?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
HEK293 transient expression systems are used to quickly generate proteins for research and pre-clinical studies. With the aim of engineering a high-producing host that grows and transfects robustly in bioreactors, we deleted the pro-apoptotic genes Bax and Bak in an HEK293 cell line. The HEK293 Bax Bak double knock-out (HEK293 DKO) cell line exhibited resistance to apoptosis and shear stress. HEK293 DKO cells sourced from 2 L seed train bioreactors were most productive when a pH setpoint of 7.0, a narrow pH deadband of ±0.03, and a DO setpoint of 30% were used. HEK293 DKO seed train cells cultivated for up to 60 days in a 35 L bioreactor showed similar productivities to cells cultivated in shake flasks. To optimize HEK293 DKO transfection cultures, we first evaluated different pH and agitation parameters in ambr15 microbioreactors before scaling up to 10 L wavebag bioreactors. In ambr15 microbioreactors with a pH setpoint of 7.0, a wide pH deadband of ±0.3, and an agitation of 630 rpm, HEK293 DKO transient cultures yielded antibody titers up to 650 mg/L in 7 days. The optimal ambr15 conditions prompted us to operate the 10 L wavebag transfection without direct pH control to mimic the wide pH deadband ranges. The HEK293 DKO transfection process produces high titers at all scales tested. Combined, our optimized HEK293 DKO 35 L bioreactor seed train and 10 L high titer transient processes support efficient, large-scale recombinant protein production for research studies.
Introduction
Monoclonal antibodies (mAbs) and other recombinant proteins have been established as successful therapeutics for many diseases, including cancer as well as immune-mediated and neurological disorders.Citation1,Citation2 With over 500 mAbs in clinical development by the biotechnology industry,Citation1 the mAb market is projected to include 70 mAb products by the year 2020.Citation3 As the industry expands and targets become more complex, larger antibody discovery campaigns are needed to screen multiple mAb variants and identify clinical candidates with the desired characteristics.
Transient transfection of mammalian cells using the cationic polymer polyethylenimine (PEI) has become a prevalent method to rapidly produce recombinant proteins for large molecule development, including antibody discovery screening studies.Citation4–Citation8 Human embryonic kidney 293 (HEK293) and Chinese hamster ovary (CHO) host cells are often used for transient transfections because they are highly transfectable and their transfection processes are scalable. While the quality of the product produced in HEK293 cells may differ compared to that from CHO cells,Citation9 HEK293 transfections can produce higher titers in half the time compared to CHOCitation10,Citation11 and are very amenable to high throughput, automated small-scale transfections.Citation12–Citation18 While numerous reports describe CHO large-scale bioreactor cultivation and transfections, fewer findings exist for HEK293 cells, and there are currently no reports of long-term cultivation of HEK293 seed train in bioreactors to support routine, high throughput transfections to generate large quantities of proteins. HEK293 transient production runs have been conducted in wavebag bioreactors up to 10 L,Citation17,Citation19,Citation20 stirred tank bioreactors as suspension cultures up to 100 L,Citation19,Citation21–Citation24 and in stirred tank bioreactors as cultures adhered to microcarriers or scaffolds.Citation25,Citation26 Some instances of HEK293 transient production in stirred tank bioreactors used a different transfection reagent, i.e., calcium phosphateCitation21,Citation23,Citation27 instead of PEI.
Literature reports HEK293 culture sensitivity to shear stress in spinner flasks.Citation28 Therefore, we hypothesized that a cell line with resistance to apoptosis would exhibit higher productivity and more robust performance in bioreactors. Here, we engineered an anti-apoptotic HEK293 cell line by deleting the pro-apoptotic genes Bax and Bak using zinc finger nuclease technology. During apoptosis, Bax and Bak permeate the mitochondrial membrane, which ultimately leads to the activation of caspase proteins that trigger programmed cell death.Citation29 We previously showed that deleting Bax and Bak in a CHO cell line correlated with higher culture viabilities and transfection titers.Citation30 Other labs have reported similar improvements to culture viabilities and productivity with suppression or deletion of Bax and Bak.Citation31–Citation33
The HEK293 Bax Bak double knock-out cell line (HEK293 DKO) showed resistance to apoptosis and shear stress. With this host, we maintained a seed train at the 35 L volume and conducted transfections up to 10 L that yielded titers up to 650 mg/L in 7 days. This is the first report that describes long-term cultivation of HEK293 seed train at pilot scale (35 L) in a stirred tank, controlled bioreactor. While there is a report of the cultivation of HEK293 cells in a 1.8 L bioreactor for 10 days,Citation34 our seed train strategy supports 35 L of culture for up to 60 days to supply routine, high throughput large-scale transient transfections.
Ambr15 bioreactors have been used for CHO stable cell line process development.Citation35–Citation39 However, at present, there are no reports describing the optimization of transfection production conditions for HEK293 cultures in ambr15 bioreactors. We used ambr15 microbioreactors to explore and optimize pH and agitation conditions for HEK293 transient transfections and production. We obtained the highest transfection titers using a wide pH deadband and a lower agitation rate. We then scaled up our transfection and production process into a 10 L wavebag, which involves simpler operations compared to a controlled bioreactor. We do not directly control pH in the wavebag process, and thus eliminate the need for probes and online measurements. Single-use wavebags also eliminate cleaning and sterilization steps between production runs. Combined, our optimized HEK293 DKO 35 L bioreactor seed train and 10 L transient transfection processes enable the high throughput generation of recombinant proteins to support research studies leading to the identification of therapeutic clinical candidates.
Results
Creating and testing a more robust HEK293 cell line
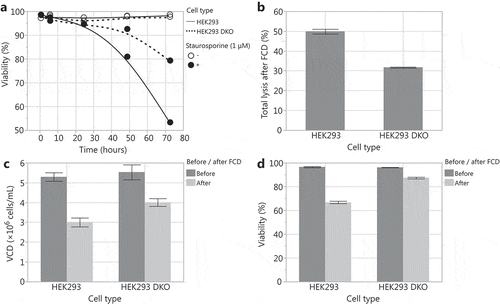
With the aim of creating an HEK293 cell line that exhibits higher productivity and robust performance in bioreactors, we used the zinc finger nuclease technologyCitation33 to delete the pro-apoptotic genes Bax and Bak in an HEK293 cell line (HEK293 DKO). We tested the susceptibility of the HEK293 DKO cell line to undergo apoptosis and its sensitivity to shear stress. To induce apoptosis, we added staurosporine to HEK293 and HEK293 DKO cultures. Upon staurosporine addition, HEK293 DKO cells maintained higher viability compared to HEK293 cells (). From this, we infer that the HEK293 DKO cells are more resistant to apoptosis. To assess the impact of shear stress, we passed HEK293 and HEK293 DKO cultures through a flow constriction device (FCD).Citation40,Citation41 The FCD subjects cells to an acute hydrodynamic force and increased shear stress equivalent to a 2.67 × 107 W/m3 energy dissipation rate (EDR) by passing the cells at a controlled flow rate through a narrow flow channel. After flowing through the FCD, the HEK293 DKO cells exhibited higher cell densities, higher viability, and reduced lysis compared to HEK293 cells (), indicating that HEK293 DKO cells are more resistant to shear stress than HEK293 cells. Thus, the HEK293 DKO cell line demonstrates the phenotypic properties that we strived to achieve through the deletion of Bax and Bak.
Figure 1. Comparison of the HEK293 Bax Bak DKO (HEK293 DKO) cell line to the parental HEK293 cell line. (A) Viability after exposure of the cell lines to 1 µM staurosporine to induce apoptosis. Using a flow constriction device (FCD) to assess sensitivity to shear stress: (B) total lysis after FCD, (C) viable cell density (VCD) before and after FCD, and (D) viability before and after FCD.
The ratio of PEI (N) to DNA (P) and the amount of PEI and DNA can significantly affect transient transfection productivity.Citation10,Citation30,Citation42,Citation43 To determine the HEK293 DKO transient transfection conditions that produce the highest titer, we seeded 30 mL tubespin production cultures at 2 × 106 cells/mL and ran a full factorial experiment to test a range of N:P ratios (5, 7.5, 10, and 12.5) and DNA concentrations (0.75, 1.0, 1.25, and 1.5 µg/mL). An N:P ratio of 7.5 and DNA concentration of 1 µg/mL yielded the highest titers (). Using this optimized condition, HEK293 DKO transient transfection performance was compared to the HEK293 in 30 mL tubespins. Both cell types showed similar growth and viability in transfection, with final day viabilities at 68.5% and 74.9% for HEK293 DKO and HEK293 cultures, respectively (). This suggests that the viability decline in these transfection cultures is induced by necrosis rather than apoptosis. With regard to productivity, the HEK293 DKO cultures expressed 40% higher titer than HEK293 cultures (). The difference in productivity could be due to the sublethal effect of shear stress on HEK293 cultures, or biological effects of deleting Bax and Bak.
Figure 2. Optimizing N:P ratio and DNA concentration for HEK293 DKO transient transfections: (A) Transfections were tested across N:P ratios of 5 to 12.5 and DNA concentrations of 0.75 to 1.5 µg/mL. Transfecting HEK293 and HEK293 DKO cells at the 30 mL tubespin scale with an N:P ratio of 7.5 and a DNA concentration of 1 µg/mL: (B) VCD and viability over the 7-day production cultures and (C) day 7 titers.
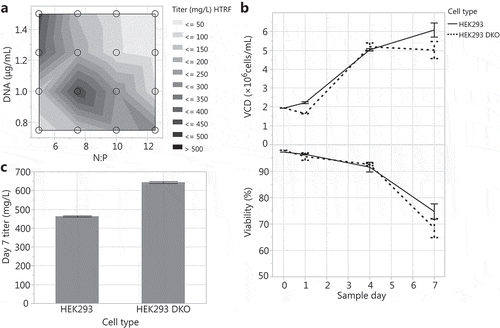
The optimized N:P ratio of 7.5 and DNA concentration of 1 µg/mL were used for all further HEK293 DKO transfections with the 30 mL scale as the control for scale up/down.
Scaling up the HEK293 seed train
To efficiently generate cell mass to support large scale (10 L) transfections, we sought to cultivate the HEK293 DKO seed train in a 35 L controlled bioreactor rather than multiple shake flasks. A regularly passaged (i.e., split every 3–4 days) seed train bioreactor with a working volume of 20–35 L would provide enough cells to start 40–70 L of transfections seeded at 2 × 106 cells/mL twice per week. This enables routine execution of high throughput, large-scale transient production runs.
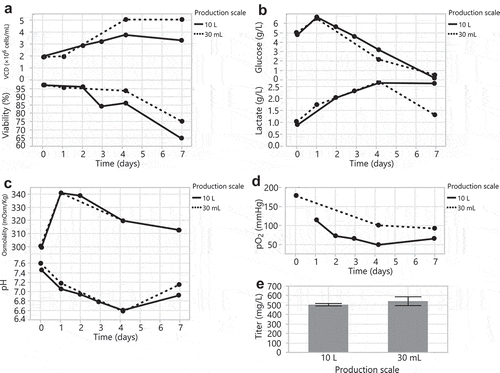
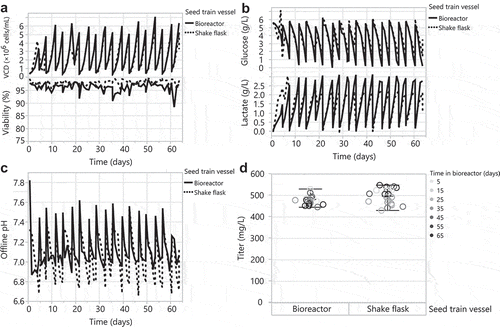
Before testing the HEK293 DKO seed train in a 35 L bioreactor, we first evaluated different pH and dissolved oxygen (DO) controlled conditions in two 2 L bioreactors. Bioreactor #1 used a pH setpoint of 7.0 with a deadband of ±0.03 and a DO setpoint of 30%; these are typical conditions for CHO stable cell line cultures.Citation44–Citation46 Bioreactor #2 used a pH setpoint of 7.0 with a deadband of ±0.4 and a DO setpoint of 60% to more closely trend with the pH and DO conditions of a shake flask. We passaged HEK293 DKO cells in a shake flask and the 2 L bioreactors in parallel, every 3–4 days for a total of 25 days and monitored growth and metabolites. Both 2 L bioreactor HEK293 DKO seed trains grew to similar peak cell densities and maintained similar viabilities compared to the shake flask seed train (). This correlates with similar glucose consumption and lactate production across the seed trains (). Per our aim, bioreactor #2 showed similar pH and DO trends to the shake flask (). We used cells from the seed trains every week for 4 weeks in 30 mL tubespin transfections. Interestingly, we observed modestly lower titers from cells sourced from bioreactor #2 that mimicked the shake flask pH and DO conditions, and similar titers from cells sourced from bioreactor #1 with tighter controls (), compared to titers from cells sourced from the shake flask seed train. Different mixing in the shake flask seed train may account for the cells’ high productivity. In the bioreactor, it is possible that the narrow pH deadband conditions affected the seed train cells and/or their spent medium such that they were more amenable for transfection. This could entail biological modifications that: 1) result in more optimal electrostatic charge interactions of the DNA/PEI complex with the cell surface during transient transfection, 2) promote intracellular trafficking of DNA/PEI complexes to the nucleus, or 3) enhance transcription, translation, and secretion of the recombinant protein.
Figure 3. Scaling up the HEK293 DKO seed train from a 1 L shake flask to controlled 2 L bioreactors. Bioreactor #1: pH setpoint of 7 with a deadband of ±0.03 and a DO setpoint of 30%. Bioreactor #2: pH setpoint of 7 with a deadband of ±0.4 and a DO setpoint of 60%. Passaging the 1 L shake flask and 2 L bioreactors every 3–4 days for 25 days: (A) VCD and viability, (B) glucose and lactate, (C) offline pH, and (D) pO2. (E) Day 7 transfection titers from 30 mL tubespins.
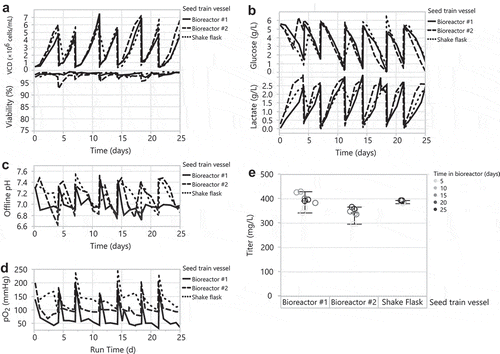
Subsequently, we scaled up the HEK293 DKO seed train into a 35 L bioreactor using bioreactor #1 conditions (pH setpoint of 7.0 with a narrow deadband of ±0.03 and DO setpoint of 30%) and matching the power input per volume of our 2 L bioreactor (13 W/m3). We passaged the HEK293 DKO shake flask and 35 L bioreactor seed trains in parallel every 3–4 days for a total of 60 days, and monitored regularly for growth and metabolites. While the HEK293 DKO cells showed comparable transfection productivity for up to 150 days after thaw (data not shown), we chose a 60-day duration for the bioreactor to balance the frequency of bioreactor breakdown/set up, which is a labor intensive operation, with ensuring that cellular debris on the glass wall of the bioreactor at the liquid-air interface does not accumulate from continuous passaging in the bioreactor. This contrasts with the shake flask seed train procedure, in which we used a new shake flask for every passage. The bioreactor HEK293 DKO seed train achieved slightly higher peak cell densities and lower viabilities compared to the shake flask seed train (). As expected, due to pH control, the bioreactor seed train consumed more glucose and produced more lactate than the shake flask (). This glucose consumption differs from the 2 L bioreactor seed trains (), and may be due to scale differences, including sparging and mixing. Except for pH spikes during passaging of the bioreactor, the bioreactor seed train maintained its pH at 7.0 with a deadband of ±0.03 (). The bioreactor maintained its DO setpoint of 30% with similar trends to the analogous 2 L bioreactor (data not shown). Every week for 9 weeks, cells from the seed trains were used for 30 mL tubespin transfections. Despite the differences noted above between the bioreactor and shake flask seed train, cells sourced from the shake flask and 35 L bioreactor produced similar titers () and product quality (Supplementary Figure 1) across 9 weeks of transfection. These data demonstrate that the HEK293 DKO seed train can be cultivated in a 35 L bioreactor up to 60 days to source weekly transfections.
Figure 4. Scale up of the HEK293 DKO seed train from a 1 L shake flask to a controlled 35 L bioreactor. Passaging the 1 L shake flask and 35 L bioreactor every 3–4 days for 60 days: (A) VCD and viability, (B) glucose and lactate, and (C) offline pH. (D) Day 7 transfection titers from 30 mL tubespins.
Optimizing and scaling up HEK293 transfections and production
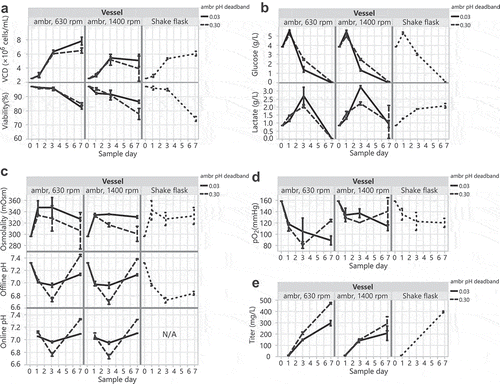
To identify optimal parameters and assess the feasibility of transfecting HEK293 DKO cells in controlled bioreactors, we performed transfections in ambr15 microbioreactors at varying agitation and pH conditions. The full factorial experiment of 4 cases in replicate evaluated agitation rates of 630 vs 1400 and pH deadbands of ±0.03 vs ±0.3 around a setpoint of 7.0. High and low agitations were selected based on 2 scale-up/down strategies:Citation36 1) 630 rpm matches the power input per volume (P/V) of our 2 L bioreactor (13 W/m3), and 2) 1400 rpm matches the maximum shear (represented by impeller tip speed) of our 2 L bioreactor (0.26 m/s). The pH deadbands were chosen to mimic bioreactor and shake flask conditions. Transfected cultures were monitored for growth and metabolites. Because of equipment limitations, 30 mL shake flasks were used instead of tubespins for the control cases. Shake flasks and tubespins produce comparable titers at the 30 mL scale (data not shown). Higher viable cell density and viability correlated with ambr agitation at 630 rpm and pH control around a tight ±0.03 pH deadband (). Despite similar glucose consumption across all vessels, the ambr bioreactor cultures had lower final lactate levels compared to shake flask cultures (), indicating that the metabolism of HEK293 DKO cells was different with pH control – lactate was consumed near the end of production. The wide pH deadband of ±0.3 correlated with lower osmolality levels due to fewer base additions and showed similar pH trends to shake flask cultures (). As expected, oxygen levels were highest and most similar to shake flasks with an ambr agitation of 1400 rpm (). Highest yields occurred in ambr bioreactors at an agitation of 630 rpm and a wide pH deadband of ±0.30 (). We suspect that these higher titers may be due to: 1) lower levels of shear stress at 630 rpm allowing for more optimal interaction of DNA/PEI complexes with cells during transfection or more conducive conditions for protein expression, and 2) a wide ±0.3 pH deadband leading to reduced base additions to maintain the pH near the end of production, which directly correlates with lower osmolality and lower final lactate.
Figure 5. HEK293 DKO transient transfections in controlled ambr15 bioreactors compared to 30 mL shake flasks. (A) VCD and viability, (B) glucose and lactate, (C) osmolality and pH, (D) pO2, and (E) titers over the 7-day production cultures.
Next, knowing that a wide ±0.3 pH deadband correlated with high yields in ambr bioreactors, we scaled up and evaluated the transfection of HEK293 DKO cells without direct pH control in 10 L wavebags and 30 mL tubespins. The wavebags and tubespins were operated without direct pH control with a gas overlay of 5% CO2 in air. The transfected cultures were monitored for growth and metabolites. The transfected cells in the 30 mL tubespin reached a higher cell density with higher final viability compared to the 10 L wavebag (). However, these cell counts may have been confounded by cell clumping. The HEK293 DKO 10 L wavebag transfection showed more significant clumping (data not shown), higher final day lactate levels (), and lower oxygen levels () compared to the 30 mL tubespin cultures. Despite differences in cell clumping, lactate, and oxygen, cells from both vessel types consumed glucose at a similar rate (), exhibited similar osmolality and pH profiles (), and produced comparable final titers (). These data demonstrate that we were able to establish HEK293 DKO transfection methodologies that produced similar titers in large-scale 10 L wavebags and 30 mL tubespins.
Discussion
In this study, we engineered an anti-apoptotic HEK293 cell line with resistance to apoptosis and shear stress by deleting the pro-apoptotic genes Bax and Bak using zinc finger nuclease technology.Citation33 Our data shows that the HEK293 DKO 35 L bioreactor seed train and 10 L transient transfection processes were capable of supporting routine, high throughput expression of recombinant proteins with titers up to 650 mg/L in 7 days.
HEK293 cells in suspension culture can be sensitive to shear stress.Citation28,Citation47 Previous studies have shown that controlling shear stress led to increased proliferation in spinner flasks.Citation28 Similarly, we previously observed that HEK293 cells cultured in bioreactors had low viabilities (data not shown), which we hypothesized to be due to their sensitivity to shear stress. Here, we show evidence that the HEK293 DKO cell line was more resistant to apoptosis and shear stress than the HEK293 parental cell line (). This property renders the HEK293 DKO cell line advantageous for high throughput transient production of recombinant proteins and potentially other HEK293 applications such as stable production of biopharmaceuticals, viral vectors, and vaccines.
Bax Bak suppression or deletion was previously shown to correlate with higher productivity than wild type in CHO cells.Citation30,Citation31,Citation33,Citation48 These studies reported observing higher viability,Citation30,Citation31,Citation33 higher DNA uptake levels,Citation30 higher transfection efficiency,Citation30 greater mitochondria mass,Citation48 and improved mitochondria membrane potentialCitation48 in CHO DKO production cultures compared to wild type. CHO DKO cells may also use an alternative intracellular trafficking pathway than wild-type CHO cells because the maximum DNA uptake occurred later in CHO DKO cells compared to wild-type cells.Citation30 Furthermore, in addition to their role in apoptosis, Bax and Bak are part of other cellular metabolism processes.Citation33 The higher productivity from HEK293 DKO () could be due to differences in resistance to stresses (i.e., shear stress, PEI toxicity) or metabolism that create conditions favorable for transient transfections, such as allowing better DNA uptake, trafficking of DNA to the nucleus, transcription, or translation. In addition to comparing the DNA uptake levels, transfection efficiency, and mitochondrial mass and potential of HEK293 DKO vs HEK293 transfections, it would be interesting to conduct –omics comparisons to gain further biologicalunderstanding of potential differences between the two hosts.
We confirmed our hypothesis that the HEK293 DKO cell line would exhibit robust performance in a seed train bioreactor. The bioreactor seed trains grew to peak cell densities that enable the use of ≤50% seed train culture to seed the production culture (at 2 × 106 cells/mL), which minimizes the volume of spent media in production. Carryover of >50% spent media with the seed train into production was shown to negatively impact transient protein expression.Citation19
We demonstrate that culturing the seed train in bioreactors with a pH setpoint of 7.0 with a narrow deadband of ±0.03 and a DO setpoint of 30% correlated with the highest transfection productivity (). This is the first report that describes long-term cultivation of HEK293 at a 35 L pilot scale in a stirred tank, controlled bioreactor to source cells for routine, high throughput transfections.
We evaluated various parameters for HEK293 DKO transfection and production in ambr15 bioreactors before scaling up to 10 L wavebag bioreactors. Our experiments show that HEK293 DKO production can be performed in controlled ambr15 microbioreactors with higher productivity than that in shake flasks when we use a pH setpoint of 7.0 with a wide pH deadband of ±0.3 and an agitation of 630 rpm (). This confirms our hypothesis that a cell line with resistance to apoptosis would exhibit higher productivity in bioreactors. It also verifies a previous study’s finding that controlled bioreactors produced higher titers than shake flasks.Citation24 In contrast with the narrow pH deadband of ±0.03 for the HEK293 DKO seed train bioreactor and for traditional CHO stable cell line cultures,Citation44–Citation46 the productivity of HEK293 DKO transfection cultures in bioreactors was highest with a wide pH deadband of ±0.3 (). In ambr15 bioreactors, a lower agitation of 630 rpm also correlated with the highest productivity (). We speculate that the reduced shear stress at 630 rpm creates more ideal conditions for DNA/PEI internalization or protein expression. Further studies should explore alternative pH, gassing, and mixing conditions in the ambr15 microbioreactor to identify parameters that promote higher titers and determine if these high titer conditions can be mimicked in other production cultures (i.e., wavebags and tubespins).
Based on our ambr15 bioreactor studies, we did not apply direct pH control to our 10 L wavebag production run; the wavebag cultures operated with a gas overlay of 5% CO2 in air. The pH in the 10 L wavebag bioreactor trended similarly to that in the wide pH deadband ambr15 cases, except for lower final pH (6.9 vs 7.3) ( and ). The pH trends and cell clumping in our wavebag transfections suggest that modification of the process to a different rock rate or gassing condition may mitigate the cell clumping and improve the productivity. Nevertheless, using the described wavebag system for production, instead of a stirred tank bioreactor, eliminates the need for probes and online measurements, as well as cleaning and sterilization steps between production runs. This provides substantial resource savings and operational benefits when high throughput large-scale transfections are used to generate material for biopharmaceutical research efforts to identify therapeutic candidates.
Materials and methods
Cell culture
The HEK293 DKO cell line was created by using zinc finger nuclease technology.Citation33 HEK293 cells and HEK293 DKO cells were cultivated as a seed train in shake flasks as previously describedCitation13 using the seed train media in .
Table 1. Seed train and production media used for HEK293 and HEK293 DKO transient transfections.
Staurosporine assay
HEK293 and HEK293 DKO seed train cultures in shake flasks were seeded at 0.8 × 106 cells/mL, and either untreated or treated with 1 µM staurosporine (Sigma, Cat# S6942).Citation49 Cultures were sampled every day for viability.
Flow constriction device
An FCDCitation41 was used to assess the impact of shear stress on HEK293 and HEK293 DKO cells. Briefly, a syringe pump (Harvard Apparatus, Model# 33) was used to pass the cells through the FCD at a flow rate of 70 mL/min or an energy dissipation rate (EDR) of 2.67 × 107 W/m3. Before passing through the FCD, whole cell samples (positive controls) were diluted 1:1 with 0.2 g/L saponin (Amresco, Cat# 0163) in water to lyse the cells and stored at −80°C. After passing through the FCD, the cultures were centrifuged at 830 × g and the supernatants were diluted 1:1 with 0.2 g/L saponin and stored at −80°C. The samples were thawed and assayed for lactate dehydrogenase using a Cedex Bio HT Analyzer (Roche). Total lysis after FCD (%) was calculated using equation 1 below. Cultures were also sampled for viable cell density (VCD) and viability before and after passing through the FCD.
Transfection process
Transient transfections were performed at a 30 mL working volume in 50 mL tubespins or 125 mL shake flasks, at a 10 L working volume in a 22 L wavebag, or at a 12 mL working volume in ambr15 microbioreactors.
For 30 mL transfections, cells were seeded at 2 × 106 cells/mL in 25.5 mL of production medium (see ) in a 50 mL tubespin (Optimum Processing, Cat# SV92050) or 125 mL nonbaffled shake flask (Corning, Cat# 431143) and equilibrated for 2 h prior to transfection at 37°C, 5% CO2 in a shaking incubator at 225 rpm with a 50 mm orbital diameter (Kuhner, Model# ISF1-X) or at 125 rpm with a 25 mm orbital diameter (e.g., Kuhner, Innova), respectively. All transfections were performed using DNA encoding a standard human IgG1 (huIgG1) antibody. To transfect, indicated amounts () of DNA and 25 kDa linear PEI at 7.5 mM (Polyplus-transfection, Cat# 101) were incubated in 3 mL of serum-free media (e.g., Opti-MEM I Reduced-Serum Medium (ThermoFisher, Cat# 31985062)) for 15 min before adding to the equilibrated cells. A 2.6 mL proprietary solution containing hydrolysates, amino acids and salts, and glucose was added 24 h post-transfection. This process was scaled proportionally for smaller or larger working volumes.
Cell count, titer, and product quality measurements
Cultures were sampled every 1–4 days for VCDs, viability, metabolites, pH, and/or gases and were measured using a Vi-CELL Cell Counter (Beckman Coulter), a BioProfile FLEX Analyzer (Nova Biomedical), or an ABL90 FLEX (Radiometer). HuIgG1 antibody titers from supernatant samples were determined using a Protein A HPLC assay. HuIgG1 antibody product quality attributes including level of aggregation, acidic and basic variants, and various glycoforms were determined using size exclusion HPLC, imaged capillary isoelectric focusing, and hydrophilic interaction liquid chromatography HPLC, respectively.
N:P experiment
Transfections were performed as described above. To determine the conditions that produce the highest titer, a full factorial experiment tested PEI:DNA (N:P) ratios of 5, 7.5, 10, and 12.5 and DNA concentrations of 0.75, 1.0, 1.25, and 1.5 µg/mL. An N:P ratio of 7.5 and a DNA concentration of 1 µg/mL yielded the highest titers () and, therefore, was used for all subsequent transfections.
2 L and 35 L bioreactor seed trains
HEK293 DKO cells were cultivated as a seed train in two controlled 2 L bioreactors (Applikon) for 25 days. Bioreactor #1 used a pH setpoint of 7.0 with a deadband of ±0.03 and a DO setpoint of 30% air saturation and bioreactor #2 used a pH setpoint of 7.0 with a deadband of ±0.4 and a DO setpoint of 60% air saturation. Culture pH was controlled using CO2 as acid and 1 M sodium carbonate as base, and DO was controlled by sparging with air and pure oxygen gas via an open pipe sparger. Temperature was maintained at a setpoint of 37°C, and a pitched blade impeller was used to agitate at 275 rpm.
Subsequently, HEK293 DKO cells were cultivated as a seed train in a 35 L bioreactor (Chemglass) for 60 days using a pH setpoint of 7.0 with a deadband of ±0.03 and a DO setpoint of 30% air saturation. Culture pH and DO were controlled the same as in the 2 L bioreactors. Temperature was maintained at a setpoint of 37°C, and a flat blade impeller was used to agitate at 50 rpm.
Ambr15 microbioreactor system
Transfections were performed in the ambr15 microbioreactor system (Sartorius Stedim Biotech)Citation36 as described above with a 12 mL final working volume, a temperature setpoint of 37°C, and a DO setpoint of 30% air saturation. SAS JMP software was used for the experimental design and analysis. The full factorial experiment of four cases in replicate evaluated agitation rates of 630 vs 1400 rpm using a pitched blade impeller and pH deadbands of ±0.03 vs ±0.3 around a setpoint of 7.0. Culture pH was controlled using CO2 as acid and 0.5 M sodium carbonate as base, and DO was controlled by sparging with air and pure oxygen gas via a sparge tube. Every 1–2 days, antifoam (Dow Corning) was added to each ambr15 bioreactor.
Wavebag bioreactor system
The wavebag bioreactor system consisted of a heated, rocking platform (GE Healthcare, Model# 20/50EHT), a gas mix box (Dasgip, Model# MX4/4), and a 22 L nominal volume wavebag (e.g., Thermo or Meissner; custom items) with inlet and outlet gas filters and a sampling port. Transfections were performed as described above with a 10 L final working volume, a temperature setpoint of 37°C, a rock rate of 20 rpm, a rock angle of 8°, and no direct pH control with a gas overlay of 5% CO2 in air at a flow rate of 27 standard liters per hour (slph).
Disclosure of potential conflicts of interest
All authors are or were employees of Genentech, a member of the Roche group, and may hold stock or other financial interests in F. Hoffmann-La Roche Ltd. Genentech has a commercial interest in therapeutic antibody products.
Supplemental Material
Download Zip (775 KB)Acknowledgments
The authors would like to thank Wendy Hsu and Nathaniel Klair for their assistance with the ambr15 microbioreactor system, and Angela Meier for assistance with the FCD. Thank you to the BioMolecular Resources group, the B6 Media Prep group, and the Analytical Operations group. Thank you to Shahram Misaghi for reviewing this manuscript, and the Research Materials Group for their continued support.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Kaplon H, Reichert JM. Antibodies to watch in 2019. MAbs. 2019;11(2):219–38. doi:10.1080/19420862.2018.1556465.
- Singh S, Kumar NK, Dwiwedi P, Charan J, Kaur R, Sidhu P, Chugh VK. Monoclonal antibodies: a review. Curr Clin Pharmacol. 2018;13(2):85–99. doi:10.2174/1574884712666170809124728.
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. 2015;7(1):9–14. doi:10.4161/19420862.2015.989042.
- Baldi L, Hacker DL, Adam M, Wurm FM. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol Lett. 2007;29(5):677–84. doi:10.1007/s10529-006-9297-y.
- Hacker DL, Kiseljak D, Rajendra Y, Thurnheer S, Baldi L, Wurm FM. Polyethyleneimine-based transient gene expression processes for suspension-adapted hek-293e and cho-dg44 cells. Protein Expr Purif. 2013;92(1):67–76. doi:10.1016/j.pep.2013.09.001.
- Stuible M, Burlacu A, Perret S, Brochu D, Paul-Roc B, Baardsnes J, Loignon M, Grazzini E, Durocher Y. Optimization of a high-cell-density polyethylenimine transfection method for rapid protein production in cho-ebna1 cells. J Biotechnol. 2018;281:39–47. doi:10.1016/j.jbiotec.2018.06.307.
- Longo PA, Kavran JM, Kim MS, Leahy DJ. Transient mammalian cell transfection with polyethylenimine (pei). Methods Enzymol. 2013;529:227–40. doi:10.1016/B978-0-12-418687-3.00018-5.
- Rajendra Y, Kiseljak D, Baldi L, Wurm FM, Hacker DL. Transcriptional and post-transcriptional limitations of high-yielding, pei-mediated transient transfection with cho and hek-293e cells. Biotechnol Prog. 2015;31(2):541–49. doi:10.1002/btpr.2064.
- Ding K, Han L, Zong H, Chen J, Zhang B, Zhu J. Production process reproducibility and product quality consistency of transient gene expression in hek293 cells with anti-pd1 antibody as the model protein. Appl Microbiol Biotechnol. 2017;101(5):1889–98. doi:10.1007/s00253-016-7973-y.
- Delafosse L, Xu P, Durocher Y. Comparative study of polyethylenimines for transient gene expression in mammalian hek293 and cho cells. J Biotechnol. 2016;227:103–11. doi:10.1016/j.jbiotec.2016.04.028.
- Chiou HC, Vasu S, Liu CY, Cisneros I, Jones MB, Zmuda JF. Scalable transient protein expression. In: Pörtner R, editor. Animal cell biotechnology: methods and protocols. Totowa (NJ): Humana Press; 2014. p. 35–55.
- Vink T, Oudshoorn-Dickmann M, Roza M, Reitsma JJ, de Jong RN. A simple, robust and highly efficient transient expression system for producing antibodies. Methods. 2014;65(1):5–10. doi:10.1016/j.ymeth.2013.07.018.
- Bos AB, Luan P, Duque JN, Reilly D, Harms PD, Wong AW. Optimization and automation of an end-to-end high throughput microscale transient protein production process. Biotechnol Bioeng. 2015;112(9):1832–42. doi:10.1002/bit.25601.
- Bos AB, Duque JN, Bhakta S, Farahi F, Chirdon LA, Junutula JR, Harms PD, Wong AW. Development of a semi-automated high throughput transient transfection system. J Biotechnol. 2014;180:10–16. doi:10.1016/j.jbiotec.2014.03.027.
- Zhao Y, Bishop B, Clay JE, Lu W, Jones M, Daenke S, Siebold C, Stuart DI, Yvonne Jones E, Radu Aricescu A. Automation of large scale transient protein expression in mammalian cells. J Struct Biol. 2011;175(2):209–15. doi:10.1016/j.jsb.2011.04.017.
- Girard P, Jordan M, Tsao M, Wurm FM. Small-scale bioreactor system for process development and optimization. Biochem Eng J. 2001;7:117–19.
- Raymond C, Tom R, Perret S, Moussouami P, L‘Abbe D, St-Laurent G, Durocher Y. A simplified polyethylenimine-mediated transfection process for large-scale and high-throughput applications. Methods. 2011;55(1):44–51. doi:10.1016/j.ymeth.2011.04.002.
- Nettleship JE, Assenberg R, Diprose JM, Rahman-Huq N, Owens RJ. Recent advances in the production of proteins in insect and mammalian cells for structural biology. J Struct Biol. 2010;172(1):55–65. doi:10.1016/j.jsb.2010.02.006.
- Tuvesson O, Uhe C, Rozkov A, Lullau E. Development of a generic transient transfection process at 100 l scale. Cytotechnology. 2008;56(2):123–36. doi:10.1007/s10616-008-9135-2.
- Geisse S, Henke M. Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production. J Struct Funct Genomics. 2005;6(2–3):165–70. doi:10.1007/s10969-005-2826-4.
- Baldi L, Muller N, Picasso S, Jacquet R, Girard P, Thanh HP, Derow E, Wurm FM. Transient gene expression in suspension hek-293 cells: application to large-scale protein production. Biotechnol Prog. 2005;21(1):148–53. doi:10.1021/bp049830x.
- Pham PL, Perret S, Doan HC, Cass B, St-Laurent G, Kamen A, Durocher Y. Large-scale transient transfection of serum-free suspension-growing hek293 ebna1 cells: peptone additives improve cell growth and transfection efficiency. Biotechnol Bioeng. 2003;84(3):332–42. doi:10.1002/bit.10774.
- Girard P, Derouazi M, Baumgartner G, Bourgeois M, Jordan M, Jacko B, Wurm FM. 100-liter transient transfection. Cytotechnology. 2002;38(1–3):15–21. doi:10.1023/A:1021173124640.
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-ebna1 cells. Nucleic Acids Res. 2002;30(2):E9. doi:10.1093/nar/30.2.e9.
- Fliedl L, Kaisermayer C. Transient gene expression in hek293 and vero cells immobilised on microcarriers. J Biotechnol. 2011;153(1–2):15–21. doi:10.1016/j.jbiotec.2011.02.007.
- Ho L, Greene CL, Schmidt AW, Huang LH. Cultivation of hek 293 cell line and production of a member of the superfamily of g-protein coupled receptors for drug discovery applications using a highly efficient novel bioreactor. Cytotechnology. 2004;45(3):117–23. doi:10.1007/s10616-004-6402-8.
- Meissner P, Pick H, Kulangara A, Chatellard P, Friedrich K, Wurm FM. Transient gene expression: recombinant protein production with suspension-adapted hek293-ebna cells. Biotechnol Bioeng. 2001;75:197–203.
- Mohd Zin NK, Sakaguchi K, Haraguchi Y, Takahashi A, Suzuki S, Yagi T, Shimizu T, Umezu M. Controlling shear stress in a suspension culture using couette flow forefficient proliferation of hek 293 cells. Fluid Mech. 2016;3:1–5.
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41.
- Macaraeg NF, Reilly DE, Wong AW. Use of an anti-apoptotic cho cell line for transient gene expression. Biotechnol Prog. 2013;29(4):1050–58. doi:10.1002/btpr.1763.
- Lim SF, Chuan KH, Liu S, Loh SO, Chung BY, Ong CC, Song Z. Rnai suppression of bax and bak enhances viability in fed-batch cultures of cho cells. Metab Eng. 2006;8(6):509–22. doi:10.1016/j.ymben.2006.05.005.
- Grav LM, Lee JS, Gerling S, Kallehauge TB, Hansen AH, Kol S, Lee GM, Pedersen LE, Kildegaard HF. One-step generation of triple knockout cho cell lines using crispr/cas9 and fluorescent enrichment. Biotechnol J. 2015;10(9):1446–56. doi:10.1002/biot.201500027.
- Cost GJ, Freyvert Y, Vafiadis A, Santiago Y, Miller JC, Rebar E, Collingwood TN, Snowden A, Gregory PD. Bak and bax deletion using zinc-finger nucleases yields apoptosis-resistant cho cells. Biotechnol Bioeng. 2010;105(2):330–40. doi:10.1002/bit.22541.
- Liste-Calleja L, Lecina M, Lopez-Repullo J, Albiol J, Sola C, Cairo JJ. Lactate and glucose concomitant consumption as a self-regulated ph detoxification mechanism in hek293 cell cultures. Appl Microbiol Biotechnol. 2015;99(23):9951–60. doi:10.1007/s00253-015-6855-z.
- Wales R, Lewis G. Novel automated micro-scale bioreactor technology: a qualitative and quantitative mimic for early process development. Bioprocess J. 2010;9:22–25. doi:10.12665/issn.1538-8786.
- Hsu WT, Aulakh RP, Traul DL, Yuk IH. Advanced microscale bioreactor system: a representative scale-down model for bench-top bioreactors. Cytotechnology. 2012;64(6):667–78. doi:10.1007/s10616-012-9446-1.
- Sokolov M, Ritscher J, MacKinnon N, Souquet J, Broly H, Morbidelli M, Butte A. Enhanced process understanding and multivariate prediction of the relationship between cell culture process and monoclonal antibody quality. Biotechnol Prog. 2017;33(5):1368–80. doi:10.1002/btpr.2502.
- Rameez S, Mostafa SS, Miller C, Shukla AA. High-throughput miniaturized bioreactors for cell culture process development: reproducibility, scalability, and control. Biotechnol Prog. 2014;30(3):718–27. doi:10.1002/btpr.1874.
- Janakiraman V, Kwiatkowski C, Kshirsagar R, Ryll T, Huang YM. Application of high-throughput mini-bioreactor system for systematic scale-down modeling, process characterization, and control strategy development. Biotechnol Prog. 2015;31(6):1623–32. doi:10.1002/btpr.2162.
- Ma N, Koelling KW, Chalmers JJ. Fabrication and use of a transient contractional flow device to quantify the sensitivity of mammalian and insect cells to hydrodynamic forces. Biotechnol Bioeng. 2002;80(4):428–37. doi:10.1002/bit.10387.
- Mollet M, Godoy-Silva R, Berdugo C, Chalmers JJ. Acute hydrodynamic forces and apoptosis: a complex question. Biotechnol Bioeng. 2007;98(4):772–88. doi:10.1002/bit.21476.
- Choosakoonkriang S, Lobo BA, Koe GS, Koe JG, Middaugh CR. Biophysical characterization of pei/DNA complexes. J Pharm Sci. 2003;92(8):1710–22. doi:10.1002/jps.10437.
- Bertschinger M, Schertenleib A, Cevey J, Hacker DL, Wurm FM. The kinetics of polyethylenimine-mediated transfection in suspension cultures of chinese hamster ovary cells. Mol Biotechnol. 2008;40(2):136–43. doi:10.1007/s12033-008-9069-0.
- Li F, Vijayasankaran N, Shen A, Kiss R, Amanullah A. Cell culture processes for monoclonal antibody production. mAbs. 2010;2:466–77.
- Li J, Wong CL, Vijayasankaran N, Hudson T, Amanullah A. Feeding lactate for cho cell culture processes: impact on culture metabolism and performance. Biotechnol Bioeng. 2012;109(5):1173–86. doi:10.1002/bit.24389.
- Yuk IH, Baskar D, Duffy PH, Hsiung J, Leung S, Lin AA. Overcoming challenges in wave bioreactors without feedback controls for ph and dissolved oxygen. Biotechnol Prog. 2011;27(5):1397–406. doi:10.1002/btpr.659.
- Rockberg J, Zhan C, Malm M, Schwarz H, Lundqvist M, Shokri A, Field R, Turner R, Chotteau V 2018. Production of biopharmaceuticals in an intensified perfusion process of hek 293 cells. Paper presented at: Cell Culture Engineering XVI. Tampa (Florida, USA).
- Misaghi S, Qu Y, Snowden A, Chang J, Snedecor B. Resilient immortals, characterizing and utilizing bax/bak deficient chinese hamster ovary (cho) cells for high titer antibody production. Biotechnol Prog. 2013;29(3):727–37. doi:10.1002/btpr.1722.
- Nagata E, Luo HR, Saiardi A, Bae BI, Suzuki N, Snyder SH. Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J Biol Chem. 2005;280(2):1634–40. doi:10.1074/jbc.M409416200.