ABSTRACT
Anti-idiotypic antibodies play an important role in pre-clinical and clinical development of therapeutic antibodies, where they are used for pharmacokinetic studies and for the development of immunogenicity assays. By using an antibody phage display library in combination with guided in vitro selection against various marketed drugs, we generated antibodies that recognize the drug only when bound to its target. We have named such specificities Type 3, to distinguish them from the anti-idiotypic antibodies that specifically detect free antibody drug or total drug. We describe the generation and characterization of such reagents for the development of ligand binding assays for drug quantification. We also show how these Type 3 antibodies can be used to develop very specific and sensitive assays that avoid the bridging format.
Abbreviations: BAP: bacterial alkaline phosphatase; CDR: complementarity-determining regions in VH or VL; Fab: antigen-binding fragment of an antibody; HRP: horseradish peroxidase; HuCAL®: Human Combinatorial Antibody Libraries; IgG: immunoglobulin G; LBA: ligand binding assay; LOQ: limit of quantitation; NHS: normal human serum; PK: pharmacokinetics; VH: variable region of the heavy chain of an antibody; VL: variable region of the light chain of an antibody.
Introduction
Therapeutic antibodies and antibody fragments are an extremely fast-growing class of drugs due to their excellent specificity, high affinity, long in vivo half-life and potent agonistic or antagonistic properties. Today, over 70 antibody-based drugs have been approved for treatment of a variety of diseases, and hundreds are in clinical development.Citation1 A number of companies develop biosimilar versions of antibodies that have lost patent protection or will soon,Citation2 and the first biosimilars of several drugs have reached the market.Citation3 Successful development of therapeutic antibodies requires consistent bioanalytical methods for characterization in pre-clinical and clinical phases. For instance, ligand binding assays (LBAs) are often performed to determine the pharmacokinetic (PK) profile by quantitating the therapeutic protein in biological matrices such as serum. Such assays are also increasingly used after market entry to monitor drug levels of patients during therapy.Citation4,Citation5 As therapeutic antibodies are often humanized or human antibodies and need to be measured in human serum, the LBAs need to provide high specificity to bind the drug antibody in the presence of a large excess of human serum immunoglobulin.
LBAs can be built using the drug target to capture the drug from serum samples, in combination with a generic anti-Ig detection reagent, but often the use of drug-specific antibodies is preferred. Anti-drug antibodies are mostly anti-idiotypic antibodies, as they recognize the idiotopes of the therapeutic antibody. The idiotope contains the hypervariable regions of the antibody, and therefore comprise epitopes that are unique for the drug molecule. Such reagents have been developed by animal immunizations and by in vitro methods such as phage display of antibody libraries.Citation6–Citation9 PK assays making use of such reagents are typically built using the bridging assay format, taking advantage of the two binding sites of the antibody drug – the therapeutic antibody is captured from serum with an anti-idiotypic antibody binding to one binding site, and detection is performed with a second labeled anti-idiotypic antibody that binds to the second binding site.Citation10 Bridging assays require a low coating density of the capture reagent to avoid the therapeutic antibody binding to the surface with both arms, which would reduce the sensitivity of the assay.Citation11 In addition, they do not work with monomeric drug antibodies like the antigen-binding fragment (Fab).
As the circulating drug antibody may interact with soluble or shed target in serum, there may be several forms of drug antibody present, either free, partially bound or fully bound drug. Consequently, it is important to determine what forms are measured with a given LBA, especially if the concentration of soluble drug target is not negligible compared to the drug concentration at the time point of measurement.Citation12 There is still an ongoing debate about what form of the drug is the most relevant to measure.Citation13
Anti-idiotypic antibodies that bind to the paratope of the therapeutic antibody typically interfere with drug-target binding and are therefore inhibitory. Such antibody reagents bind to free drug. Non-inhibitory anti-idiotypic antibodies bind outside the paratope and do not interfere with target binding, thereby allowing detection of free, partially bound and fully bound drug.Citation13–Citation15
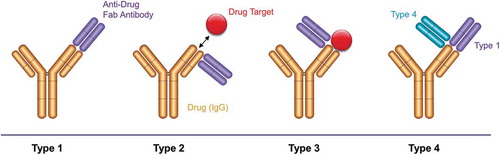
Here, we introduce another type of antibody reagent, which is not an anti-idiotypic antibody but a reagent that recognizes the complex of drug and drug target (). We have named this reagent as Type 3 antibody, to distinguish it from inhibitory and non-inhibitory anti-idiotypic antibodies, which we refer to as Type 1 and Type 2, respectively. Type 3 reagents are highly specific for the complex and recognize neither free drug nor target. They can be obtained by in vitro panning of phage display libraries on the drug-target complex with a suitable blocking protocol. We show here the generation and characterization of Type 3 antibodies for several approved drug antibodies using the Human Combinatorial Antibody Libraries (HuCAL®) technology.Citation16,Citation17 We discuss strategies for obtaining such reagents, and show detailed characterization using several assay formats. We show that Type 3 reagents can be used to detect free or bound drug, or to develop LBAs without using the bridging assay format, which typically leads to higher assay sensitivity and also works for antibody fragments, such as ranibizumab. Finally, we introduce a Type 3 related antibody specificity (named Type 4) binding to a complex of a drug antibody with a Type 1 reagent, which can be useful if the drug target is not easily available or if Type 3 antibodies cannot be generated against a given drug-target complex.
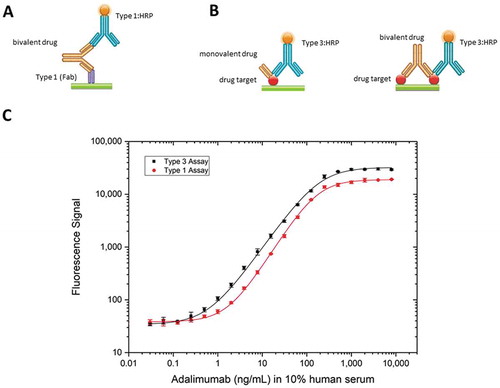
Figure 1. Binding modes of anti-biotherapeutic antibodies. Type 1 anti-idiotype antibodies bind the paratope of the drug (yellow). They inhibit drug-target binding and detect free drug. Type 2 anti-idiotype antibodies bind outside the drug paratope and do not interfere with target (red) binding. They are used to detect total drug levels. Type 3 antibodies are specific for the drug-target complex and exclusively detect bound drug. The anti-drug antibodies (purple) are shown as Fabs for clarity. Type 4 antibodies are specific for a complex formed between the drug and a Type 1 antibody.
Results
Selection of Type 3 antibodies (ranibizumab, adalimumab, golimumab, trastuzumab, omalizumab)
The Fab phage display library HuCAL PLATINUMCitation16 was used for the generation of drug-target complex specific antibodies shown in . All selections of Type 3 antibodies were performed on the antibody drug-target complex () that was formed by immobilizing either drug or drug target and subsequent incubation with drug target or drug, respectively. Blocking of the antibody phage library was performed with drug target and isotype matched antibodies, and for some selections, with human serum and/or drug antibody (not used together with drug target in the same panning round) to guide the selection towards antibodies specific for the complex that do not bind drug or drug target alone. After the selection rounds the antibody genes were sub-cloned as a pool into a vector for expression of a monovalent Fab equipped with a FLAG- and a His-tag for detection and purification. Primary screening of 368 expression lysates from each selection was performed by ELISA on drug-target complex, drug, drug target and isotype control antibodies. Clones that only showed binding to the complex were sequenced and unique antibodies were expressed and purified by affinity chromatography.
Table 1. Details on generation and affinity of Type 3 and 4 antibodies used in this study.
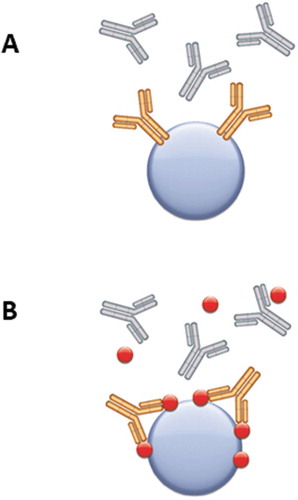
Figure 2. Guided selection strategies for generation of Type 1, 2 and 3 anti-biotherapeutic antibodies by phage display. (a) For Type 1 antibodies, selection on the drug (yellow) coupled to magnetic beads (blue sphere) in the presence of isotype/subclass matched antibodies (gray) avoids enrichment of specificities that bind to the constant regions of the antibody drug. (b) For Type 2 and Type 3 antibodies, selections are performed on the drug-target complex, and blocking is done with isotype/subclass matched control antibodies plus the drug target (red).
Three Type 3 antibodies (AbD18754, AbD20893 and AbD28276) were affinity matured by inserting pre-built trinucleotide librariesCitation18,Citation19 into the heavy chain complementarity-determining region (CDR) 2 and/or the light chain CDR3. Selections were carried out using increased stringency, i.e., more and longer washing steps during the panning rounds, and in all cases the affinity of the antibodies was improved () while maintaining the specificity of the parental antibody. The highest affinity improvement was 22-fold for the maturation of the anti-adalimumab/tumor necrosis factor (TNF) antibody AbD18754.
Some of the antibodies were converted from the monovalent Fab into a bivalent format because this usually leads to an increased functional affinity, and therefore also better sensitivity in assays when used as detection reagent.Citation20,Citation21 Type 3 antibodies AbD20349, AbD20893 and AbD20760 and the Type 1 antibody AbD18655 were therefore converted into human immunoglobulin G isotype 1 (IgG1) and produced in mammalian cell culture. The bivalent anti-trastuzumab/ErbB2 Type 3 antibody AbD25279 in the Fab-A-FH format was converted from the monovalent antibody AbD24937. Here the Fd chain (consisting of the variable domain (VH) and the first constant domain (CH1) of the antibody heavy chain) is fused to dimeric bacterial alkaline phosphatase (BAP) followed by FLAG- and His-tag.
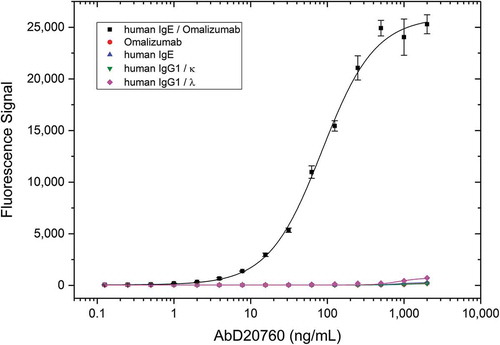
The specificity of the purified antibodies for the respective drug-target complexes was confirmed by titration ELISA; shows an example for the anti-omalizumab/IgE complex specific antibody AbD20760. Specificity titration ELISAs for the other Type 3 antibodies used in this study are shown in Supplementary Figure 1.
Figure 3. Demonstration of Type 3 antibody specificity. A microtiter plate was coated overnight with the omalizumab target human IgE, omalizumab, human IgG1/κ or human IgG1/λ at a concentration of 5 µg/mL. After washing and blocking with PBST + 5% BSA, the omalizumab/human IgE complex was formed by adding 2 µg/mL omalizumab to the wells coated with human IgE. Detection was performed with HRP-conjugated Type 3 anti-omalizumab/hIgE antibody AbD20760 in HISPEC assay diluent, followed by QuantaBlu fluorogenic peroxidase substrate.
Use of secondary reagents for detection
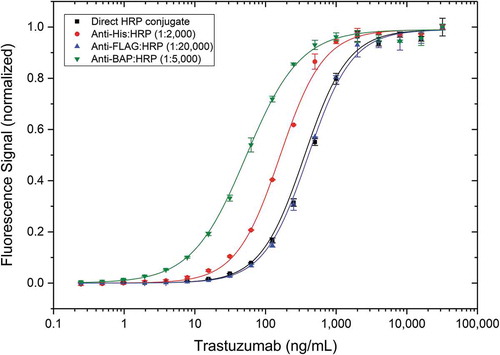
Due to the recombinant nature of the Type 3 antibodies with several tags attached as described here, detection of the drug-target complex can be performed with a range of secondary reagents. Type 3 antibody AbD25279 in the format Fab-A-FH was used to evaluate various suitable secondary reagents and compare them with directly horseradish peroxidase (HRP)-conjugated reagent (). The trastuzumab/ErbB2 complex was detected using either the directly HRP-conjugated Type 3 antibody or by addressing different tags of this antibody (His, FLAG or BAP) in combination with a corresponding HRP-conjugated anti-tag secondary antibody. A concentration-dependent signal was generated with all reagents, albeit with different sensitivities. Detection with high-affinity anti-BAP:HRP resulted in an about 10-fold higher sensitivity than detection with anti-FLAG:HRP or detection with directly HRP-conjugated AbD25279. Detection with anti-His:HRP led to intermediate sensitivity.
Figure 4. Use of different secondary antibodies in a Type 3 PK assay. Human ErbB2 was coated at 5 µg/mL on a microtiter plate overnight. After washing and blocking, trastuzumab spiked into 10% human serum was added. Anti-trastuzumab/ErbB2 antibody AbD25279 was added at 2 µg/mL in HISPEC assay diluent, either directly HRP-conjugated (black), or followed by different HRP-conjugated secondary antibodies in their recommended dilutions, and QuantaBlu fluorogenic peroxidase substrate. Obtained signals were normalized for better comparison of assay curves.
Influence of serum concentration on PK assay activity
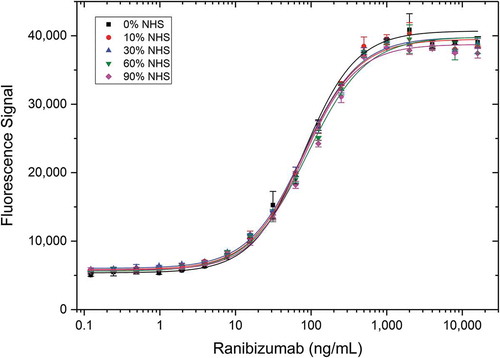
As patient samples are mostly provided as serum for PK assays, we tested whether serum matrix components may have an influence on the assay using a Type 3 antibody for drug quantification. Type 3 antibody AbD29928 directed against the ranibizumab/vascular endothelial growth factor (VEGF) complex was evaluated using an antigen capture assay with increasing serum levels. Ranibizumab in different concentrations was spiked in 0 to 90% normal human serum (NHS) and added to immobilized VEGF to form the drug-target complex. Detection of the complex was then performed with the Type 3 antibody (). All curves display the same binding behavior, indicating that the assay performance is not influenced by matrix components in up to 90% human serum. In addition, demonstrates detection of an antibody fragment (ranibizumab) by a Type 3 reagent.
Figure 5. Detection of ranibizumab (Fab antibody drug) in different serum concentrations using a Type 3 antibody. VEGF was coated at 5 µg/mL on a microtiter plate overnight. After washing and blocking, ranibizumab spiked into different concentrations of normal human serum (NHS) was added in increasing amounts and incubated for 1 hour at room temperature. Detection was performed with HRP-conjugated anti-ranibizumab/VEGF antibody AbD29928 in HISPEC assay diluent, followed by QuantaBlu fluorogenic peroxidase substrate.
Type 3 antigen capture assay compared with bridging assay
The bridging assay is the most common LBA for PK studies. This assay requires the antibody drug to be bivalent. However, some drugs such as ranibizumab are developed as monovalent Fabs, which is incompatible with the bridging assay setup. Also, bridging assays are limited by the coating density of the capture reagent, as binding of the drug with both arms to the surface needs to be avoided, which in turn may reduce assay sensitivity. As an alternative to the bridging assay format (), a Type 3 antibody can be used in a PK antigen capture assay () with either a monovalent or a bivalent drug and equivalent high sensitivity can be achieved ( for monovalent and () for bivalent antibody drugs). Comparison of a typical bridging assay for adalimumab with a Type 3 antigen capture assay for recognizing the adalimumab/TNF complex showed a slightly higher sensitivity for the Type 3 assay (). Using the Type 3 antibody AbD18754, the limit of quantitation (LOQ) achieved for the antigen capture assay was 0.7 ng/mL, about 2.5-fold lower than the LOQ of 1.8 ng/mL achieved with the bridging assay using the Type 1 antibody AbD18655. Remarkably, the affinity of the Type 3 detection antibody (AbD18754, KD = 67 nM) is more than 1,000-fold lower than the high-affinity Type 1 detection antibody (AbD18655, KD = 0.06 nM) used in the bridging assay, illustrating the advantage of the Type 3 antigen capture assay.
Figure 6. Comparison of assay formats. (a) Schematic view of the assay set-up: bridging assay set-up using a Type 1 reagent for capture and an HRP-conjugated Type 1 antibody for detection of a bivalent drug and (b) antigen capture assay using an HRP-conjugated Type 3 antibody for detecting a monovalent or bivalent drug in complex with its drug target. (c) Detection of adalimumab using a Type 3 antibody in antigen capture assay (black), or Type 1 capture and detection antibodies in a bridging assay (red). For the antigen capture assay, human TNF was coated at 5 µg/mL on a microtiter plate overnight. After washing and blocking, adalimumab spiked into 10% human serum was added. HRP-conjugated anti-adalimumab/TNF antibody AbD18754 (KD = 67 nM) was added at 2 µg/mL in HISPEC assay diluent, followed by QuantaBlu fluorogenic peroxidase substrate. For the adalimumab bridging assay, the anti-adalimumab Type 1 antibody AbD18654 (KD = 0.16 nM) was coated at 1 µg/mL on a microtiter plate overnight. After washing and blocking, adalimumab spiked into 10% human serum was added, followed by HRP-conjugated Type 1 anti-adalimumab antibody AbD18655 (KD = 0.06 nM) at 2 µg/mL in HISPEC assay diluent and QuantaBlu fluorogenic peroxidase substrate.
Immunocapture of drug-target complexes by Type 3 antibodies
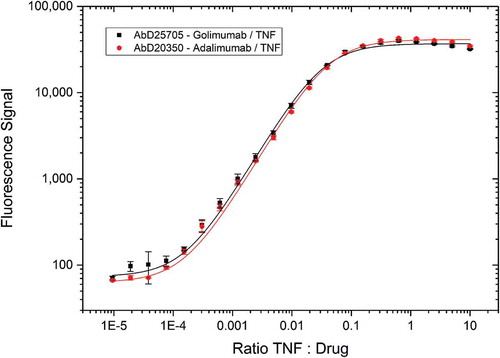
To evaluate the use of Type 3 antibodies for capturing soluble drug-target complexes, Type 3 reagents specific either for the golimumab/TNF or the adalimumab/TNF complex were tested in combination with increasing drug target concentrations (). For both Type 3 antibodies the curves are similar, with the signal starting at drug-to-target ratios of 10,000:1 and saturation reached above 10:1 drug-to-target ratios. Hence, the reagents could capture drug-target complexes from the solution even when free drug is present in 10,000-fold higher concentrations.
Figure 7. Immunocapture of drug/TNF complexes by Type 3 antibodies. Type 3 anti-golimumab/TNF antibody AbD25705 and Type 3 anti-adalimumab/TNF antibody AbD20350 were coated on a microtiter plate at 1 µg/mL. Golimumab and adalimumab at a fixed concentration (1 µg/mL = 7 nM) were incubated for 1 hour with an increasing amount of TNF and added to the plate. Detection was performed with anti-human IgG:HRP antibody specific for the CH2 domain that detects golimumab or adalimumab but not the Fab antibodies, followed by QuantaBlu fluorogenic peroxidase substrate.
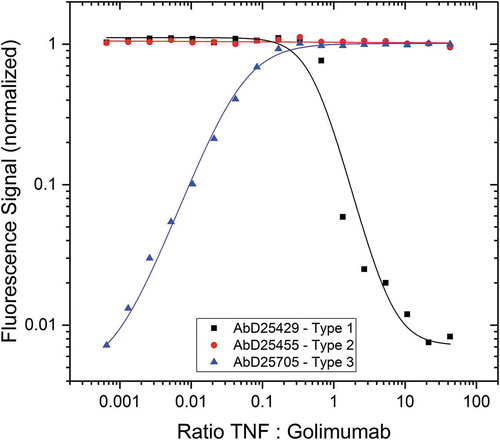
To demonstrate the different binding modes of the anti-idiotypic antibody formats Type 1 and 2 and the complex-specific format Type 3, we compared binding curves when capturing soluble golimumab under conditions of increasing concentrations of TNF (). Capture with Type 1 results in maximum signal until the (trimeric) TNF-to-golimumab ratio is about 1:2, indicating that at that ratio no free drug is available for binding. Type 2 antibodies bind to the drug regardless of the amount of drug target present, resulting in maximum signal regardless of drug target concentration. In contrast, the signal obtained from Type 3 antibody binding increases with increasing TNF amounts until saturation is reached, again at a TNF-to-golimumab ratio of about 1:2.
Figure 8. Immunocapture of golimumab/TNF complex by Type 1, 2 and 3 antibodies. The anti-golimumab/TNF antibodies AbD25429 (Type 1), AbD25455 (Type 2) and AbD25705 (Type 3) were coated on a microtiter plate. Golimumab at fixed concentration (300 ng/mL = 2 nM) was incubated for 1 hour with an increasing amount of TNF and added to the plate. Detection was performed with an HRP-conjugated anti-human IgG:HRP antibody specific for the Fc-CH2 domain that detects golimumab but not the Fab antibodies, followed by QuantaBlu fluorogenic peroxidase substrate.
Drug targets of some antibody drugs are relatively abundant in human serum. Therefore, a significant fraction of the drug will be present in complexed form. An example is the drug omalizumab, which binds to IgE. The level of IgE in plasma is usually 1 µg/mL or less,Citation22 with a mean level of about 300 ng/mL in healthy individuals. To determine the level of complexed omalizumab in serum, and thereby indirectly determining the IgE concentration, the corresponding Type 3 antibody AbD20760 was used as a capture antibody for normal serum spiked with omalizumab and the signals with a standard curve obtained from measuring the omalizumab/IgE complexes with increasing IgE concentrations were compared. Detection of omalizumab/IgE complexes was performed with an HRP-conjugated anti-human IgE antibody. Maximum fluorescence is seen when the omalizumab-to-IgE ratio is below 1, indicating that under these conditions all human IgE is present in its complexed form (Supplementary Figure 2). Fluorescence decreased with reducing IgE levels until background was reached at omalizumab-to-IgE ratios of 53:1 (equivalent to an LOQ of 0.7 ng/mL IgE). The amount of complexed omalizumab in NHS at different dilutions was quantified using the standard curve, and the IgE concentration in the NHS sample used for this experiment was calculated to be 186 ng/mL.
Robustness of Type 3 antigen-capture PK assays
The Type 3 antigen-capture PK assay was validated with regard to its precision and accuracy. Type 3 antibody AbD20893 directed against the golimumab/TNF complex was tested both as monovalent Fab and as bivalent IgG1. Recovery at three golimumab concentrations (500, 63 and 8 ng/mL) was assessed during assay qualification. Overall, the mean recovery rates ranged from 88–100%. Assays for both antibody formats were performed in triplicate for evaluation of the intra-assay precision and were repeated six times with independent assay setup to determine the inter-assay precision. Intra-assay precision was consistently at 7% or better and inter-assay precision was consistently less than 16% ().
Table 2. Assay accuracy (SD, %CV, Recovery).
A Type 4 antibody specifically detects the complex of drug bound to a Type 1 reagent
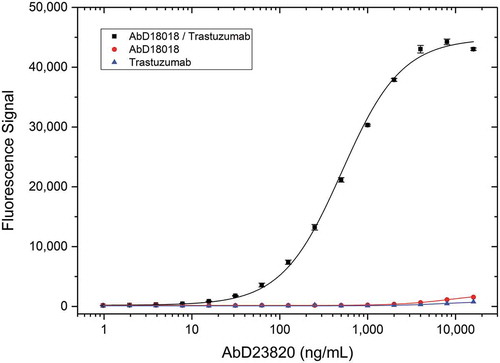
The Type 3 PK assay setup requires access to the drug target for capturing free drug from serum samples, but the drug target is not always available, or it may be quite expensive. We wondered whether the drug target could be replaced by a Type 1 antibody in this assay format. As a proof-of-concept, we isolated AbD23820 in a panning on the drug trastuzumab bound by the previously identified high-affinity Type 1 antibody AbD18018 used in IgG1 format. AbD23820 is the first example of a specialized derivative of the drug-target complex-specific Type 3 reagent, as it specifically recognizes a complex formed between the drug and a Type 1 reagent. To demonstrate specificity, AbD23820 was tested in a titration ELISA (). Antibody clone AbD23820 bound to the drug-Type 1 complex, but failed to bind to trastuzumab or the Type 1 antibody (AbD18018 in human IgG1 format) alone. We named this new type of specificity Type 4.
Figure 9. Type 4 specificity titration. Type 1 anti-trastuzumab antibody AbD18018 in IgG1 format or trastuzumab were coated on a microtiter plate at a concentration of 5 µg/mL. After washing and blocking, the trastuzumab/Type 1 complex was formed by adding 5 µg/mL trastuzumab to the wells coated with the Type 1 antibody. Type 1 and trastuzumab negative controls were incubated with buffer only. After washing Type 4 antibody AbD23820 specific for the trastuzumab/Type 1 complex was titrated in PBST and added. Detection was performed using HRP-conjugated anti-Strep-tag antibody in HISPEC assay diluent followed by QuantaBlu fluorogenic peroxidase substrate.
Discussion
When measuring the antibody drug concentration in a patient sample, it is critical to understand which drug species is being measured, i.e., free or (target-) bound drug.Citation23 When low amounts of drug target are present in serum, total and free drug species are often equivalent, and their detection is less dependent on the assay format. However, some drug targets are present in substantial amounts in patient serum,Citation24,Citation25 and others may accumulate during treatment and can in turn influence the measurement of drug concentration, depending on the assay.Citation26 The drug species being measured is dependent on the reagents available and used for assay development. Anti-idiotypic antibodies are frequently used for the development of PK assays because they provide excellent drug specificity and are useful tools to assess drug exposure and safety, as well as to characterize PK/pharmacodynamic (PD) relationships. Such antibody reagents have been made by animal immunization and by in vitro library methods.Citation7,Citation8 Monoclonal anti-idiotypic antibodies can either bind to the drug antibody paratope, competing with drug target binding (named Type 1 in this study), or they bind the drug outside the paratope independent of the presence of target (Type 2). Type 1 reagents detect free drug, whereas Type 2 can bind to both the free and the bound form of the drug and therefore detect total drug levels. Reagents that exclusively recognize the bound form of the drug have not been available so far.
Here, we report the generation and characterization of a series of antibodies specific for several approved therapeutic antibodies when bound to their respective targets. We have successfully isolated such specificities using the HuCAL PLATINUM antibody library in combination with CysDisplay,Citation16 a modified phage display method, by panning on drug-target complexes with simultaneous blocking of the library with both isotype-matched antibodies and drug target, thereby preferentially enriching clones with the desired specificity. Such guided selection strategies require an in vitro method of antibody generation such as phage display. A recent publication reports the isolation of a Type 3-like reagent using a traditional immunization approach based on immunization with drug-target complex and screening of hybridoma supernatants.Citation27 The majority of clones from this approach were not complex-specific, as expected, and the frequency of identifying Type 3 specificities was not reported. Our hit rate for selecting Type 3 specificities was on average about five-fold lower than with selections for Type 1 antibodies. This indicated that such specificities are quite rare, even when a large naïve library is used that usually yields several hundred hits in a standard selection. Indeed, in addition to the antibodies described here, we have performed such selections on numerous other antibody drugs and drug candidates. Our overall success rate was about 50%, whereas Type 1 antibodies are routinely selected with a 100% success rate. Type 3 antibodies require an epitope that is shared between the drug and the target in its complexed form, and the binding strength for each of the two sub-epitopes needs to be too low for detectable binding of the individual components in a typical LBA. If the bound drug target completely shields the drug idiotopes, it is not possible to select such specificities. This is apparently not dependent on the size of the target, as we successfully selected Type 3 specificities against large targets such as IgE (MW = 190 kDa) in the case of omalizumab. Another possible explanation for the lower success rate for selecting Type 3 specificities could be that the affinity between drug and target is sometimes not high enough to ensure a stable complex formation during the antibody selection process. However, we have not observed such correlation between affinity and success rate, and antibody drugs usually show a high affinity to their targets.
We performed several strategies for generation of Type 3 antibodies, using either drug-target (drug immobilized, followed by incubation with drug target) or target-drug complex (drug target immobilized, followed by incubation with drug) as antigen and using such complexes alternating in the panning rounds. Furthermore, different blocking conditions were used, e.g., for generation of the anti-ranibizumab/VEGF Type 3 antibody AbD29928, ranibizumab was also used for blocking in one panning round to avoid selection of Type 2 antibodies. So far, we have not found one superior strategy. But since only one strategy was successful for some drugs, it is advisable to try several strategies in parallel.
Drug-target complex-specific Type 3 antibodies show several properties that make them useful tools for quantification of free or bound drug levels. First, they are very specific for the complex and do not show binding for the drug or the target alone, even at high drug concentrations. Second, they can be used to detect free drug in a simple assay setup where the drug is captured by immobilized drug target and the complex is detected with a Type 3 reagent. This setup avoids the more sophisticated bridging format and does not suffer from background issues, typically seen when the captured drug is detected with an anti-human Fc reagent. Due to the assay setup the affinity requirements of the Type 3 antibodies are not very high and similar sensitivities are seen when compared with a bridging setup using Type 1 antibodies with several hundred-fold higher affinities. The assay was shown to be very robust with low CV values and good recovery. We also demonstrated that the high specificity prevents matrix effects with no change in performance when the drug is measured in 0% or 90% serum. If the Type 3 reagent is used as a Fab antibody equipped with suitable detection tags, a variety of secondary reagents for detection can be used. Third, the opportunity to avoid the bridging format makes it possible to quantify drug antibodies that are not of bivalent nature such as ranibizumab, which is a Fab. This should be also true for other fragment types such as the single-chain variable fragment format, or biotherapeutic drugs based on non-antibody protein scaffolds. Bispecific antibodies that would require two different anti-idiotypic reagents in a bridging assay may also benefit from the Type 3 assay setup. Fourth, Type 3 antibodies are complex-specific and can be used to specifically detect bound drug. We have demonstrated that complexes can be selectively captured by Type 3 antibodies even under conditions where free drug is present in large excess. Such specific detection of bound drug exclusively has not been possible so far with the Type 1 or 2 anti-idiotypic antibodies. With this additional specificity, further assay setups are conceivable. For instance, a combination of a Type 3 antibody as capture reagent with a Type 1 for detection could be used to detect exclusively half-bound drug, albeit different stoichiometries of drug-target complex formation dependent on concentration of the components would need to be considered.Citation28 Using golimumab and TNF as an example, we also show the different binding behavior of Type 1, 2 and 3 antibodies when used as capture reagents with increasing amounts of target present: Type 1 antibodies compete with target binding and lose the ability to bind the drug in the presence of high concentrations of target; Type 2 reagents bind the drug independent of the target concentration; Type 3 reagents capture the complex, the concentration of which increases with increasing target concentration until the drug is present exclusively as complex, leading to a constant signal even when the target concentration is further increased. Finally, we introduced a derivative of the Type 3 specificity, termed Type 4, where the drug target is replaced by a previously isolated Type 1 antibody, so that Type 4 reagents recognize the complex between drug and Type 1 anti-drug antibody. We used trastuzumab and the high-affinity Type 1 anti-trastuzumab antibody AbD18018 as an example for isolating the Type 4 specificity. We envisage the use of Type 4 for drug monitoring assays in cases where the drug target is not easily available or very expensive to produce, or where selection of Type 3 reagents is not possible.
In summary, the drug-target complex specific antibodies described here will be a valuable addition to the LBA toolbox for developing more sophisticated assays for PK analyses of biotherapeutics. The simplicity and robustness of directly recognizing the drug-target complex (for Type 3 reagents) or the drug/Type 1 complex (for Type 4 reagents) may allow development of simplified rapid tests for therapeutic drug monitoring during treatment.
Material and methods
Materials used for antibody generation
Commercially available antibody drugs adalimumab, golimumab, omalizumab, trastuzumab, rituximab, ustekinumab, alemtuzumab and ranibizumab were obtained from pharmacies in Munich and Puchheim, Germany. Drug targets and other materials used in antibody generation were: human TNF (recombinant human TNF-α, BioLegend 570108), human ErbB2-Fc (Sino Biological 10004-H02H), human IgE (Bio-Rad HCA171), human VEGF (Bio-Rad, produced in E. coli, amino acids Ala27-Glu140 plus amino acids Pro186-Arg191 with a C-terminal His- tag), normal human serum (NHS) (Sigma H4522), AbD18705 (hIgG1κ; Bio-Rad HCA192), AbD15790 (hIgG1λ; Bio-Rad HCA218) and human IgG1κ (Sigma I5154).
Antibody selection and affinity maturation
Anti-idiotypic antibodies were selected from the HuCAL PLATINUM phage display library.Citation16 summarizes panning and blocking conditions used for generation of Type 3 and 4 antibodies described in this publication. For the generation of drug/drug target complex specific antibodies (Type 3), either drug target or drug antibody were immobilized and incubated with drug antibody and drug target, respectively, to form the complex. Immobilization was done either by coating of drug or drug target onto a microtiter plate (solid phase panning) or by coupling to magnetic beads (Life Technologies #14011) for bead panning. Generation of Type 1 anti-idiotypic antibodies AbD18654 (Bio-Rad HCA202), AbD18655 (Bio-Rad HCA204) and AbD25429 (Bio-Rad HCA286) was as describedCitation29 by panning on immobilized drug in all three panning rounds and blocking with isotype-matched control antibody and human serum. The Type 2 anti-golimumab antibody AbD25455 (Bio-Rad HCA289) was selected from an alternating panning on the TNF/golimumab complex and golimumab, whereby blocking of the phage antibody library was done with hIgG1κ isotype control, ustekinumab, human serum and TNF (blocking only in the panning rounds performed on the complex). Selection of the Type 4 antibody AbD23820 was performed on complex formed between AbD18018 as IgG1 and trastuzumab. For standard panning, three rounds of phage display selection were performed, essentially as described by Jarutat et al.Citation30 For generation of anti-ranibizumab antibody AbD28276 (), RapMAT technologyCitation31,Citation32 was included in the antibody generation process, since we were aiming for antibodies with high affinity due to the low amounts of ranibizumab in patient sera. Three Type 3 antibodies () were affinity matured using the single binder maturation procedure as described by Steidl et al.Citation19 The two Type 1 antibodies AbD25429 (Bio-Rad HCA286) and AbD18018 (Bio-Rad HCA177) are derived from maturation pannings. Generation of AbD18018 was described by Ylera et al.,Citation29 AbD25429 was from a maturation of AbD20710 (Bio-Rad HCA240). More and longer washing steps were performed in the maturation pannings to increase the selection stringency. After panning, the selection output was sub-cloned into the expression vector pMorph11_Fab-FH,Citation16 which leads to expression of monovalent antibody Fabs with a FLAG- and a His-tag (Fab-FH format) or into expression vector pMorph11_Fab-V5Sx2, which leads to expression of monovalent antibody Fabs with a V5Citation33 and Twin-Strep-tagCitation34 (Fab-V5Sx2 format). E. coli TG1F- (TG1 without F plasmid) was transformed with the ligation mixture and plated. From each panning, 368 colonies were picked into a 384-well microtiter plate with a MegaPix2 robot (Molecular Devices). A copy of this plate was used for Fab expression. Crude bacterial lysates from the expression cultures were preparedCitation30 and 368 clones (one column of the 384 well plate was used for controls) were screened by ELISA on immobilized antigens or antigen complexes used for antibody generation. Antibodies specific for the antigen(s) used in panning were considered positive, e.g., for Type 3 antibodies, clones that bind drug-target complex with a signal greater than five-fold over background and do not bind to the drug antibody, the drug target and the other control proteins. Such clones were sequenced, and the resulting unique antibodies were expressed and purified as described.Citation35 Briefly, E. coli TG1F- cultures (250 mL) containing the chosen antibody genes were grown at 30°C until OD600nm reached 0.5, and the antibody expression was induced by adding isopropyl β-D-1-thiogalactopyranoside to a final concentration of 1 mM. After further incubation for at least 14 hours at 30°C, the cells were harvested, chemically lysed, and the soluble crude extract was subjected to one-step affinity chromatography (Ni-NTA agarose; Qiagen #30250 for all His-tagged antibodies; Strep-Tactin Superflow high capacity resin; IBA #2–1208-025 for all Strep-tagged antibodies). After elution of the purified antibodies, the buffer was changed from elution buffer to 3 x phosphate-buffered saline (PBS), pH 7.4, and the concentration was determined by UV280nm measurement. Purity and activity were subsequently tested by Coomassie-stained SDS-PAGE and indirect ELISA, respectively.
Off-rate screening
For antibodies from single binder maturation pannings, an off-rate screening was performed on an Octet RED384 instrument (Pall ForteBio) as described before.Citation29 All measurements were performed at 30°C, agitated at 1000 rpm in 384-well microplates (Greiner Bio-One #781900). Lysates of 95 selected positive clones from the primary screening were transferred to a new 384-well microtiter plate for secondary screening. Human TNF (10 µg/mL) in 10 mM sodium acetate, pH 6, or human VEGF (7.9 µg/mL) in 10 mM sodium acetate, pH 5, were covalently immobilized on amine-reactive second-generation sensors (Pall ForteBio, AR2G sensors, #18–5092) via EDC/Sulfo-NHS coupling chemistry for 10 min; excess reactive esters were blocked with ethanolamine (Sigma-Aldrich #02400) afterwards. Typical immobilization levels were 2.7 ± 0.2 nm for hTNF and 3.3 ± 0.3 nm for hVEGF. For complex formation sensors were dipped into 15 µg/mL adalimumab or 10 µg/mL golimumab or 12 µg/mL ranibizumab, respectively, each in PBST/BSA running buffer (PBS + 0.02% v/v Tween20 + 0.1% w/v bovine serum albumin) for 5 min. The sensors were blocked with E. coli mock lysate containing no antibody for 5 min, transferred to the wells containing the antibody lysate for the association step (7.5 min), and transferred back to the mock lysate for the measurement of the dissociation step (7.5 min). Using new sensors for each sample, a total of 16 samples were measured in parallel. Dissociation rate constants (kd) for each antibody were calculated applying a 1:1 interaction model (fitting local, full) using the ForteBio data analysis software 8.2.0.7. Curves that could not be reliably fitted with the software (mostly full RCitation2 < 0.96), usually caused by heterogeneous binding, were excluded from further analysis.
Antibody conversions
Some Fabs were converted into full-length immunoglobulins by sub-cloning VH and VL (variable domains of the heavy and light chain, respectively) gene fragments into the pMORPH2_h_Ig vector series for human IgG1 expression.Citation19,Citation36 These vectors carry the human IgG1 constant region and the human lambda or kappa constant region, respectively. Human HKB11 cellsCitation37 were transiently transfected with the human IgG1 and the human light-chain expression constructs. Cell culture supernatants were subjected to protein A affinity chromatography. The purified antibody was rebuffered to PBS and finally sterile-filtered. Anti-trastuzumab/ErbB2 antibody AbD24937 was converted from the monovalent Fab format into the bivalent Fab format Fab-A-FH (Fd chain fused to bacterial alkaline phosphatase followed by FLAG- and His-tag) by sub-cloning the complete region coding for light chain and Fd chain into the pMORPH expression vectorCitation38 containing the gene coding for BAP fused to a region coding for FLAG- and His-tag. Expression of bivalent Fab was exactly as described for monovalent Fab.
General ELISA information
Antigens were coated in PBS on Maxisorp plates (Thermo Fisher Scientific #10395991) and incubated overnight at 4°C. The plates were washed five times with PBS containing 0.05% (v/v) Tween20 (PBST) before blocking and after each incubation step. After blocking with 5% (w/v) BSA in PBST for 1 hour (h) at room temperature (RT), the drug target/drug complex was formed by adding the drug to the wells coated with the corresponding drug target and incubated for 1 h at RT. Detection was performed by adding HRP-conjugated Type 3 detection antibodies (conjugated with LYNX rapid HRP antibody conjugation kit, Bio-Rad LNK002P) or by adding Type 3 antibodies followed by HRP-conjugated anti-tag secondary antibodies in HISPEC assay diluent (Bio-Rad BUF49A) for 1 h at RT. Plates were washed ten times with PBST, followed by the addition of QuantaBlu fluorogenic peroxidase substrate (Thermo Fisher Scientific #15169). Fluorescence signal was recorded after 30 min on a photometer (Tecan Infinite; excitation at 320 ± 25 nm, emission at 430 ± 35 nm). All assays were performed in triplicate, and the average values and error-bars are shown.
All titration curves were generated with Origin 2017 software (OriginLab) using a four-parameter logistic curve-fit program (Levenberg Marquardt algorithm). The limit of quantitation (LOQ) was calculated as 10-times standard deviation of the background signal.
Specificity titration ELISA
Antigens were coated at 5 µg/mL in PBS. After washing and blocking, the human IgE/omalizumab complex was formed by adding 2 µg/mL omalizumab to the corresponding wells coated with human IgE. Detection was performed by adding HRP-conjugated Type 3 antibody AbD20760 (Bio-Rad HCA237) at concentrations from 2,000 to 0.1 ng/mL in HISPEC assay diluent.
Testing of different secondary detection antibodies
Human ErbB2 (also known as HER2) was coated at 5 µg/mL in PBS. After washing and blocking, the ErbB2/trastuzumab complex was formed by adding trastuzumab at concentrations from 32 µg/mL to 0.06 ng/mL in PBST spiked with 10% NHS. The Type 3 antibody AbD25279 (Bio-Rad HCA263) was added at 2 µg/mL in HISPEC assay diluent, either directly HRP-conjugated or followed by a commercially available HRP-conjugated secondary antibody using the dilutions recommended by the suppliers: anti-His:HRP (1:2,000, Bio-Rad MCA5995P), anti-BAP:HRP (1:5,000, Bio-Rad HCA275P) and anti-FLAG:HRP (1:20,000, Sigma A8592).
PK assay for recognizing the VEGF/ranibizumab complex spiked in different human serum levels
Human VEGF was coated at 5 µg/mL in PBS. After washing and blocking, the VEGF/ranibizumab complex was formed by adding ranibizumab ranging from 8,000 ng/mL to 0.008 ng/mL in PBST with different concentrations of NHS (0, 10, 30, 60 and 90%). Detection was performed with HRP-conjugated Type 3 antibody AbD29928 (Bio-Rad HCA304) in HISPEC assay diluent.
Type 1 and Type 3 PK assays
Adalimumab was detected either using a Type 3 antibody in an antigen capture assay or using Type 1 capture and detection antibodies in a bridging assay. For the antigen capture assay, human TNF (recombinant human TNF alpha, Bio-Rad PHP051) was coated at 5 µg/mL in PBS. After washing and blocking, the TNF/adalimumab complex was formed by adding adalimumab ranging from 8,000 ng/mL to 0.03 ng/mL in PBST with 10% NHS. The HRP-conjugated Type 3 antibody AbD18754 (Bio-Rad HCA207) was added at 2 µg/mL in HISPEC assay diluent. For the bridging assay, the Type 1 antibody AbD18654 (Bio-Rad HCA202) was coated at 1 µg/mL in PBS. After washing and blocking, adalimumab spiked into 10% NHS was added, followed by the HRP-conjugated anti-adalimumab antibody AbD18655 (Bio-Rad HCA204) at 2 µg/mL in HISPEC assay diluent and QuantaBlu fluorogenic peroxidase substrate.
Immunocapture of drug/TNF complexes by Type 3 antibodies
Type 3 anti-golimumab/TNF antibody AbD25705 (Bio-Rad HCA274), and Type 3 anti-adalimumab/TNF antibody AbD20350 (Bio-Rad HCA231) were coated with 1 µg/mL in PBS. Golimumab and adalimumab at a fixed concentration (1 µg/mL = 7 nM) in PBST were incubated for 1 h with an increasing amount of TNF and then added to the plate with the immobilized antibodies and incubated for 1 h before washing. Detection of golimumab and adalimumab levels was performed with an HRP-conjugated anti-human IgG (Fc-CH2 domain specific) antibody (Bio-Rad MCA647P) with a dilution factor of 1:1000 in HISPEC assay diluent.
Immunocapture by Type 1, 2 and 3 antibodies
The anti-golimumab/TNF antibodies AbD25429 (Type 1, Bio-Rad HCA286), AbD25455 (Type 2, Bio-Rad HCA289) and AbD25705 (Type 3, Bio-Rad HCA274) were coated at 1 µg/mL in PBS. Golimumab at a fixed concentration (300 ng/mL = 2 nM) in PBST was incubated for 1 h with an increasing amount of TNF and then added to the plate with the immobilized antibodies for 1 h. Detection of golimumab levels was performed with an HRP-conjugated anti-human IgG (Fc-CH2 domain specific) antibody (Bio-Rad MCA647P) at 1:1000 in HISPEC assay diluent.
Immunocapture of omalizumab/human IgE complexes in serum
Type 3 anti-omalizumab/human IgE antibody AbD20760 (Bio-Rad HCA238) was coated at 5 µg/mL in PBS. Omalizumab at a fixed concentration (100 ng/mL = 0.7 nM) in PBST was incubated for 1 h with an increasing amount of human IgE (Bio-Rad HCA171) or NHS (1:2 dilution series with a starting concentration of 45% NHS) and then added to the plate with the immobilized antibody for 30 min. Detection of bound human IgE was performed with 2 µg/mL of HRP-conjugated anti-human IgE antibody (Bio-Rad 0100-0413P) in HISPEC assay diluent.
Robustness of Type 3 antigen-capture PK assay
Human TNF was coated at 5 µg/mL in PBS. After washing and blocking, the TNF/golimumab complex was formed by adding golimumab ranging from 16 µg/mL to 0.03 ng/mL in PBST with 10% NHS. The HRP-conjugated Type 3 antibody AbD20893 in its monovalent Fab and bivalent IgG format (Bio-Rad HCA245) was added at 2 µg/mL in HISPEC assay diluent. The assay was performed in triplicate for both antibody formats. In addition, the recovery of golimumab at three different concentrations (500, 63 and 8 ng/mL spiked into 10% NHS) was tested. Recovery in terms of accuracy was calculated as observed concentration of golimumab divided by the added concentration of golimumab. Observed concentrations were determined by correlating the respective fluorescence signals to the equation from standard curve fitting. The assay was repeated six times with independent assay setups for determining the inter-assay precision. For intra-assay and inter-assay precision the coefficient of variation (CV) was determined as the ratio of the standard deviation of corresponding values to the mean.
Affinity determination
Affinity determination was performed on an Octet RED384 instrument (Pall ForteBio) under the same measurement conditions as described above, except for AbD18754, which was determined on an Attana 200 QCM instrument (Attana). Each of the purified Fab antibodies (Mw = 52 kDa) was measured at five concentrations between 200 and 3 µg/mL diluted in running buffer (PBST/BSA). Human TNF or VEGF was immobilized on the sensors under the same conditions as described for the off-rate screening; golimumab (7.5 µg/mL) in 10 mM sodium acetate, pH 6, omalizumab (10 µg/mL) in 10 mM sodium acetate, pH 6, and trastuzumab (15 µg/mL) in 10 mM sodium acetate, pH 5. For complex formation, sensors were dipped into 15 µg/mL adalimumab or 25 µg/mL TNF or 10 µg/mL IgE or 9.6 µg/mL ErbB2 or 12 µg/mL ranibizumab, respectively, each in running buffer. Between measurements the golimumab biosensor surfaces were regenerated three times by exposing them to 10 mM glycine, pH 2.0, for 5 s followed by PBST/BSA for 10 s. For VEGF biosensors, the same procedure but using 10 mM glycine, pH 1.5, was applied. The other sensors were used without regeneration. Association phase was measured for 300–600 s, dissociation phase depending on the interaction for 900–1800 s. All measurements were corrected for baseline drift by subtracting a control sensor exposed to running buffer only. Data were analyzed using a 1:1 interaction model (fitting global, Rmax unlinked by sensor) on the ForteBio data analysis software 8.2.0.7. The sensorgrams of all affinity determinations are summarized in Supplementary Figure 3.
Disclosure of potential conflicts of interest
All authors are employees of Bio-Rad AbD Serotec that markets reagents for drug monitoring. C.F. and A.K. are listed as inventors on a patent submission that discloses complex-specific antibodies.
Supplemental Material
Download Zip (2 MB)Acknowledgments
We thank Claudia Ganser and Michaela Eick-Werner for excellent technical assistance and Amanda Turner for carefully reading the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7:9–14. doi:10.4161/19420862.2015.989042.
- McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91:405–417. doi:10.1038/clpt.2011.343.
- Beck A, Reichert JM. Approval of the first biosimilar antibodies in Europe: a major landmark for the biopharmaceutical industry. MAbs. 2013;5:621–623. doi:10.4161/mabs.25864.
- Mok CC, van der Kleij D, Wolbink GJ. Drug levels, anti-drug antibodies, and clinical efficacy of the anti-TNFalpha biologics in rheumatic diseases. Clin Rheumatol. 2013;32:1429–1435. doi:10.1007/s10067-013-2336-x.
- Mazor Y, Almog R, Kopylov U, Ben Hur D, Blatt A, Dahan A, Waterman M, Ben-Horin S, Chowers Y. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn’s disease. Aliment Pharmacol & Therapeut. 2014;40:620–628. doi:10.1111/apt.12869.
- Cohen ES, Dobson CL, Kack H, Wang B, Sims DA, Lloyd CO, England E, Rees DG, Guo H, Karagiannis SN, et al. A novel IgE-neutralizing antibody for the treatment of severe uncontrolled asthma. MAbs. 2014;6:756–764. doi:10.4161/mabs.28394.
- Chin SE, Ferraro F, Groves M, Liang M, Vaughan TJ, Dobson CL. Isolation of high affinity, neutralizing anti-idiotype antibodies by phage and ribosome display for application in immunogenicity and pharmacokinetic analyses. J Immunol Methods. 2015;416:49–58.
- Tornetta M, Fisher D, O’Neil K, Geng D, Schantz A, Brigham-Burke M, Lombardo D, Fink D, Knight D, Sweet R, et al. Isolation of human anti-idiotypic antibodies by phage display for clinical immune response assays. J Immunol Methods. 2007;328:34–44. doi:10.1016/j.jim.2007.08.008.
- Hentrich C, Ylera F, Frisch C, Ten Haaf A, Knappik A. Chapter 3 – Monoclonal antibody generation by phage display: history, state-of-the-art, and future. In: Vashist SK, Luong JHT, editors. Handbook of immunoassay technologies. London: Academic Press; 2018:47–80.
- Damen CW, Schellens JH, Beijnen JH. Bioanalytical methods for the quantification of therapeutic monoclonal antibodies and their application in clinical pharmacokinetic studies. Hum Antibodies. 2009;18:47–73. doi:10.3233/HAB-2009-0206.
- Bourdage JS, Lee TN, Taylor JM, Willey MB, Brandt JT, Konrad RJ. Effect of double antigen bridging immunoassay format on antigen coating concentration dependence and implications for designing immunogenicity assays for monoclonal antibodies. J Pharm Biomed Anal. 2005;39:685–690. doi:10.1016/j.jpba.2005.03.037.
- Verch T, Chilewski S, Bouaraphan S, Yarovoi H, Yin KC, Chen D, Washabaugh MW. Pharmacokinetic immunoassay methods in the presence of soluble target. J Immunol Methods. 2010;361:75–81. doi:10.1016/j.jim.2010.07.014.
- Kuang B, King L, Wang HF. Therapeutic monoclonal antibody concentration monitoring: free or total? Bioanalysis. 2010;2:1125–1140. doi:10.4155/bio.10.64.
- Mayer AP, Hottenstein CS. Ligand-Binding assay development: what do you want to measure versus what you are measuring? Aaps J. 2016;18:287–289. doi:10.1208/s12248-015-9855-0.
- Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT, Lu JF, Kamerud J, Ahene A, Myler H, et al. Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. Aaps J. 2011;13:99–110. doi:10.1208/s12248-011-9251-3.
- Prassler J, Thiel S, Pracht C, Polzer A, Peters S, Bauer M, Norenberg S, Stark Y, Kolln J, Popp A, et al. HuCAL PLATINUM, a synthetic Fab library optimized for sequence diversity and superior performance in mammalian expression systems. J Mol Biol. 2011;413:261–278. doi:10.1016/j.jmb.2011.08.012.
- Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, Hoess A, Wölle J, Plückthun A, Virnekäs B. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86. doi:10.1006/jmbi.1999.3444.
- Virnekas B, Ge L, Pluckthun A, Schneider KC, Wellnhofer G, Moroney SE. Trinucleotide phosphoramidites: ideal reagents for the synthesis of mixed oligonucleotides for random mutagenesis. Nucleic Acids Res. 1994;22:5600–5607.
- Steidl S, Ratsch O, Brocks B, Durr M, Thomassen-Wolf E. In vitro affinity maturation of human GM-CSF antibodies by targeted CDR-diversification. Mol Immunol. 2008;46:135–144. doi:10.1016/j.molimm.2008.07.013.
- Pack P, Pluckthun A. Miniantibodies: use of amphipathic helices to produce functional, flexibly linked dimeric FV fragments with high avidity in Escherichia coli. Biochemistry. 1992;31:1579–1584.
- Rheinnecker M, Hardt C, Ilag LL, Kufer P, Gruber R, Hoess A, Lupas A, Rottenberger C, Pluckthun A, Pack P. Multivalent antibody fragments with high functional affinity for a tumor-associated carbohydrate antigen. J Immunology. (Baltimore, Md: 1950). 1996;157:2989–2997.
- Amarasekera M. Immunoglobulin E in health and disease. Asia Pac Allergy. 2011;1:12–15. doi:10.5415/apallergy.2011.1.1.12.
- Fischer SK, Yang J, Anand B, Cowan K, Hendricks R, Li J, Nakamura G, Song A. The assay design used for measurement of therapeutic antibody concentrations can affect pharmacokinetic parameters: case studies. MAbs. 2012;4:623–631. doi:10.4161/mabs.20814.
- Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi:10.1038/nbt1344.
- Ito R, Gon Y, Nunomura S, Atsuta R, Harada N, Hattori T, Maruoka S, Okayama Y, Ra C, Hashimoto S. Development of assay for determining free IgE levels in serum from patients treated with omalizumab. Allergol International: Official Journal Jpn Soc Allergol. 2014;63(Suppl 1):37–47. doi:10.2332/allergolint.13-OA-0643.
- Talbot JJ, Calamba D, Pai M, Ma M, Thway TM. Measurement of free versus total therapeutic monoclonal antibody in pharmacokinetic assessment is modulated by affinity, incubation time, and bioanalytical platform. Aaps J. 2015;17:1446–1454. doi:10.1208/s12248-015-9807-8.
- Lowe J, Wakshull E, Shek T, Chuntharapai A, Elliott R, Rusit J, Maia M. Development and validation of a novel semi-homogenous clinical assay for quantitation of ranibizumab in human serum. J Immunol Methods. 2018;461:44–52. DOI:10.1016/j.jim.2018.05.007
- Krayukhina E, Noda M, Ishii K, Maruno T, Wakabayashi H, Tada M, Suzuki T, Ishii-Watabe A, Kato M, Uchiyama S. Analytical ultracentrifugation with fluorescence detection system reveals differences in complex formation between recombinant human TNF and different biological TNF antagonists in various environments. MAbs. 2017;9:664–679. doi:10.1080/19420862.2017.1297909.
- Ylera F, Harth S, Waldherr D, Frisch C, Knappik A. Off-rate screening for selection of high-affinity anti-drug antibodies. Anal Biochem. 2013;441:208–213. doi:10.1016/j.ab.2013.07.025.
- Jarutat T, Frisch C, Nickels C, Merz H, Knappik A. Isolation and comparative characterization of Ki-67 equivalent antibodies from the HuCAL phage display library. Biol Chem. 2006;387:995–1003. doi:10.1515/BC.2006.123.
- Whiteaker JR, Zhao L, Frisch C, Ylera F, Harth S, Knappik A, Paulovich AG. High-affinity recombinant antibody fragments (Fabs) can be applied in peptide enrichment immuno-MRM assays. J Proteome Res. 2014;13:2187–2196. doi:10.1021/pr4009404.
- Prassler J, Steidl S, Urlinger S. In vitro affinity maturation of HuCAL antibodies: complementarity determining region exchange and RapMAT technology. Immunotherapy. 2009;1:571–583. doi:10.2217/imt.09.23.
- Dunn C, O’Dowd A, Randall RE. Fine mapping of the binding sites of monoclonal antibodies raised against the Pk tag. J Immunol Methods. 1999;224:141–150.
- Schmidt TG, Batz L, Bonet L, Carl U, Holzapfel G, Kiem K, Matulewicz K, Niermeier D, Schuchardt I, Stanar K. Development of the Twin-Strep-tag(R) and its application for purification of recombinant proteins from cell culture supernatants. Protein Expr Purif. 2013;92:54–61. doi:10.1016/j.pep.2013.08.021.
- Knappik A, Brundiers R. Recombinant antibody expression and purification. In: J.M. W, editor. The protein protocols handbook. Totowa (NJ): Humana Press; 2009. p. 14.
- Ostendorp R, Frisch C, Urban M. Generation, engineering and production of human antibodies using Hucal®. In: Subramanian G, editor. Antibodies. New York: Springer US; 2004. p. 13–52.
- Cho MS, Yee H, Chan S. Establishment of a human somatic hybrid cell line for recombinant protein production. J Biomed Sci. 2002;9:631–638. doi:10.1159/000067294.
- Rauchenberger R, Borges E, Thomassen-Wolf E, Rom E, Adar R, Yaniv Y, Malka M, Chumakov I, Kotzer S, Resnitzky D. Human combinatorial Fab library yielding specific and functional antibodies against the human fibroblast growth factor receptor 3. J Biol Chem. 2003;278:38194–38205. doi:10.1074/jbc.M303164200.
