ABSTRACT
Multivalent mono- or bispecific antibodies are of increasing interest for therapeutic applications, such as efficient receptor clustering and activation, or dual targeting approaches. Here, we present a novel platform for the generation of Ig-like molecules, designated diabody-Ig (Db-Ig). The antigen-binding site of Db-Ig is composed of a diabody in the VH-VL orientation stabilized by fusion to antibody-derived homo- or heterodimerization domains, e.g., CH1/CL or the heavy chain domain 2 of IgE (EHD2) or IgM (MHD2), further fused to an Fc region. In this study, we applied the Db-Ig format for the generation of tetravalent bispecific antibodies (2 + 2) directed against EGFR and HER3 and utilizing different dimerization domains. These Db-Ig antibodies retained the binding properties of the parental antibodies and demonstrated unhindered simultaneous binding of both antigens. The Db-Ig antibodies could be purified by a single affinity chromatography resulting in a homogenous preparation. Furthermore, the Db-Igs were highly stable in human plasma. Importantly, only one short peptide linker (5 aa) per chain is required to generate a Db-Ig molecule, reducing the potential risk of immunogenicity. The presence of a fully functional Fc resulted in IgG-like pharmacokinetic profiles of the Db-Ig molecules. Besides tetravalent bispecific molecules, this modular platform technology further allows for the generation of other multivalent molecules of varying specificity and valency, including mono-, bi-, tri- and tetra-specific molecules, and thus should be suitable for numerous applications.
Introduction
Bispecific antibodies combine the activity of two parental monoclonal antibodies, such as dual targeting, but can also utilize novel mechanisms of action, including effector cell retargeting, shuttling the blood-brain-barrier, or mimicking the activity of natural proteins.Citation1-Citation6 Two bispecific antibodies are currently approved for therapy, blinatumomab for the treatment of acute myeloid leukemia and emicizumab as a substitute of factor VIII for the treatment of hemophilia A, and more than 50 bispecific antibodies are currently in clinical development.Citation7,Citation8 A wide variety of different formats are available to generate bispecific antibodies, which can be classified according to the absence or presence of an Fc region (IgG-like molecules), their overall architecture (symmetric or asymmetric), or the number of antigen-binding sites (valency).Citation7,Citation9
In the past, the correct assembly of heavy and light chains to generate an intact bispecific IgG molecule was a challenge. Genetic engineering forcing the heterodimerization of the two heavy chains (heavy chain problem) and of the heavy and light chain (light chain problem) solved these issues, and allowed the generation of asymmetric IgG or Ig-like molecules.Citation10-Citation19 Symmetric IgG-like molecules can be generated by fusion of different binding sites to an Fc region or an IgG, resulting in bispecific and tetravalent molecules,Citation7 including IgG-single-chain variable fragment (scFv) fusion proteins,Citation20 single-chain diabody-Fc fusion proteins,Citation21 dual-variable-domain antibody (DVD-Ig),Citation22 and cross-over dual variable Ig-like proteins (CODV-Ig).Citation23 However, these tetravalent bispecific formats often suffer from poor stability, restricted antigen-binding, or the extensive use of linker sequences to connect the variable domains.
Here, we present a novel and versatile platform for the generation of tetravalent symmetric Ig-like antibody molecules, designated diabody-Ig (Db-Ig). The binding sites of these molecules are formed by a bivalent diabody (either mono- or bispecific), fusing a VH domain with a short, five amino acid long linker to a VL domain. The diabody unit is stabilized by fusion to either homodimerizing domains, such as the second heavy chain domains of IgE (EHD2) or IgM (MHD2),Citation24,Citation25 or heterodimerizing domains, such as the CH1/CL domains or a modified EHD2 domain (hetEHD2). Furthermore, the fusion of these bivalent building blocks (Db-Fab) to an Fc results in Ig-like tetravalent molecules. We used the Db-Ig platform to generate tetravalent bispecific antibodies targeting epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 3 (HER3). These Db-Igs retained the antigen-binding properties of parental antibodies, and demonstrated the ability to bind simultaneously both antigens, resulting in strong anti-proliferative activity and induction of apoptosis. In addition, the Db-Ig molecules showed high stability in human plasma, and the use of the Fc of an IgG revealed IgG-like pharmacokinetic properties in mice.
Results
The Db-Ig format
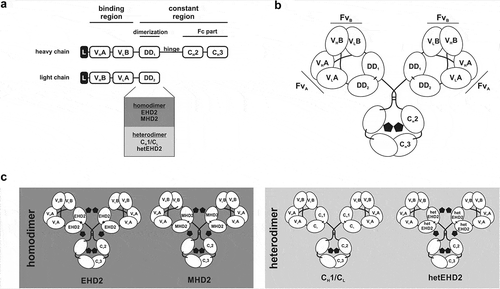
The Db-Fab arm of a Db-Ig molecule is formed by fusing a diabody moiety in the VH-VL configuration to the N-terminus of a dimerization domain (DD), which can form either a homo- or a heterodimer (). Here, the second constant domain of the heavy chain of IgM (MHD2)Citation24 or IgE (EHD2)Citation24,Citation25 was engaged as homodimerization domains, whereas CH1/CL and a mutated version of EHD2 (hetEHD2) were used as heterodimerization domains. With respect to the hetEHD2 domain, cysteine residues forming one of the two interdomain disulfide bonds formed between residues C247 and C337 were substituted by serine residues, leading to the formation of heterodimers. Heterodimerization of the hetEHD2 domain was confirmed in SDS-PAGE analysis under non-reducing conditions using His-tagged scFv-EHD2(C247S) and Flag-tagged EHD2(C337S), which were co-transfected in HEK293E cells and purified with anti-Flag M2-agarose (Fig. S1). Importantly, the formation of homodimers of scFv-EHD2(C247S) of EHD2(C337S) was not detected in SDS-PAGE analysis. Fusion of one chain of the Db-Fab arm to the N-terminus of an Fcγ1 results in dimeric assembly and formation of tetravalent molecules ().
Figure 1. Tetravalent Db-Fc fusion proteins. (a) the schematic construction of a heavy and light chain of Db-Ig molecules. Variable domains are fused via G4S linker to build a diabody as binding region. The dimerization domain (DD1 and DD2) originate from either a heterodimer (CH1/CL or hetEHD2) or a homodimer (EHD2 or MHD2). The Fc part is formed by the hinge region and CH2/CH3 of an IgG. (b) schematic illustration of a tetravalent Db-Ig molecule with symmetric architecture. In dependence of the variable domains, this molecule can be either mono- (FvA = FvB) or bispecific (FvA≠FvB). N-glycans are shown as black pentagons. (c) Schematic illustration of tetravalent Db-Ig molecules using either homodimerization domains (EHD2 or MHD2) or heterodimerization domains (CH1/CL or hetEHD2). N-glycans are shown as black pentagons.
Modeling of crystal structures of a diabody moiety (VH-VL orientation)Citation26 with the CH1 and CL domains of an IgGCitation27 revealed close proximity of the C-termini of the diabody moiety and the N-termini of the constant domains enabling a direct genetic fusion without the insertion of an additional linker sequence (Fig. S2A). In addition, an alignment of the different crystal structures of human CH1/CL, human EHD2, and mouse MHD2 (as no human structure was available) showed high structural similarity, including the position of the N-terminus of each domain (Fig. S2B). Thus, for the generation of tetravalent Db-Ig molecules, only one short linker (e.g., 5 aa) connecting both variable domains (VH-VL) in the diabody moiety is required within each chain of the molecule.
Tetravalent bispecific Db-Igs targeting EGFR and HER3
The variable domains of a humanized version of anti-EGFR antibody cetuximab (hu225)Citation28 and anti-HER3 antibody IgG 3–43Citation29 were used to generate tetravalent bispecific Db-Ig molecules. In total, four different tetravalent bispecific molecules were produced using different dimerization domains (DD): CH1/CL, EHD2, MHD2, and hetEHD2 (). Thus, each tetravalent molecule consists of two antigen-binding sites for EGFR and two binding sites for HER3 (2 + 2 format). All Db-Ig molecules were produced in HEK293-6E suspension cells, which were co-transfected with both plasmids encoding for the heavy chain (VH-VL-DD-CH2-CH3) and for the light chain (VH-VL-DD). Proteins were purified for further analysis via a one-step affinity chromatography using either CH1-CaptureSelect (using CH1/CL as DD) or FcXL-CaptureSelect (using EHD2, MHD2, or hetEHD2 as DD). Productivities of the CH1/CL-based Db-Ig molecules were 6.4 mg/l or 9.9 mg/l using either CH1-CaptureSelect or FcXL-CaptureSelect, respectively. In comparison, using FcXL-CaptureSelect, higher productivities of 35.3 mg/l, 21.6 mg/l, and 21.3 mg/l were obtained for Db-Ig molecules utilizing EHD2, hetEHD2, and MHD2, respectively, as dimerization domain.
Figure 2. Biochemical characterization of Db3-43xhu225-Ig molecules. (a) Schematic illustration of Db3-43xhu225-Ig molecules using CH1/CL, EHD2, MHD2, or hetEHD2 as dimerization module. (b) SDS-PAGE analysis of Db3-43xhu225-Ig molecules under reducing (1) or non-reducing (2) conditions (4–12% PAA gradient). Proteins were stained with Coomassie blue. (c) Size-exclusion chromatography of Db3-43xhu225-Ig molecules by HPLC using a Tosoh TSKgelSuperSW column.
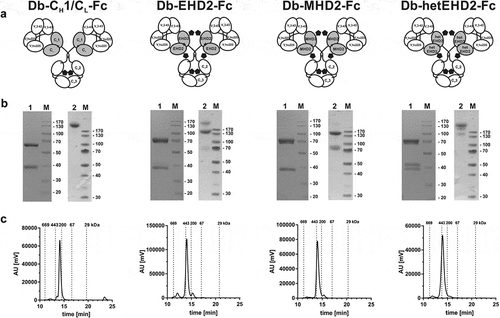
Size-exclusion chromatography of the different tetravalent antibodies under native conditions confirmed correct assembly of the tetravalent antibodies, indicating protein preparations with high purity for the different Db-Ig molecules, with only very low amounts of multimers (below 5% using CH1/CL, hetEHD2, or MHD2 as DD; below 10% using EHD2 as DD) (). The apparent Stokes radii (SR) were ~5.4 nm for Db3–43xhu225-CH1/CL-Fc and ~5.8 nm for the Db3–43xhu225-Ig molecules using EHD2, MHD2, or hetEHD2 as dimerization domain. SDS-PAGE analysis confirmed purity of the molecules, revealing two bands of ~ 40 kDa and 70 kDa under reducing conditions, representing the light and heavy chain (calculated molecular weight: 37.3 and 60.8 kDa), respectively (). In the case of EHD2, MHD2, and hetEHD2, a more or less pronounced double band was detected for each chain, which is most likely due to the presence or absence of N-glycans (all three domains exhibit one potential N-glycosylation site).Citation24,Citation25 Under non-reducing conditions, a major band of approximately 220 kDa was observed for Db-CH1/CL-Fc, confirming disulfide-linkage of all four chains. This band was also observed for the other molecules, but bands of lower molecular weights were also observed to a varying extent, indicating a partial disulfide-bond formation between the light and heavy chains, which was especially observed for the EHD2 and MHD2-derived Db-Igs. This was confirmed by Western Blot analysis of the different Db-Ig molecules using horseradish peroxidase (HRP)-conjugated anti-human Fc detection antibody (Fig. S3).
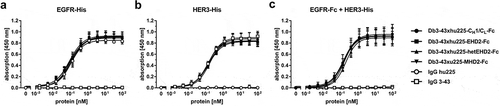
Binding of the tetravalent antibodies was analyzed by ELISA using either EGFR-His or HER3-His as immobilized antigen. All Db-Igs showed a concentration-dependent binding to the two antigens, similar to the parental monospecific antibodies anti-EGFR IgG hu225 and anti-HER3 IgG 3–43 included as controls. The EC50 values were in the range of 92 to 118 pM for EGFR binding and 140 to 173 pM for HER3 binding (, ). This finding confirms the correct assembly of the individual antigen-binding sites in all four Db-Ig formats. Furthermore, simultaneous binding of both antigens was confirmed in a second ELISA binding assay using EGFR-Fc fusion protein as immobilized antigen and HER3-His as second soluble antigen. Similar EC50 values in the range of 160 to 204 pM were obtained for the tetravalent bispecific molecules, whereas, as expected, no binding was detected for the parental antibodies (, ).
Table 1. EC50 values [pM] of bispecific Db3–43xhu225-Ig molecules determined by ELISA binding experiments or flow cytometry analysis. Mean ± SD, n = 3.
Figure 3. Binding of different Db3-43xhu225-Ig molecules to EGFR and HER3 by ELISA. A + B Binding of Db3-43xhu225-Ig molecules to immobilized EGFR-His (a) or HER3-His (b). Parental antibodies IgG hu225 and IgG 3-43 were included as a control. Bound antibodies were detected with an HRP-conjugated anti-human Fc secondary antibody. Mean ± SD, n = 3. (c) Simultaneous binding of both antigens was analyzed using EGFR-Fc as immobilized antigen. Serial dilution of different antibodies was added to the wells. Finally, the second antigen, HER3-His, was added to the wells and bound HER3-His was detected using an HRP-conjugated anti-His secondary antibody. Mean ± SD, n = 3.
We further generated a Db-Ig with a VL-VH orientation of the diabody moiety using CH1/CL as heterodimerization module. Production and purification of the molecule were performed identically as described above for the Db-Ig molecule in the VH-VL configuration. Of note, the Db3–43xhu225-CH1/CL-Fc molecule in the VL-VH orientation showed similar binding to both antigens in ELISA binding experiments (EGFR: EC50 ~218 pM; HER3: EC50 ~240 pM) compared to the VH-VL Db-Ig molecule, but the protein preparation was associated with a heterogeneous purification profile with an increased portion of multimers (above 20%) (Fig. S4).
Cell binding of the bispecific antibodies was analyzed by flow cytometry using the head and neck cancer cell line FaDu (). The parental antibodies IgG hu225 and IgG 3–43 were included as a control. Similar to the ELISA binding studies, concentration-dependent binding was detected for all analyzed antibodies. As FaDu cells express very high amount of EGFR (~ 140,000 receptors per cell) and low amounts of HER3 (~ 3,000 receptors per cell) on their surface,Citation29 cell binding was obviously dominated by EGFR, which resulted in binding of IgG hu225 and of all bispecific antibodies with similar EC50 values in the range between 129 and 238 pM ().
Figure 4. Binding and bioactivity of bispecific Db3-43xhu225-Ig molecules on FaDu cells. (a) Binding of different antibodies to FaDu cells was analyzed by flow cytometry. Bound antibodies were detected using a PE-labeled anti-human Fc secondary antibody. Mean ± SD, n = 3. (b) Proliferation assay of different bispecific Db3-43xhu225-Ig molecules (50 nM) using FaDu cells in starvation medium after incubation of 7 days. Cells were kept either unstimulated (w/o ligand) or stimulated with heregulin (HRG). Parental antibodies were included as control (single treatment: 50 nM; combination: 50 nM each). Cell viability was measured using CellTiter-Glo 2.0. Mean ± SD, n = 3. (c) Flow cytometry analysis of annexinV/PI staining using FaDu cells. Bispecific Db3-43xhu225-CH1/CL-Fc molecule (50 nM), or parental antibodies (single treatment: 50 nM; combination: 50 nM each) were incubated with cells in complete medium for 24 h. Mean ± SD, n = 3. *p < .05; **p < .01; ***p < .001; ****p < .0001.
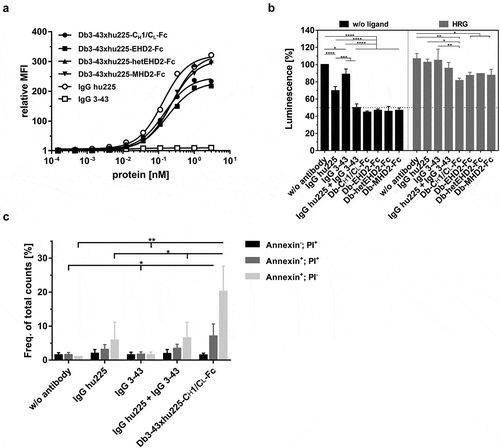
Bioactivity of the Db-Igs was investigated in a proliferation assay using FaDu cells in starvation medium kept either unstimulated or stimulated with heregulin (HRG). Here, all bispecific antibodies, as well as the combination of both parental antibodies (IgG hu225 and IgG 3–43), showed a strong reduction of proliferation, with strongest effects observed for the bispecific antibodies under HRG-stimulation (). The bispecific Db-Ig antibody harboring CH1/CL as dimerization domain was further analyzed for the induction of apoptosis in FaDu cells. FaDu cells were incubated with monospecific antibodies, the combination thereof, or the bispecific Db-Ig antibody for 24 h in rich medium. Induction of apoptosis was analyzed by flow cytometry using annexinV and PI staining. Increased amounts of annexinV-stained (early apoptosis) and annexin V/PI-stained (late apoptosis) cells were measured for IgG hu225 and the combination of the monospecific antibodies compared to untreated and IgG 3-43-treated cells, indicating the dominant effect of EGFR-targeting on FaDu cells. Interestingly, the bispecific Db-Ig antibody revealed the highest amount of cells in the early and late apoptosis (; Fig. S5).
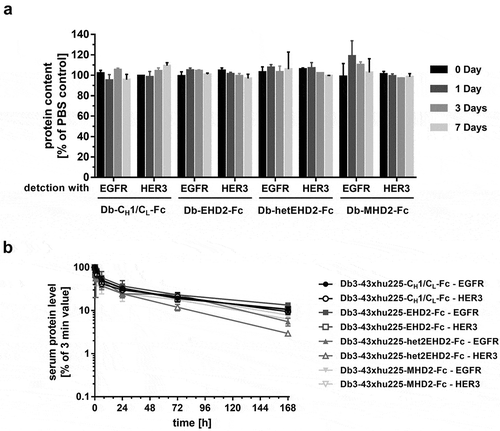
In vitro stability of the tetravalent bispecific Db-Ig antibodies was tested by incubating proteins in human plasma at 37°C. All tetravalent antibodies showed retained the binding capacity to both antigens after 7 days of incubation (), indicating high stability of these molecules in human plasma. In addition, pharmacokinetic profiles of tetravalent bispecific antibodies were determined in immunocompetent CD-1 mice receiving one single dose intravenous (i.v.) injection. Except for Db3-43xhu225-hetEHD2-Fc, which revealed a slightly reduced pharmacokinetic profile, all other Db-Ig molecules showed similar terminal half-lives in the range of 71 and 105 h, as well as similar drug exposure (AUC), independent of the antigen used for detection (; ).
Table 2. Determination of initial (t½α) and terminal (t½β) half-lives as well as of drug exposure (area under the curve; AUC) of bispecific Db3-43xhu225-Ig molecules. Mean ± SD, n = 3.
Figure 5. Plasma stability and PK analysis of bispecific Db3-43xhu225-Ig molecules. (a) The Db-Ig molecules were diluted in 50% human plasma and incubated at 37°C for, 1, 3, or 7 days. Protein content was determined by ELISA using both antigens, EGFR-His or HER3-His. (b) Pharmacokinetic profile of Db-Ig molecules were analyzed in female CD-1 mice (n = 3). Mice received a single i.v. injection of 25 µg of the protein. Serum protein concentrations were determined by ELISA using both antigens, EGFR-His or HER3-His.
Discussion
With the Db-Ig platform, we established a new format for the generation of tetravalent bispecific IgG-like molecules. Variable domains of monoclonal antibodies can be fused directly into the platform, generating novel multivalent antibodies. We found that the diabody moiety in the VH-VL orientation is ideally suited for the fusion to the N-terminus of the different dimerization domains. Advantages include: 1) the C-termini of both Db-assembled VL domains are closely located to the N-termini of the different dimerization domains; 2) both antigen-binding sites are facing away from each other enabling unhindered access to both antigens; 3) Db exhibit (besides a VH-VL pairing within each Fv head) a strong VH-VH back-to-back interaction stabilizing a Fab-like, closed conformation of the antigen-binding sites.Citation26 This particular structure of a diabody in the VH-VL orientation was further confirmed by other studiesCitation30-Citation32 and nicely reviewed by Kwon et al.Citation33 In general, we assume that Db-Ig molecules should exhibit Ig-like flexibility, for example, the elbow angle, within each Db-Fab arm or the hinge angle between both Db-Fab arms,Citation34,Citation35 due to the high structural similarity within the utilized dimerization domains (CH1, CL, MHD2, and EHD2). In our examples, neither the inter-peptide linker within the antigen-binding site (diabody moiety) nor the composition of the antigen-binding sites within one Db-Fab arm of the antibody needed to be optimized for the generation of novel bispecific antibodies. In contrast, a requirement for linker optimization has been described for other formats, e.g., DVD-Ig,Citation22,Citation36 CODV-Ig.Citation23 Here, the four different linkers with a length of up to 30 aa, which connect the variable domains with each other or with the constant domains, as well as the arrangement of the variable domains, need to be adapted for optimal binding of the antibody.
Of note, the orientation of the variable domains on the diabody moiety seems to be critical for correct assembly of the Db-Igs. Carmichael and coworkers published the crystal structure of a diabody in the VL-VH orientation.Citation37 In comparison to the crystal structure of the VH-VL orientation (1LMKCitation26), the position and orientation of the different domains are rearranged. This divergent structure resulted most likely from the hydrophilic interactions of the loops originated from both, VH and VL, domains.Citation33 As a result, the C termini of this VL-VH diabody are located ~ 60 Å from each other. With a distance of ~ 40 Å for the N-termini of a CH1/CL heterodimer originating form an IgG1 (1HZHCitation27), the direct (linker-less) fusion with a diabody in the VL-VH orientation results in a discrepancy of ~ 20 Å, which could be responsible for the heterogeneous preparation compared to fusion with a diabody in the VH-VL configuration (~ 40 Å between both C-termini).
Different dimerization domains were used in the Db-Ig platform for the stabilization of the diabody moiety: CH1/CL, hetEHD2, EHD2, and MHD2. Homogeneous protein preparation, as well as similar binding to both antigens and FaDu cells or similar plasma stability, was associated with all bispecific antibodies independent of the utilized dimerization domains. Besides EHD2 and MHD2, other constant domains of an antibody, which tend to form homodimers like CL/CL, IgG-derived CH3/CH3, or IgM- or IgE-derived CH4/CH4, could potentially also be used as dimerization domains in the Db-Ig platform. In addition to these homodimers, we also designed and generated a EHD2-based heterodimerization domain, hetEHD2. In general, the two EHD2 domains are disulfide-linked between residue C241 (domain a) and C337 (domain b) and vice versa between C337 (domain a) and C241 (domain b). Besides the two disulfide bridges, the interface of the EHD2 dimer is dominated by polar residues and involves only 11 hydrogen bonds.Citation38 In the absence of these dimer-forming disulfide bridges, the EHD2 domains had no measurable affinity to each other,Citation39 indicating the importance of the disulfide bridges for dimerization. Thus, eliminating one disulfide bond by substituting the involved cysteine residues with serine residues forces the formation of heterodimers. As these mutated cysteine residues are located at the interface of the EHD2 dimer, the conformational structure of the hetEHD2, including structurally dependent properties like plasma stability, should be mainly unaffected by the introduction of this mutational change. Recently, Cooke and co-workers developed a heterodimerization domain, EFab, on the basis of EHD2 by introducing knob-into-hole-like mutations.Citation19 Asymmetric bivalent and bispecific molecules were generated using this EFab arm in combination with a ‘classical’ second Fab arm and a heterodimeric Fc part (knob-into-hole). Besides the formation of multimers (and half antibodies), disordered regions were identified in the fusion of the variable domains with EFab. In line with these results, EFab showed increased proteolysis in the presence of proteinase K compared to the wild-type EHD2 or a ‘classical’ Fab.
We show that the different Db-Ig formats can be purified via a one-step purification procedure using either CH1-CaptureSelect or FcXL-CaptureSelect with less than 5% to 10% of multimers formation. Interestingly, different dimerization domains affected the production of Db-Ig molecules, with EHD2 showing the highest productivity (~ 35 mg/l). This productivity is within the range of our in-house production of monospecific IgG antibodies (30 to 50 mg/l). In addition, as protein G exclusively binds to the constant domains of the heavy chain of IgGs,Citation40,Citation41 this affinity chromatography can also be used for purification of Db-Ig molecules. In contrast, standard purification of antibodies via protein A affinity chromatography purification resulted in heterogeneous preparation of antibodies (not shown), as VH, which can be also bound by protein A,Citation42,Citation43 is present in both chains of the antibody, resulting also in the purification of the light chain dimer.
ELISA binding studies confirmed unhindered accessibility of both antigen-binding sites of the Db-Ig platform to their antigens, resulting in retained binding properties of the parental antibodies, and in simultaneous binding of both antigens. For other multivalent antibody platforms, for example, the DVD-Ig platform, reduced binding of the inner Fv compared to the parental antibody was reported for a molecule targeting tumor necrosis factor (TNF), most likely due to steric hindrances.Citation36
Results from the plasma stability assay as well as IgG-like PK profiles underline correct assembly of the polypeptide chains for all four analyzed Db-Ig molecules, with high stability in vitro and in vivo. The half-life of the different anti-EGFR x anti-HER3 Db-Ig antibodies (2 to 4 days) is similar to that of the parental anti-HER3 antibody IgG 3-43 (2.5 days).Citation29 Due to the cross-reactivity of IgG 3-43 to mouse HER3, target-mediated disposition could be an explanation for these pharmacokinetic properties. With respect to stability, the importance of the introduction of dimerization domains within the Db-Igs becomes apparent when compared with other diabody-based molecules, which lack an additional dimerization domain. For example, the tetravalent ‘Di-Diabody’ showed reduced antigen binding either after incubation in mouse serum at 37°C for three daysCitation44 or after circulation in mice for 7 days,Citation45 most likely due to the dissociation of the two non-covalently linked polypeptide chains. In line with these results, reduced serum stability was also reported for non-covalently linked bivalent diabody molecules,Citation46 as well as for tetravalent tandem diabodies (tandAbsCitation47).
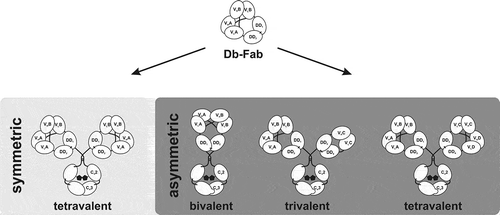
In summary, we present a novel Db-Ig platform for the generation of tetravalent bispecific IgG-like antibodies, with a diabody as a binding moiety. Db-Ig molecules are stabilized either by homo- or heterodimerization domains, resulting in high stability. In total, only one short inter-peptide linker was introduced within the binding moiety, whereas the other domains, which are human antibody-derived, are ‘naturally’ fused to each other. Our results indicate that variable domains of parental antibodies can be directly inserted into the Db-Ig platform without time-consuming optimization of the composition of antigen binding sites or optimization of the inter-peptide linker,Citation22,Citation23 retaining parental binding properties. Our results need to be validated in future studies that include other variable domains. Of note, the Db-Ig platform also allows the generation of asymmetric molecules by introducing a heterodimeric assembled Fc.Citation10 Thus, tetravalent asymmetric molecules, for example, tetraspecific (1 + 1 + 1 + 1), trispecific (2 + 1 + 1), or bispecific (2 + 2; each Db-Fab arm is monospecific), can be generated by using two different dimerization domains in each Db-Fab arm (). Due to the modular composition of the Db-Ig platform, other moieties can be fused to one chain of the heterodimerized Fc part, including scFv or Fabs, resulting in trivalent molecules (1 + 2, 1 + 1 + 1), or cytokines such as single-chain derivatives of the TNF superfamily, resulting in bifunctional molecules. In addition, bivalent Db-Ig molecules (1 + 1) can be generated by fusing both chains of the Db-Fab arm directly to a heterodimeric Fc part. Thus, the Db-Ig format represents a versatile novel platform for the generation of molecules with varying valency and specificity for a plethora of applications.
Figure 6. Schematic overview of symmetric and asymmetric Db-Ig molecules. Symmetric tetravalent molecules are designed by fusing a wild-type Fc part to one chain of the Db-Fab arm resulting in mono- (VA = VB) or bispecific (VA≠VB) antibodies. By introducing a heterodimeric assembled Fc part (e.g., knob-into-hole), asymmetric molecules can be engineered, which can be bivalent (1 + 1), trivalent (3 + 0; 2 + 1; 1 + 1 + 1), or tetravalent (2 + 2; 2 + 1 + 1; 1 + 1 + 1 + 1).
Materials and methods
Material
HRP- and phycoerythrin (PE)-conjugated anti-human Fc antibodies were purchased from Sigma-Aldrich (A0170; P9170), whereas anti-His-HRP (HIS-6 His-Probe-HRP, sc-8036) was purchased from Santa Cruz Biotechnology. Antibodies for immunoblotting were purchased at Cell Signaling (Phospho-EGF Receptor (Tyr1068) (D7A5) XP® Rabbit mAb #3777; Phospho-HER2/ErbB2 (Tyr1221/1222) (6B12) Rabbit mAb #2243; Phospho-HER3/ErbB3 (Tyr1289) (21D3) Rabbit mAb #4791; Akt (pan) (40D4) Mouse mAb #2920; Phospho-Akt (Thr308) (D25E6) XP® Rabbit mAb #13038; Phospho-Akt (Ser473) (D9E) XP® Rabbit mAb #4060; p44/42 MAPK (Erk1/2) (3A7) Mouse mAb #9107; Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) Antibody #9101; Cell Signaling Technology Europe B.V), at Santa Cruz (EGFR (sc-03) Rabbit mAb #sc-03), at Thermo Fisher Scientific (HER-2/c-erbB-2/neu Ab-17, Mouse Monoclonal Antibody # MS-730-P-A; ErbB3, clone: 2F12, Invitrogen™ Mouse mAb #MA5-12675), at Sigma-Aldrich (α-Tubulin Mouse mAb #T6793) and Dianova (Goat IgG anti-Mouse IgG (H + L)-HRPO, MinX Hu,Bo,Ho #115–035-062).
Antibody production and purification
Heavy and light chains of different tetravalent bispecific antibodies were cloned into variants of pSecTagA vector, and were transiently transfected into HEK293-6E suspension cells using polyethylenimine (PEI; linear, 25 kDa, Sigma-Aldrich) for transfection. HEK293-6E cells were provided by National Research Council of Canada (Ottawa, Canada), and cultivated in F17 Freestyle expression medium (ThermoFisher) supplemented with 0.1% Kolliphor P-118 (Sigma), 4 mM GlutaMAX (ThermoFisher), and 25 µg/ml G418. By adding TN1 (20% tryptone N1 (Organotechnie S.A.S., France) in F17 medium) to feed the culture 24-h post transfection, protein production was initiated; cells were cultivated for additional 4 days at 37°C. Proteins were purified from the supernatant using CH1-CaptureSelcet or FcXL-CaptureSelect (2943452010, 194328010, ThermoFisher) or protein G agarose (ProteinMods) affinity chromatography. Preparations were dialyzed against phosphate-buffered saline (PBS) at 4°C.
Antibody characterization
Antibodies were analyzed by SDS-PAGE (4 µg) and stained with Coomassie-Brilliant Blue G-250. The purity and integrity of molecules (9–30 µg in 30 µl) were analyzed via size-exclusion chromatography using a Waters 2695 HPLC in combination with a TSKgel SuperSW mAb HR column (822854, Sigma Aldrich) at a flow rate of 0.5 ml/min using 0.1 M Na2HPO4/NaH2PO4, 0.1 M Na2SO4, pH 6.7 as mobile phase. Standard proteins: thyroglobulin (669 kDa, RS 8.5 nm), apoferritin (443 kDa, RS 6.1 nm), β-amylase (200 kDa, RS 5.4 nm), bovine serum albumin (66 kDa, RS 3.55 nm), carbonic anhydrase (29 kDa, RS 2.35 nm). Stokes radii of antibodies were interpolated from standard proteins.
Enzyme-linked immunosorbent assay (ELISA)
Ninety-six-well plates were coated with EGFR-Fc,Citation24 EGFR-His (extracellular domain (ECD) of EGFR: aa 25–645 with C-terminal His-tag), or HER3-His (ECD of HER3: aa 20–643 with C-terminal His-tag) (200 ng/well in PBS) overnight at 4°C and residual binding site were blocked with 2% (w/v) skim milk powder in PBS (MPBS, 200 µl/well). The antibodies were diluted in MPBS and titrated 1 to 3 in duplicates starting from 100 nM and incubated for 1 h at room temperature. Bound antibodies were detected with HRP-conjugated antibodies specific for human Fc (for the bispecific antibodies) or His-tag (for bound HER3-His). Detection antibodies were incubated for one additional hour at room temperature. TMB (1 mg/ml; 0.006% (v/v) in 100 mM Na-acetate buffer, pH 6) was used as the substrate, reaction was terminated using 50 µl 1 M H2SO4 and absorption was measured at a wavelength of 450 nM. In general, plates were washed three times with PBST (PBS + 0.005% Tween20) and twice with PBS between each incubation step and in advance of the detection.
Flow cytometry analysis
FaDu cellsCitation29 (1x105 per well) were incubated with a serial dilution of different antibodies (starting from 3 nM) for 1 h at 4°C. After washing cells twice, bound antibodies were detected using a PE-labeled anti-human Fc antibody (9170, Sigma-Aldrich). Flow cytometry was performed using MACSQuant Analyzer 10 or MACSQuant VYB (both Miltenyi Biotec). Relative mean fluorescence intensities (MFI) were calculated as followed: rel. MFI = ((MFIsample-(MFIdetection-MFIcells))/MFIcells).
Cell proliferation assays
FaDu cellsCitation29 (2x103 per well) were seeded in 96-well plate and cultivated at 37°C in rich medium (10% fetal bovine serum (FBS)) for 24 h, and subsequent medium exchange to starvation medium (0.2% FBS and 1x penicillin/streptomycin). After 24 h of starvation, cells were treated with different antibodies (single: 50 nM; combination: 50 nM each) for 60 min at 37°C. Cells were kept unstimulated or were stimulated with heregulin (HRG; 30 ng/ml; Sigma-Aldrich: St. Louis, MO, USA) and cultivated for 5 days at 37°C. Cell viability was measured using CellTiter-Glo 2.0 Assay (Promega). Luminescence of untreated and unstimulated cells was set as 100%.
Induction of apoptosis assay
FaDu cellsCitation29 (5x104 per well) were seeded in 12-well plates and cultivated at 37°C in rich-medium (10% FBS) for 24 h, and subsequently medium exchanged to rich medium (10% FBS and 1x penicillin/streptomycin) with added antibodies (single: 50 nM; combination: 50 nM each). After 24 h of incubation at 37°C, supernatant was collected, and the cells were washed with PBS and harvested using Trypsin-EDTA (1x). The trypsin-EDTA reaction was stopped using matched collected supernatant. After centrifugation (5 min, 500 x g), supernatant was discarded and cells were resuspended in 100 µl AnnexinV Binding Buffer (556454; Heidelberg, BD Biosciences). Next, 5 µl of PI (556463, Heidelberg, BD Biosciences) and 5 µl of annexinV-GFP were added, following 15 min of incubation at room temperature. Flow cytometry was performed using MACSQuant Analyzer 10 (both Miltenyi Biotec). Gates were set for untreated cells.
Plasma stability
Proteins were diluted in 50% human plasma obtained from healthy donors (Klinikum Stuttgart, Germany) with a concentration of 200 nM. Proteins were either directly stored at −20°C (day 0) or incubated at 37°C for 1, 3, or 7 days prior storing at −20°C. Levels on intact protein were analyzed via ELISA using either EGFR-His or HER3-His as antigen. Bound antibodies were detected using an HRP-conjugated anti-human Fc antibody as described above.
Pharmacokinetics
Animal care and all performed experiments were in accordance with Federal and European guidelines and were approved by university and state authorities. Twenty-five micrograms of proteins were injected into the tail vein of female CD-1 mice (Charles River, three animals per molecule) in a total volume of 100 µl. Blood samples were taken after 3 min, 30 min, 1 h, 2 h, 6 h, 24 h, 72 h, and 168 h after injection, and incubated on ice immediately to obtain serum samples after centrifugation (16.000 x g, 4°C, 20 min), which were stored at −20°C until analysis. Serum concentration of antibodies were determined via ELISA using either EGFR-His or HER3-His as antigen. Bound antibodies were detected using HRP-conjugated anti-human Fc antibody.
Statistics
All data are represented as mean ± SD. Significances were calculated by GraphPad Prism 5.0.1 and results were compared by one-way ANOVA followed by Tukey’s multiple comparison test (post-test).
Abbreviations
Disclosure of potential conflicts of interest
O.S., F.R., and R.E.K. are named inventors on patent applications covering multivalent binding proteins.
Supplemental Material
Download Zip (2.1 MB)Acknowledgments
We would like to thank Nadine Heidel, Doris Göttsch, Ana Margarida Cardoso Henriques, and Sabine Münkel (Institute of Cell Biology and Immunology, University of Stuttgart) for excellent technical assistance, as well as Beatrice Reiser and Alexandra Kraske (Institute of Cell Biology and Immunology, University of Stuttgart) for maintenance of the animal facility.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4:182–97. PMID:22453100. doi:10.4161/mabs.4.2.19000.
- Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20:838–47. PMID:25728220. doi:10.1016/j.drudis.2015.02.008.
- Zhukovsky EA, Morse RJ, Maus MV. Bispecific antibodies and CARs: generalized immunotherapeutics harnessing T cell redirection. Curr Opin Immunol. 2016;40:24–35. PMID:26963133. doi:10.1016/j.coi.2016.02.006.
- Runcie K, Budman DR, John V, Seetharamu N. Bi-specific and tri-specific antibodies- the next big thing in solid tumor therapeutics. Mol Med. 2018;24:50. PMID:30249178. doi:10.1186/s10020-018-0051-4.
- Dahlén E, Veitonmäki N, Norlén P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother. 2018;6:3–17. PMID:29998217. doi:10.1177/2515135518763280.
- Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the ‘high-hanging fruit‘. Nat Rev Drug Discov. 2018;17:197–223. PMID:29192287. doi:10.1038/nrd.2017.227.
- Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9:182–212. PMID:28071970. doi:10.1080/19420862.2016.1268307.
- Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA. Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther. 2018;12:195–208. PMID:29403265. doi:10.2147/DDDT.S151282.
- Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67:95–106. PMID:25637431. doi:10.1016/j.molimm.2015.01.003.
- Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific IgG. Nat Biotechnol. 1998;16:677–81. PMID:9661204. doi:10.1038/nbt0798-677.
- Choi H-J, Kim Y-J, Lee S, Kim Y-S. A heterodimeric Fc-based bispecific antibody simultaneously targeting VEGFR-2 and Met exhibits potent antitumor activity. Mol Cancer Ther. 2013;12:2748–59. PMID:24132142. doi:10.1158/1535-7163.MCT-13-0628.
- Davis JH, Aperlo C, Li Y, Kurosawa E, Lan Y, Lo K-M, Huston JS. SEEDbodies: fusion proteins based on strand-exchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel. 2010;23:195–202. PMID:20299542. doi:10.1093/protein/gzp094.
- de Nardis C, Hendriks LJA, Poirier E, Arvinte T, Gros P, Bakker ABH, de Kruif J. A new approach for generating bispecific antibodies based on a common light chain format and the stable architecture of human immunoglobulin G1. J Biol Chem. 2017;292:14706–17. PMID:28655766. doi:10.1074/jbc.M117.793497.
- Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, Ng SB, Born T, Retter M, Manchulenko K, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J Biol Chem. 2010;285:19637–46. PMID:20400508. doi:10.1074/jbc.M110.117382.
- Schaefer W, Regula JT, Bähner M, Schanzer J, Croasdale R, Dürr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011;108:11187–92. PMID:21690412. doi:10.1073/pnas.1019002108.
- Wu X, Sereno AJ, Huang F, Zhang K, Batt M, Fitchett JR, He D, Rick HL, Conner EM, Demarest SJ. Protein design of IgG/TCR chimeras for the co-expression of Fab-like moieties within bispecific antibodies. MAbs. 2015;7:364–76. PMID:25611120. doi:10.1080/19420862.2015.1007826.
- Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014;32:191–98. PMID:24463572. doi:10.1038/nbt.2797.
- Golay J, Choblet S, Iwaszkiewicz J, Cérutti P, Ozil A, Loisel S, Pugnière M, Ubiali G, Zoete V, Michielin O, et al. Design and validation of a novel generic platform for the production of tetravalent IgG1-like bispecific antibodies. J Immunol. 2016;196:3199–211. PMID:26921308. doi:10.4049/jimmunol.1501592.
- Cooke HA, Arndt J, Quan C, Shapiro RI, Wen D, Foley S, Vecchi MM, Preyer M. EFab domain substitution as a solution to the light-chain pairing problem of bispecific antibodies. MAbs. 2018;10:1248–59. PMID:30215570. doi:10.1080/19420862.2018.1519631.
- Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15:159–63. PMID:9035142. doi:10.1038/nbt0297-159.
- Alt M, Müller R, Kontermann RE. Novel tetravalent and bispecific IgG-like antibody molecules combining single-chain diabodies with the immunoglobulin gamma1 Fc or CH3 region. FEBS Lett. 1999;454:90–94. PMID:10413102.
- Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu -R-R, Santora L, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25:1290–97. PMID:17934452. doi:10.1038/nbt1345.
- Steinmetz A, Vallée F, Beil C, Lange C, Baurin N, Beninga J, Capdevila C, Corvey C, Dupuy A, Ferrari P, et al. CODV-Ig, a universal bispecific tetravalent and multifunctional immunoglobulin format for medical applications. MAbs. 2016;8:867–78. PMID:26984268. doi:10.1080/19420862.2016.1162932.
- Seifert O, Plappert A, Heidel N, Fellermeier S, Messerschmidt SKE, Richter F, Kontermann RE. The IgM CH2 domain as covalently linked homodimerization module for the generation of fusion proteins with dual specificity. Protein Eng Des Sel. 2012;25:603–12. PMID:22988132. doi:10.1093/protein/gzs059.
- Seifert O, Plappert A, Fellermeier S, Siegemund M, Pfizenmaier K, Kontermann RE. Tetravalent antibody-scTRAIL fusion proteins with improved properties. Mol Cancer Ther. 2014;13:101–11. PMID:24092811. doi:10.1158/1535-7163.MCT-13-0396.
- Perisic O, Webb PA, Holliger P, Winter G, Williams RL. Crystal structure of a diabody, a bivalent antibody fragment. Structure. 1994;2:1217–26. PMID:7704531. doi:10.1016/S0969-2126(94)00123-5.
- Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–59. PMID:11498595. doi:10.1126/science.1061692.
- Siegemund M, Pollak N, Seifert O, Wahl K, Hanak K, Vogel A, Nussler AK, Göttsch D, Münkel S, Bantel H, et al. Superior antitumoral activity of dimerized targeted single-chain TRAIL fusion proteins under retention of tumor selectivity. Cell Death Dis. 2012;3:e295. PMID:22495350. doi:10.1038/cddis.2012.29.
- Schmitt LC, Rau A, Seifert O, Honer J, Hutt M, Schmid S, Zantow J, Hust M, Dübel S, Olayioye MA, et al. Inhibition of HER3 activation and tumor growth with a human antibody binding to a conserved epitope formed by domain III and IV. MAbs. 2017;9:831–43. PMID:28421882. doi:10.1080/19420862.2017.1319023.
- Moraga I, Wernig G, Wilmes S, Gryshkova V, Richter CP, Hong W-J, Sinha R, Guo F, Fabionar H, Wehrman TS, et al. Tuning cytokine receptor signaling by re-orienting dimer geometry with surrogate ligands. Cell. 2015;160:1196–208. PMID:25728669. doi:10.1016/j.cell.2015.02.011.
- Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer J-P, Liu J, Lim JS, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest. 2016;126:2610–20. PMID:27294525. doi:10.1172/JCI81603.
- Kim JH, Song DH, Youn S-J, Kim JW, Cho G, Kim SC, Lee H, Jin MS, Lee J-O. Crystal structures of mono- and bi-specific diabodies and reduction of their structural flexibility by introduction of disulfide bridges at the Fv interface. Sci Rep. 2016;6:34515. PMID:27682821. doi:10.1038/srep34515.
- Kwon N-Y, Kim Y, Lee J-O. Structural diversity and flexibility of diabodies. Methods. 2018. PMID:30261312. doi:10.1016/j.ymeth.2018.09.005.
- Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1997;159:3372–82. PMID:9317136.
- Roux KH, Strelets L, Brekke OH, Sandlie I, Michaelsen TE. Comparisons of the ability of human IgG3 hinge mutants, IgM, IgE, and IgA2, to form small immune complexes: a role for flexibility and geometry. J Immunol. 1998;161:4083–90. PMID:9780179.
- Digiammarino EL, Harlan JE, Walter KA, Ladror US, Edalji RP, Hutchins CW, Lake MR, Greischar AJ, Liu J, Ghayur T, et al. Ligand association rates to the inner-variable-domain of a dual-variable-domain immunoglobulin are significantly impacted by linker design. MAbs. 2011;3:487–94. PMID:21814039. doi:10.4161/mabs.3.5.16326.
- Carmichael JA, Power BE, Garrett TPJ, Yazaki PJ, Shively JE, Raubischek AA, Wu AM, Hudson PJ. The crystal structure of an anti-CEA scFv diabody assembled from T84.66 scFvs in V(L)-to-V(H) orientation: implications for diabody flexibility. J Mol Biol. 2003;326:341–51. PMID:12559905. doi:10.1016/S0022-2836(02)01428-6.
- Wan T, Beavil RL, Fabiane SM, Beavil AJ, Sohi MK, Keown M, Young RJ, Henry AJ, Owens RJ, Gould HJ, et al. The crystal structure of IgE Fc reveals an asymmetrically bent conformation. Nat Immunol. 2002;3:681–86. PMID:12068291. doi:10.1038/ni811.
- McDonnell JM, Calvert R, Beavil RL, Beavil AJ, Henry AJ, Sutton BJ, Gould HJ, Cowburn D. The structure of the IgE Cepsilon2 domain and its role in stabilizing the complex with its high-affinity receptor FcepsilonRIalpha. Nat Struct Biol. 2001;8:437–41. PMID:11323720. doi:10.1038/87603.
- Derrick JP, Wigley DB. Crystal structure of a streptococcal protein G domain bound to an Fab fragment. Nature. 1992;359:752–54. PMID:1436040. doi:10.1038/359752a0.
- Kato K, Lian LY, Barsukov IL, Derrick JP, Kim H, Tanaka R, Yoshino A, Shiraishi M, Shimada I, Arata Y. Model for the complex between protein G and an antibody Fc fragment in solution. Structure. 1995;3:79–85. PMID:7743134. doi:10.1016/S0969-2126(01)00136-8.
- Vidal MA, Conde FP. Alternative mechanism of protein A-immunoglobulin interaction the VH-associated reactivity of a monoclonal human IgM. J Immunol. 1985;135:1232–38. PMID:3925002.
- Sasso EH, Silverman GJ, Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J Immunol. 1989;142:2778–83. PMID:2495325.
- Lu D, Jimenez X, Zhang H, Atkins A, Brennan L, Balderes P, Bohlen P, Witte L, Zhu Z. Di-diabody: a novel tetravalent bispecific antibody molecule by design. J Immunol Methods. 2003;279:219–32. PMID:12969563. doi:10.1016/S0022-1759(03)00251-5.
- Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, Corcoran E, Mangalampalli V, Bassi R, Anselma D, et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665–72. PMID:15757893. doi:10.1074/jbc.M500815200.
- Kipriyanov SM, Moldenhauer G, Braunagel M, Reusch U, Cochlovius B, Le Gall F, Kouprianova OA, von der Lieth CW, Little M. Effect of domain order on the activity of bacterially produced bispecific single-chain Fv antibodies. J Mol Biol. 2003;330:99–111. PMID:12818205. doi:10.1016/S0022-2836(03)00526-6.
- Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, von der Lieth CW, Matys ER, Little M. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol. 1999;293:41–56. PMID:10512714. doi:10.1006/jmbi.1999.3156.
- Müller R, Gräwert MA, Kern T, Madl T, Peschek J, Sattler M, Groll M, Buchner J. High-resolution structures of the IgM Fc domains reveal principles of its hexamer formation. Proc Natl Acad Sci U S A. 2013;110:10183–88. PMID:23733956. doi:10.1073/pnas.1300547110.
