B) Pain assessment scores were determined three times daily per patient and cycle. Data represent mean ± SEM. C) Usage of i.v. morphine in µg/kg/h was determined daily per patient and cycle and presented as mean ± SEM. When error bars are not visible, they are covered by the symbol. Total morphine usage per cycle ± SEM is indicated in mg/kg/cycle.
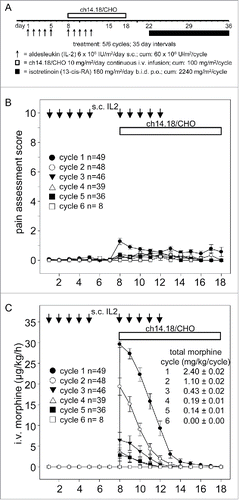
In summary, LTI of ch14.18/CHO is associated with acceptable pain toxicity profile and can be delivered in an outpatient setting.
We also evaluated the overall toxicity profile with this new treatment regimen. For this purpose, the frequency of Grade ≥3 adverse events (AEs) observed in >10% of our 53 patients treated by LTI (10 d; 100 mg/m2/cycle ch14.18/CHO combined with IL-2) was determined (). The incidence of Grade ≥3 neuropathic pain was 37.7% in this cohort. The most frequently observed AEs concerned the gastrointestinal system (constipation, vomiting, abdominal pain, diarrhea), general conditions (pain, pyrexia), skin (pruritus, dry skin), vascular disorders (capillary leak syndrome), and the musculoskeletal system (pain). Other frequent Grade ≥3 AEs considered related to ch14.18/CHO given in combination with IL-2 were cough, pruritus and capillary leak syndrome (). There were in total 16 Serious Treatment Emergent Adverse Events (SAEs) in 12 patients: 6 gastrointestinal disorders, 3 general disorder and administration site condition, 3 infections and infestations, 1 metabolism and nutrition disorder, 2 nervous system disorders, 1 respiratory disorder. The most common severity grade of SAE was Grade 3. Only two SAEs, convulsion and hyperkalemia, were of Grade 4 severity, and both recovered without sequelae. A total of 8 SAEs were considered to be at least possibly related to the study medication treatment. One of these SAEs was a patient developing floppy paresis of the lower extremities as a result of myelitis. This SAE also resolved without sequelae. There was no Grade 5 toxicity observed with this treatment. These results provide a base line to compare observed toxicity profiles with other reported regimen.Citation3
Analysis of response and survival
Disease overall response assessment was evaluable in 37/53 patients with measurable disease at baseline according to iodine-123-meta-iodobenzylguanidine (mIBG) scan or measurable disease by mIBG- or CT/MRI-scan evaluated by RECIST (). Five of 37 (13.5%) patients achieved complete response (CR) and 10/37 (27%) patients had partial response (PR) as the best response. Thus, in this population the best response rate was 40.5% (). The responses were confirmed by external review.
Progression-free survival (PFS) and overall survival (OS) observed with the LTI treatment regimen was 33.1% at 4.9 y (mean: 2.2 y; median: 1.6 y; range: 0.1- 4.9 y) () and 47.7% at 5.2 y (mean: 3.6 y; median: 3.5 y, range: 0.27- 5.20 y) (), respectively. Within our cohort, all relapsed patients (N = 29) were identified in order to compare them with a matched patient cohort according to stage, age and MYCN amplification available from the AIEOP data base (). Patients with relapsed disease treated by LTI had lower PFS and OS rates than the entire cohort of 18.1% at 4.9 y (mean: 1.6 y; median: 0.6 y; range: 0.11- 4.92 y) () and of 41.2% at 4.9 y (mean: 3.2 y; median: 3.4 y; range: 0.27- 4.92 y) (), respectively. This observation is in line with reports that relapsed NB patients have an inferior outcome compared to patients with refractory disease.Citation11
Figure 2. Analysis of survival and time to progression following LTI of ch14.18/CHO. Patients treated by LTI of 100 mg/m2 ch14.18/CHO in combination with 6 × 106 IU/m2 s.c. IL-2 (d1–5; 8–12) and oral 13-cis RA (d19–32) were analyzed for progression-free survival (PFS) (A) and overall survival (OS) (B) using the Kaplan-Meier method. Patients of the entire cohort (n = 53) and patients with relapsed status at base line (n = 29) were analyzed separately. A) PFS curves of the entire LTI cohort (red) and relapsed patients (blue) (top panel). B) OS of the entire LTI cohort (red) and relapsed patients (blue) was compared to relapsed patients of the AIEOP data base not treated with ch14.18/CHO (green).Citation21 The starting point of the AIEOP relapsed patients equals to the date of first relapse plus the median time between relapse and start of ch14.18/CHO therapy for the LTI patients (1 y 7 d). Patients in the AIEOP relapsed group who died before the auxiliary starting point were excluded. The difference between LTI relapsed- and AIEOP relapsed- patients was statistically significant (P = 0.002).
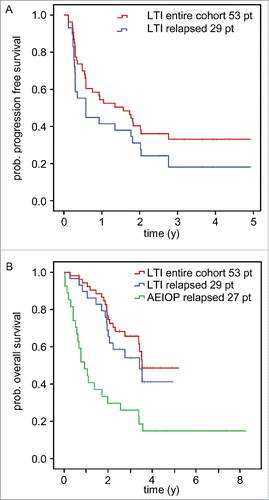
Patients (n = 29) with relapsed disease treated by LTI were matched with a control patient population (n = 27) from the AIEOP data base (). Patients of both groups were not different with regard to demographics and baseline characteristics (). The OS rate of the control group was 14.8% at 8.2 y (mean: 2.2 y; median: 0.9 y; range: 0.38- 8.27 y). The OS rates at 1-, 2-, 3-, 4 y of the LTI- and the control-group were 90%, 69%, 54%, 41% and 48%, 30%, 26%, 15%, respectively. The difference in OS between the groups was statistically significant (P = 0.002). When adding prognostic factors for OS that are used for risk-group assignment to a Cox model (i.e., categorized age at diagnosis, gender, MYCN amplification, and INSS stage), the difference in OS between LTI patients and the historic control group remained significant (P = 0.002) in favor of LTI.
Discussion
Application of anti-GD2 Ab ch14.18 is a treatment option for children with NB,Citation1–3 although the induction of pain is an on-target side effect. In order to improve the toxicity profile, we investigated a new delivery method by long-term continuous infusion of 100 mg/m2 per cycle over 10 days, which was described to be a clinically active and effective cumulative dose in the treatment of NB.Citation3,10
We compared the frequencies of Treatment Emergent Adverse Events (TEAEs) of Grade ≥3 observed in our cohort () to published results of STI of ch14.18 produced in SP2/0 cells (ch14.18/SP2/0)Citation3 and found a lower frequency of neuropathic pain with LTI vs. STI (37.7% vs. 51.8%, respectively). Other TEAEs of Grade ≥3 were also less frequent in our cohort compared to that study: capillary leak syndrome (13.2% vs. 22.6%), pyrexia (9.4% vs. 38.7%), hypoxia (5.7 vs. 13.1%), diarrhea (3.8 vs. 13.1%), hypotension (1.9 vs. 17.5%). None of the TEAEs of Grade ≥3 frequencies reported in the study with STI of ch14.18/SP2/0Citation3 were lower compared to LTI of ch14.18/CHO as observed here ().
In another study, STI of hu14.18 K322 was assessed in a Phase 1 study to determine the safety profile. Hu14.18K322A is a humanized anti-GD2 mAb with a single point mutation (K322A) that reduces complement-dependent lysis. The induction of neuropathic pain by anti-GD2 Ab was reported to be associated with complement activation, based on a comparison of ch14.18 with hu14.18K332A in an allodynia animal model.Citation12 However, in the clinical study of hu14.18 K322, a frequency of grade ≥3 neuropathic pain occurred in 68% of patients,Citation10 which is in sharp contrast to the observation with ch14.18/CHO LTI of 37.7% (). This observation suggests that the delivery method of anti-GD2 Ab by LTI has a great impact on reduction of on-target pain toxicity, and may be more important than deletion of the complement binding site by antibody engineering.
When changing the delivery method of a given treatment, clinical activity and efficacy of the drug are the critical aspects. We previously showed that application of ch14.18/CHO by LTI results in trough concentration of ≥ 1 µg/ml at time points preceding subsequent Ab infusions, allowing a persistent activation of Ab effector mechanisms over the entire treatment period of 6 monthsCitation13 Here, we report that the new delivery method also induced objective clinical responses (). Importantly, this clinical activity translated into a significant prolongation of the OS rate compared to historical controls ().
We also compared our observations with a reported historical gold standard for time-to-progression (TTP) and PFS from relapsed/refractory NB modern era patients (2002 – 2014)Citation14 To form this gold standard, 384 distinct patients on Phase 1/2 Children's Oncology Group trials were analyzed for PFS (relapse, progression, death from disease), OS (death- any cause), and TTP, starting from Phase 1/2 trial enrollmentCitation14 A standard was defined with 1 y and 4 y PFS of 19±2% and 8±3%, an OS of 56±3% and 14±4% and a median TTP of 63 d (95% CI: 56.8 d). Patients treated by LTI revealed higher percentages in 1 y and 4 y PFS- (entire cohort 54±7% and 33±7%; relapsed 41±9% and18±8%) and OS rates (entire cohort 93±4% and 48±9%; relapsed 90±6% and 41±11%) and showed a prolongation of TTP for the entire cohort (>9 fold) or the relapsed patients (>3 fold).
Despite the limitations of our study, which was a first single-center experience, without control group, that required historic controls and literature reports for comparison, we observed a strong effect size of clinical activity and efficacy, as well as decreased toxicity. In conclusion, the toxicity and efficacy profile of ch14.18/CHO LTI reported here may constitute for the first time a substantial step forward for this type of immunotherapy in patients with NB, which is currently subject to prospective clinical trials.
Patients and methods
Fifty-three patients with high-risk NB according to the INSS criteriaCitation15 were treated by using a 10 d continuous LTI of 100 mg/m2 ch14.18/CHO (d8–17) combined with 6 × 106 IU/m2 s.c. IL-2 (d1–5; 8–12) and 160 mg/m2 oral 13-cis RA (d19–32) (treatment schematic , patient characteristics ). Patients were treated in a compassionate use program reviewed by the ethical committee of the medical University of Greifswald. A total of 244 cycles were applied.
Table 1. Demographics and characteristics of high-risk NB patients treated by long term infusion (LTI) of ch14.18/CHO at initial diagnosis and at the time of LTI treatment start.
Table 2. Treatment Emergent Adverse Events (TEAEs) of Grade ≥3 related to ch14.18/CHO LTI combined with IL-2.
Table 3. Treatment response in 37 evaluable relapsed/refractory patients with measurable disease at baseline.
Table 4. Demographics and baseline characteristics of relapsed NB patients evaluable for historical comparison.
Co-medication included i.v. morphine (cycle 1: 30 µg/kg/h, d8 as long as needed; cycle ≥2 as needed); oral gabapentin (all cycles: 10 mg/kg/d d5; 2 × 10 mg/ kg/d d6; 3 × 10 mg/kg/d d8–15 or longer as needed) and metamizole (all cycles: 80 mg/kg/d continuous d8–15 or longer as needed).
Patient characteristics: Median age at diagnosis and at treatment start was 4.41 y [0.5–24.1] and 7.08 [1.9–25.5 y], respectively. INSS Stage 4: 88%, % MYCN amplified: 31% (). Of the 53 patients, 29 patients had relapsed disease, 19 were primary refractory (≥ 2 lines of conventional treatment) and 5 patients were front-line patients treated after completion of autologous stem cell transplantation (). As first-line therapy, all 53 patients received intensive multimodality treatment including high dose chemotherapy followed by autologous stem cell transplantation.
Clinical assessments
The clinical study protocol defined safety and tolerability assessments by recording pain intensity, morphine use, incidence, grade and type of AEs, vital signs and changes in clinical laboratory findings. The response rate was determined in patients with measurable/evaluable disease (skeletal lesions, soft tissue lesions, lymph nodes and/or primary tumor site, bone marrow) as measured by 123I mIBG scan, CT/MRI and/or bone marrow examination at the end of cycle 3 and at the end of treatment (after 5th or 6th cycle). OS and PFS were calculated as number of days from starting the CU-LTI treatment until relapse or disease progression. Detection of disease progression was done by any of the 3 methods, CT/MRI, 123I mIBG scan or bone marrow examination.
Safety and tolerability assessments
Pain toxicity was evaluated by a three times daily assessment of the patient using a validated age-adapted pain scoring system. It was applied by parents or the medical team using the validated KUSS, MOPS and Ramsay scores.Citation16–19 Furthermore, the daily use of i.v. morphine in µg/kg/h was recorded. Complete data sets were available for 49/53 patients. All patients were included in an overall safety assessment of AEs using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0).
Efficacy evaluation
Response rate was measured by RECIST Criteria Version 1.1 using CT/MRI after 3 cycles, at end of treatment (after 5th or 6th cycle) and at follow up evaluations. CTs or MRIs were used for assessment of soft tissue lesions only. Response rate was also measured by 123I mIBG after 3 cycles, at end of treatment (after 5th or 6th cycle) and at follow up evaluations. Response rate measurements also included bone marrow examination (cytology, immunocytology, flow cytometry or MRD analysis – Automatic Immunofluorescence plus FISH (AIPF).Citation20 Patients with measurable bone marrow disease at any examination were considered as evaluable for efficacy evaluation. With these parameters, an overall response was determined following International Neuroblastoma Risk Group criteria.Citation15 External review of mIBG scans was performed to validate tumor response (Keosys, 1 Impasse Augustin Fresnel, 44800 Saint-Herblain, France).
Survival analysis in comparison to the AIEOP data base
In order to obtain a historical control population comparable to our patient cohort, only patients with relapsed NB as baseline disease status were compared between our patient cohort and patients registered in the AIEOP database. This group was selected because relapsed patients constitute the largest group in our cohort and relapsed patients are different from refractory patients associated with an inferior outcome;Citation11 It is therefore the largest and most homogeneous group.
The selection criteria date of initial diagnosis ≥ 1999, age at initial diagnosis ≥12 months, age at first relapse ≥ 12 months and INSS stage 4 at initial diagnosis or type of first relapse “not local” were applied to the patient group described.Citation21 OS was defined as time from starting the LTI treatment until death or last evaluation, regardless of disease status. PFS was calculated as number of days from starting the LTI treatment until disease progression, and progression was evaluated by any of the 3 methods, CT/MRI, 123I mIBG scan or bone marrow examination (cytology, immunocytology, flow cytometry and/or MRD analysis – AIPF).
The starting point of our single center program patients was the day of starting the LTI treatment. Since the historical control patients had not been treated with ch14.18/CHO, an auxiliary starting point had to be defined. For the historical control patients, the starting point was the date of first relapse plus the median time (1 y 7 d) between first relapse/progression and start of Ab therapy for the single center program patients. Patients in the historical control group who died before the auxiliary starting point were excluded.
Conflict of interest statement
The authors declare no conflict of interest.
Financial support
This work was supported by the “Hector Stiftung”, the “Kinderkrebsstiftung” and Apeiron Biologics.
Acknowledgments
Funding provided by the Hector Stiftung, Kinderkrebsstiftung and Apeiron Biologics was used for data collection and analysis. Apeiron Biologics provided ch14.18/CHO antibody for the treatment of patients. We thank Sandra Pasewald and Ergomed (CRO) for data collection and management and Keosys for external review of response data.
References
- Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13(6):397–411. PMID:23702928
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, Berthold F. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21. PMID:21244693
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334.
- Zeng Y, Fest S, Kunert R, Katinger H, Pistoia V, Michon J, Lewis G, Ladenstein R, Lode HN. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol. 2005;42(11):1311–1319.
- Ladenstein R, Weixler S, Baykan B, Bleeke M, Kunert R, Katinger D, Pribill I, Glander P, Bauer S, Pistoia V, et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: a SIOPEN Phase 1 study. MAbs. 2013;5(5):801–809.
- Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, Spitzer T, Strandjord SE, Coccia PF, Berger NA. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5(9):1430–1440. PMID:3625258
- Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, Kung FH. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16(6):2169–2180. PMID:9626218
- Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, Reuland P, Gillies SD, Reisfeld RA, Neithammer D. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur J Cancer. 1995;31A(2):261–267. PMID:7718335
- Xiao WH, Yu AL, Sorkin LS. Electrophysiological characteristics of primary afferent fibers after systemic administration of anti-GD2 ganglioside antibody. Pain. 1997;69(1–2):145–151. PMID:9060025
- Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, Gan J, Hutson P, Seo S, Kim K, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32(14):1445–1452. doi:10.1200/JCO.2013.50.4423. PMID:24711551
- Moreno L, Rubie H, Varo A, Le Deley MC, Amoroso L, Chevance A, Garaventa A, Gambart M, Bautista F, Valteau-Couanet D, et al. Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer. 2017;64(1):25–31. doi:10.1002/pbc.26192. PMID:27555472
- Sorkin LS, Otto M, Baldwin WM, III, Vail E, Gillies SD, Handgretinger R, Barfield RC, Ming Yu H, Yu AL. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149(1):135–142. doi:10.1016/j.pain.2010.01.024. PMID:20171010
- Siebert N, Eger C, Seidel D, Juttner M, Zumpe M, Wegner D, Kietz S, Ehlert K, Veal GJ, Siegmund W, et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. MAbs. 2016;8(3):604–616. doi:10.1080/19420862.2015.1130196. PMID:26785755
- London WB, Bagatell R, Weigel B, Fox E, van Ryn C, Naranjo A, et al. Historical gold standard for time-to-progression (TTP) and progression-free survival (PFS) from relapsed/refractory neuroblastoma modern era (2002–2014) patients. J Clin Oncol. 2014;32(5s):5–6. PMID:24190110
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27(2):289–297. doi:10.1200/JCO.2008.16.6785. PMID:19047291
- Buttner W, Breitkopf L, Miele B, Finke W. [Initial results of the reliability and validity of a German-language scale for the quantitative measurement of postoperative pain in young children]. Anaesthesist. 1990;39(11):593–602. PMID:2288408
- Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2(5920):656–659. doi:10.1136/bmj.2.5920.656. PMID:4835444
- Zernikow B, Hechler T. Pain therapy in children and adolescents. Dtsch Arztebl Int. 2008;105(28–29):511–521. PMID:19626208
- Wilson GA, Doyle E. Validation of three paediatric pain scores for use by parents. Anaesthesia. 1996;51(11):1005–1007. doi:10.1111/j.1365-2044.1996.tb14991.x. PMID:8943588
- Mehes G, Luegmayr A, Hattinger CM, Lorch T, Ambros IM, Gadner H, Ambros PF. Automatic detection and genetic profiling of disseminated neuroblastoma cells. Med Pediatr Oncol. 2001;36(1):205–209. doi:10.1002/1096-911X(20010101)36:1%3c205::AID-MPO1050%3e3.0.CO;2-G. PMID:11464886
- Garaventa A, Parodi S, De BB, Dau D, Manzitti C, Conte M, Casale F, Viscardi E, Bianchi M, D'Angelo P, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45(16):2835–2842. doi:10.1016/j.ejca.2009.06.010. PMID:19616426
