ABSTRACT
Hu14.18K322A is a humanized anti-GD2 monoclonal antibody with a single point mutation that reduces complement-mediated cytotoxicity, with a maximum tolerated dose (MTD) of 60 mg/m2 daily for 4 days in children with recurrent/refractory neuroblastoma. We report additional results of a Phase 1 trial to determine the MTD and safety profile of hu14.18K322A in patients with osteosarcoma, and of an alternative schedule of weekly hu14.18K322A administration in patients with neuroblastoma or osteosarcoma. Eligible patients with recurrent/refractory osteosarcoma received hu14.13K22A daily x4 every 28 days in a Phase 1 traditional 3 + 3 dose escalation design. Additional patients with osteosarcoma were then enrolled to receive hu14.18K322A once weekly for 4 weeks per course. Patients with recurrent/refractory neuroblastoma were also enrolled on the weekly schedule at 50 mg/m2/dose. Six patients with osteosarcoma treated on the daily schedule received a median of 2 (range 1–6) courses; the recommended daily dose was established as 60 mg/m2. Three patients had stable disease (SD) as best overall response. Five patients (3 neuroblastoma, 2 osteosarcoma) enrolled on the weekly schedule received a median of 1 (1–3) course; 2 achieved SD as best overall response. Pain, fever, hematologic toxicities, hyponatremia, and ocular/visual abnormalities were common toxicities among both schedules. Dose-limiting toxicities attributed to hu14.18K322A included anorexia and fatigue (n = 1). Pharmacokinetic profiles were similar between daily and weekly schedules. The recommended dose for patients with osteosarcoma receiving daily hu14.18K322A x4 is 60 mg/m2. Patients receiving the weekly schedule experienced similar pharmacokinetics and toxicity profile as the daily schedule.
Introduction
Treatment of children with high-risk neuroblastoma now incorporates the use of agents targeting disialoganglioside GD2, a surface molecule uniformly expressed on most neuroblasts, resulting in improved survival rates.Citation1 Currently, the chimeric monoclonal antibody dinutuximab (ch14.18) is the only U.S. Food and Drug Administration-approved product targeting GD2. Dinutuximab induces cell lysis via antibody-dependent cell-mediated cytotoxicity (ADCC), but also induces complement-mediated cytotoxicity and is associated with significant toxicities, including pain and hypersensitivity reactions. Alternative infusion schedules of anti-GD2 antibodies have been proposed to reduce toxicity profiles.Citation2
Hu14.18K322A is a novel anti-GD2 monoclonal antibody that retains the antigen-binding specificity of dinutuximab but is 98% human, and has a single point mutation to reduce complement cascade-associated pain.Citation3 Additionally, hu14.18K322A is produced in a YB2/0 rat myeloma cell line that reduces fucosylation and enhances ADCC activity.Citation4,Citation5 We have previously reported the results of 38 patients with recurrent/refractory neuroblastoma treated on a Phase 1 trial with hu14.18K322A given daily for 4 consecutive days of a 28-day course.Citation6 Simulations using population pharmacokinetic modeling from the daily schedule suggested that a weekly schedule of hu14.18K322A may provide higher sustained plasma and tissue concentrations while lowering the plasma Cmax concentration, potentially resulting in less toxicity.
In this new study, we sought to define the maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of hu14.18K322A when administered once weekly for 4 weeks per course in patients with recurrent or refractory neuroblastoma or osteosarcoma. Since expression of GD2 has also been observed in frozen samples of osteosarcoma,Citation7 and early phase studies of anti-GD2 antibodies included responses in osteosarcoma patients,Citation8,Citation9 we included patients with osteosarcoma to investigate the role of anti-GD2 therapy on a daily schedule as well as on the weekly schedule of administration in that patient population.
Results
Patient characteristics are summarized in . Six patients (1–6) with recurrent or refractory osteosarcoma were treated on the daily schedule, and 5 patients (7–11; 3 with neuroblastoma, 2 with osteosarcoma) were treated with weekly administration of antibody. Patients on the osteosarcoma daily treatment cohort, as expected, were older [median age 18.6 years (range 12.2–20.9 years)] and primarily presented with macroscopic pulmonary nodules; other involved sites included bone, hilar lymph nodes, and mediastinal/pleural soft tissue disease. Patients on the weekly treatment were more heavily pre-treated (3 with >3 prior lines of therapy) and included two patients with neuroblastoma with marrow-positive disease. Three patients on the weekly schedule (1 neuroblastoma and 2 osteosarcoma) and four patients on the daily schedule had a history of prior thoracic surgery. Enrollment to the weekly schedule was limited due to both slow accrual and lack of availability of hu14.18K322A.
Table 1. Patient characteristics.
Osteosarcoma patients who were treated on the daily schedule received a total of 14 courses (median 2, range 1–6). Three patients achieved stable disease (SD) as best overall response. Five of 6 patients experienced progressive disease (PD) while on study; one patient with SD after six courses refused further treatment and was taken off therapy. Patients treated on the weekly schedule cumulatively received seven total courses (median 1, range 1–3) of hu14.18K322A. Best overall response for the weekly patients included 2 SD and 3 PD. One patient with neuroblastoma who had persistent iodine-123 metaiodobenzylguanidine (MIBG)-avid disease in the orbit and scapula after completion of standard high-risk therapy (chemotherapy, stem cell transplantation, radiation and cis-retinoic acid) received one course of weekly treatment but was taken off therapy after 3 weeks due to toxicity; best response was SD at discontinuation of study treatment. Six months later the known MIBG findings had resolved, and the patient remains disease-free 7 years off therapy.
Significant treatment-related toxicities (≥Grade 3) are listed in . Pain was the most common toxicity; all patients in both daily and weekly cohorts experienced grade 3 pain during initial courses, typically during infusion. Hyponatremia and hematologic toxicities (lymphopenia and neutropenia) were also observed in both the daily and weekly schedules of administration. Fever, tachycardia and tachypnea were experienced with antibody infusions regardless of schedule. Patients receiving weekly administration of hu14.18K322A were less commonly observed to have fatigue, hypertension, cough and hypoxia (Supplemental Table 1). Ocular or visual abnormalities such as Adie’s pupil (mydriasis, photophobia, poor accommodation) were common among both treatment cohorts (10 of 11 total patients), but were typically mild, persistent, and did not worsen with subsequent exposures. DLTs observed with weekly hu14.18K322A therapy included anorexia (n = 1) and fatigue (n = 1) in a single patient with neuroblastoma treated at a dose of 50 mg/m2 (). Following discontinuation of hu14.18K322A due to poor tolerance, the patient experienced additional unrelated DLTs within the study evaluation period due to an extravasation of parenteral nutrition from the patient’s central venous access, with resultant local reaction, infection, and gastrointestinal toxicity including pancreatitis. A second patient with neuroblastoma was observed to have anorexia and hypoxia that were deemed to be related to rapid progression of disease by the study team; the patient declined further therapy after receiving the third weekly dose and died of progressive disease 8 days after the last treatment administration. One patient with osteosarcoma on the daily schedule experienced aspiration pneumonia and hypoxia secondary to emesis while intubated for a sedated procedure, with acute respiratory distress syndrome, respiratory failure, and acute renal insufficiency as DLTs; these were considered unrelated to the administration of hu14.18K322A. The recommended dose for the osteosarcoma daily dosing schedule was determined to be 60 mg/m2/dose; the MTD could not be established for the weekly schedule due to insufficient number of enrolled subjects.
Table 2. Summary of Grade ≥3 Adverse Events of hu14.18K322A for daily and weekly schedules.
Table 3. Observed dose-limiting toxicities (DLTs).
Pharmacokinetics
The pharmacokinetics of hu14.18K322A were best modeled using the previously described, two-compartment model with clearance and distribution volume as a function of body surface area.Citation6 No significant differences in pharmacokinetics were observed utilizing hu14.18K322A unit per weight (mg/kg). Area under the curve (AUC) increased proportionally with dose increases from 40 to 60 mg/m2 (data not shown). Inclusion of human anti-human antibody (HAHA) status, dose number, or disease did not improve predictions of clearance. One patient with osteosarcoma was excluded from the pharmacokinetic analysis because the second dose was delayed and pharmacokinetic samples were not taken.
The dose-normalized hu14.18K322A exposure was compared between subjects treated for neuroblastoma vs. osteosarcoma (). No clear differences in dose-adjusted exposure were noted with one exception: one subject with neuroblastoma treated on the weekly schedule had a clearance approximately 1/3 that of the other subjects, with correspondingly higher AUC from the first 4 doses. Despite this finding, the patient did not experience increased toxicity and completed three courses of therapy before disease progression.
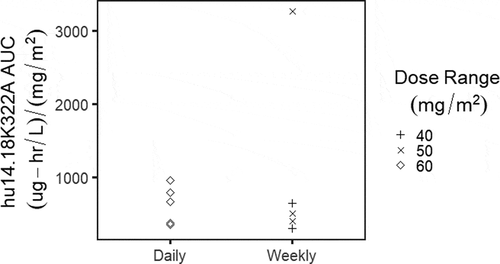
Figure 1. Dose-normalized hu14.18K322A area under the curve (AUC) based on diagnosis of neuroblastoma (n = 3) vs. osteosarcoma (n = 7).
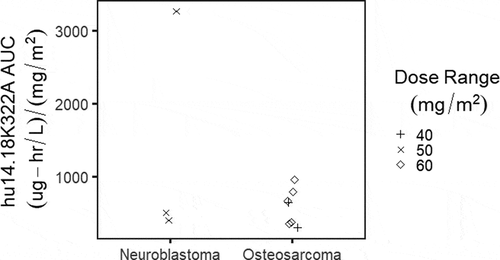
As shown in , there were no observed differences when the dose-normalized AUC is assessed by diagnosis. Similarly, we did not observe any clear distinctions in hu14.18K322A dose-normalized AUC exposure from the two dosing schedules (). compares the plots of the individual subjects, with the fitted data as a red dashed line overlaid on the black points representing observed data. The pharmacokinetic fits suggest that for most of the subjects, either the daily x4 or weekly x4 dosing schedule was able to maintain concentrations over the 4 week period above 1 ng/mL, which was considered the threshold level for detection of ADCC in preclinical studies of hu14.18K322A.Citation10 The exceptions were subjects 7 and 11, both treated on the weekly schedule, which were also the two subjects who developed HAHA after the start of therapy; both had detectable HAHA response at day 15. Neither of these two subjects had prior anti-GD2 exposure or therapy before enrollment. As shown in for these two subjects (the blue vertical line representing the first blood sample with detectable HAHA), there is a suggestion that the onset of HAHA may decrease the circulating concentrations of hu14.18K322A; both subjects had lower than expected hu14.18K322A trough levels prior to day 15 dosing, and more rapid clearance following subsequent administration.
Figure 3. Concentration-time curves of individual subjects receiving hu14.18K322A based on daily (a) vs. weekly (b) schedules. Predicted curves are demonstrated by continuous red lines; circles represent observed data.
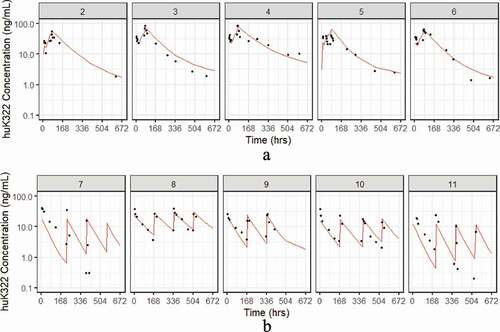
Figure 4. Expanded concentration-time curves [patients 7(a) and 11(b)] with human-anti-human antibody (HAHA) response to hu14.18K322A. Predicted curves are demonstrated by dashed red lines; blue line represents timepoint of first detection of HAHA response; circled points indicate hu14.18K322A concentrations below threshold level of activity (1 ng/mL).
![Figure 4. Expanded concentration-time curves [patients 7(a) and 11(b)] with human-anti-human antibody (HAHA) response to hu14.18K322A. Predicted curves are demonstrated by dashed red lines; blue line represents timepoint of first detection of HAHA response; circled points indicate hu14.18K322A concentrations below threshold level of activity (1 ng/mL).](/cms/asset/1af22d7b-a1ed-4b98-a6a8-cb8536e3006b/kmab_a_1773751_f0004_oc.jpg)
Discussion
This study investigated alternative schedules for administration of anti-GD2 antibody therapy, with the goal of administering treatment with similar efficacy based on predicted pharmacokinetic models while exploring differences in toxicity profiles. We also report on the use of anti-GD2 therapy in patients with osteosarcoma, who have previously been shown to express GD2 but whose outcomes have not been described in a prospective clinical trial. We determined that the recommended dose for patients with osteosarcoma receiving hu14.18K322A daily for 4 days was 60 mg/m2, consistent with the MTD in children with refractory neuroblastoma treated on the same schedule.Citation6 Although these patients did not experience DLTs attributable to hu14.18K322A, they were perceived to have poor tolerance of the antibody primarily due to pain. As a result, osteosarcoma patients enrolled on the weekly schedule received 40 mg/m2 per dose. All patients receiving weekly treatment also experienced grade 3 pain, equivalent to those patients who received the daily schedule. Correlation of hu14.18K322A dose and maximum pain score was previously established;Citation11 it is unclear whether the older age, larger body surface area or prior opioid use of osteosarcoma patients may have contributed to the higher severity of reported pain. Overall patterns of toxicity were similar to those previously reported for patients with neuroblastoma receiving a daily x 4 schedule of hu14.18K322A.Citation6 One patient with neuroblastoma on the weekly schedule experienced anorexia and fatigue as DLTs, both commonly experienced toxicities in our current report (6 of 11 reporting both in course 1) and in the previously reported neuroblastoma cohort. Other DLTs were either related to unexpected clinical events or reflective of significant disease burden. For those patients reported here, we noted similar patterns of toxicity between the daily and weekly schedules of administration, specifically for pain, fever and inflammatory responses, and hematologic abnormalities. While limited ≥grade 3 toxicites other than pain were experienced in both schedules, patients who received the weekly schedule appeared to experience fewer episodes of grade 1–2 fatigue, hypertension, respiratory symptoms and hypoxia. Respiratory distress is a common indication for escalation of care to a critical care setting for patients receiving anti-GD2 therapy, frequently secondary to capillary leak syndrome. A reduction of respiratory symptoms via weekly administration would support clinical feasibility of providing hu14.18K322A in an outpatient setting with less need of aggressive supportive interventions. However, our findings are limited by the small number of subjects enrolled on the weekly schedule, which likely occurred due to a combination of factors, including concomitant efforts to combine GD2 therapy with cytotoxic chemotherapy for neuroblastoma,Citation12–Citation14 general acceptance of the daily schedule based on prior experience, and limited availability of hu14.18K322A. We therefore cannot readily assess whether these results represent a difference of toxicity between the two schedules, or a difference of tolerance in osteosarcoma patients for daily administration compared to a mixed histology group receiving weekly dosing, and further study is needed in a larger cohort.
Alternative schedules of administration of anti-GD2 therapy remain of interest to clinical investigators in efforts to achieve efficacious treatment for high-risk neuroblastoma while mitigating significant inflammation-induced co-morbidities of therapy such as hypersensitivity reactions. Recent prospective clinical trials of newly diagnosed high-risk neuroblastoma patients conducted by a European neuroblastoma collaborative group have assessed the tolerability and efficacy of a 10-day continuous infusion of dinutuximab beta (ch14.18-CHO).Citation2,Citation15 Estimated 2-year event-free survival was similar to standard dosing.Citation16 Predictive population pharmacokinetic modeling from neuroblastoma patients treated on the daily scheduleCitation6 suggested that a weekly schedule of hu14.18K322A may provide higher sustained plasma and tissue concentrations of antibody while lowering the plasma Cmax concentration. However, in our study, pharmacokinetic analysis did not reveal substantial differences in AUC based on schedule of administration. We also did not observe evidence of differences in AUC between the two enrolled disease histologies. Given the relative stability in outcomes observed between standard and long-term infusions of anti-GD2 therapy in recent SIOPEN and COG trials,Citation1,Citation15,Citation16 altered administration schedules could be evaluated further with a goal to demonstrate non-inferiority of efficacy with potential reduction of toxicity profile. While interpretation of the available clinical and pharmacokinetic data are limited by the patient sample size, the similarity in AUC among schedules suggests that it may be possible to safely administer hu14.18K322A on a weekly basis without limiting the patient’s overall drug exposure. With a suggestion of potential reduction in respiratory symptoms, which necessitates close observation and potential transfer to critical care settings, a weekly schedule may offer a logistical convenience for certain patients residing within a closer radius to their treatment facility. Minimizing their length of inpatient admission may allow them to attend school, participate in additional outpatient activities and improve health-related quality of life. Conversely, patients who reside beyond proximity to their treating institution may be better served by completing all therapy in a single inpatient setting on the daily schedule. Caution is warranted with interpretation of our results; however, as data suggesting less pulmonary toxicity with weekly administration may reflect important differences in the six patients that received the daily schedule versus the five that received the weekly schedule (rather than a difference in the schedule itself); this study is not powered to be able to make this distinction.
We previously demonstrated a HAHA response during the first course of therapy in 40% of patients with neuroblastoma on the daily schedule of hu14.18K322A, and that no correlation of HAHA existed with dose or toxicity.Citation6 However, in this study the presence of HAHA may have influenced pharmacokinetic parameters in their first course, as observed in two patients with detectable HAHA whose serum hu14.18K322A levels declined below threshold levels of activity following the detection of HAHA in their weekly serum samples. While only two patients on the weekly schedule developed HAHA response and none was observed among the daily cohort in this analysis, we cannot infer differences in likelihood of development of HAHA between schedules. Both patients had detectable HAHA after day 8 dosing, similar to those who developed HAHA in the prior study with most occurring at ≥day 10.Citation6 The increased clearance of hu14.18K322A in these 2 subjects after detection of HAHA raises concern for potential impact on drug efficacy, and highlights the importance of monitoring for immune response against monoclonal antibody-based therapy.
Heterogeneity in our study population, which was composed of two histologies with distinct patterns of clinical presentation, resulted in wide variation of patient age and size (weight/body surface area). Two separate dosing schedules were studied with multiple dose levels, creating additional heterogeneity. In order to limit the impact on results, pharmacokinetic data for AUC were dose-normalized by weight and administered dose ( and ).
Currently, the role of anti-GD2 therapy for osteosarcoma is undefined. A recent study of dinutuximab in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) has been conducted by the Children’s Oncology Group for pediatric patients with completely resected pulmonary recurrence of osteosarcoma; tumor GD2 positivity was not included as an eligibility criteria for enrollment (NCT02484443). Additional studies of GD2-targeted therapy in osteosarcoma are ongoing, including a humanized monoclonal antibody (Hu3F8) with GM-CSF (NCT02502786). While GD2 has been reported to be broadly expressed in newly diagnosed and recurrent osteosarcoma,Citation7,Citation17,Citation18 intratumoral heterogeneity may result in variable expression on individual cells. Preclinical evaluation of [64Cu]Cu-Bn-NOTA-hu14.18K322A, a positron emission tomography (PET) radiotracer for GD2, established a spectrum of uptake in animal models in vivo that correlated with flow cytometry of GD2-positive cells with a higher dimension of specificity than immunohistochemistry methods.Citation19 The demonstration of diverse patterns of GD2 expression in preclinical models suggest an opportunity to explore responses to anti-GD2 therapy based on tumor GD2 status. Incorporating quantifiable methods of GD2 expression (PET detection, flow cytometry, or surrogate methods such as expression of the GD2 synthase B4GALNT1 via RNA sequencing) as a biomarker for enrollment on prospective clinical trials of GD2-targeted agents such as hu14.18K322A could enrich the likelihood of response to therapy.
Recent data from a prospective Phase 2 clinical trial now demonstrate feasibility of combining hu14.18K322A with cytotoxic induction chemotherapy for patients with newly diagnosed high-risk neuroblastoma, with resultant improvement in rates of tumor response.Citation12 Additional maturation of data will elucidate the impact of this combinatorial approach on survival outcomes, but these results have spawned additional prospective efforts incorporating other GD2-targeting therapies such as dinutuximab (NCT03686783). While we recognize the small size of our current study, we believe it has implications for larger collaborative approaches to neuroblastoma therapy by providing cues for further hypothesis testing. Broader study of alternative schedules of anti-GD2 antibodies in maintenance therapy could explore comparisons of efficacy, toxicity, and quality of life in a high-risk population. Given the widespread acceptance of anti-GD2 therapy as a critical component of therapy for high-risk neuroblastoma in the upfront setting, as well as its demonstration of activity when administered with salvage chemotherapy for patients with recurrent disease,Citation13,Citation14 future studies of alternative schedules of hu14.18K322A or other anti-GD2 agents should be conducted prospectively in the context of multimodal therapy rather than single-agent use in order to optimize the likelihood of derived benefit for these patients.
Methods
Patient population
Two schedules of hu14.18K322A antibody were evaluated in our study. Eligibility criteria for patients enrolled on the weekly schedule of hu14.18K322A included diagnoses of recurrent or refractory neuroblastoma or osteosarcoma. Patients with recurrent or refractory osteosarcoma and age ≤21 years were eligible for the daily schedule. Additional eligibility criteria were reported in a prior publication of neuroblastoma patients treated on a daily schedule of hu14.18K322A.Citation6 The protocol (ClinicalTrials.gov NCT00743496) was approved by the St. Jude Children’s Research Hospital Institutional Review Board. Informed consent was obtained from parents or legal guardians, and patient assent was obtained as appropriate.
Drug supply and administration
YB2/0 rat myeloma cells transfected with the expression plasmid pdHL7 s-hu14.18 (K322A; hu14.18 antibody with lysine-322 in the CH2 domain replaced by alanine) were provided by Merck Serono (Darmstadt, Germany), and hu14.18K322A was manufactured for clinical use by Children’s GMP (Memphis, TN).
For osteosarcoma patients on the daily schedule, hu14.18K322A was administered on an inpatient basis intravenously over 4 hours daily for 4 consecutive days every 28 days (one course). For patients on the weekly schedule (neuroblastoma or osteosarcoma), hu14.18K322A was administered on an inpatient basis intravenously over 4 hours once weekly for 4 weeks per course. Patients were premedicated with an antihistamine and acetaminophen prior to each infusion. At the discretion of the treating physician, premedication with morphine sulfate or an alternative analgesic was administered; continuous infusions of morphine or alternative opioid were provided based on symptoms, if needed.
Study design
For the cohorts of patients with neuroblastoma and osteosarcoma treated on the weekly schedule of hu14.18K322A, the planned study design was a traditional Phase 1 with 3 + 3 dose escalation. Neuroblastoma patients reported here were all enrolled on the weekly schedule at a dose level of 50 mg/m2/dose, one dose level below the MTD of the daily x4 schedule. Patients with osteosarcoma were initially enrolled on the daily schedule of hu14.18K322A at the MTD of 60 mg/m2/dose previously established for daily use in neuroblastoma,Citation6 with plans to reduce to 50 mg/m2/dose if ≥2 of 6 patients experienced a DLT. The highest dose at which fewer than 2 of 6 patients experienced a DLT during course 1 would be considered the tolerable dose for the osteosarcoma cohort. After six osteosarcoma patients showed acceptable tolerance of the daily schedule, subsequent osteosarcoma patients were enrolled on the weekly schedule starting at a reduced 40 mg/m2/dose.
Toxicities were graded according to the Common Terminology Criteria for Adverse Events (version 3.0).Citation20 Pain was graded based on the reported pain level per the age-appropriate pain scale (e.g., numeric or Faces Pain Scale; pain level 1 to 4 was considered grade 1; 5 to 7, grade 2; 8 to 10; grade 3). Pretreatment and on-study evaluations to monitor toxicity and criteria for subsequent courses and dose modifications and definitions for duration of response were previously described in the publication describing treatment of neuroblstoma patients on a daily schedule.Citation6 Tumor response was reported using RECIST (version 1.0)Citation21 and, for neuroblastoma patients, inclusion of Curie score (mIBG) score).Citation22,Citation23
Pharmacokinetic and human anti-human antibody studies
For patients treated on the weekly schedule, serial blood samples for pharmacokinetic studies were collected on day 1 (preinfusion and 1, 8, and 20 hours after infusion), and on days 4, 6, and 8. Additional samples were obtained weekly during subsequent courses on day 1 (preinfusion and 1 and 20 hours after infusion) and day 8. Samples were also obtained for HAHA prior to each weekly infusion.
For osteosarcoma patients on the daily schedule, serial blood samples for pharmacokinetic studies were collected on days 1 and 4 of the first two courses (preinfusion and 1, 2, 4, 8 (day 1 only), 12 and 20 hours after infusion) and on days 8, 11, 15, 21 and 28. Patients were also monitored for the development of HAHA. With each course of treatment, blood samples (3 mL) were collected before the first infusion and at days 8, 15, and 28. Serum concentration of hu14.18K322A and HAHA were measured by enzyme-linked immunosorbent assay, similarly to those used previously.Citation24–Citation26
The NONMEM 7.4 population pharmacokinetic programCitation27 was used to perform a compartmental analysis of the first course hu14.18K322A concentration data. The two-compartment model identified in the previous analysis of Phase 1 dose-escalation in neuroblastoma patientsCitation6 was used as the basis for this evaluation of aggregate data of hu14.18K322A. Pharmacokinetic parameters for individual patients such as clearance (CL) and volume distribution (Vc) were estimated from the empirical Bayesian post hoc in a two-compartment model (ADVAN3, TRANS4) that included body surface area as a proportional covariate model with first-order conditional estimation. The possible effect of the individual studies upon the pharmacokinetic model was assessed by adding inter-occasion variables to CL and Vc, as well as by examination of diagnostic plots.
Cmax was estimated at the end of each infusion using the two-compartment model to predict the concentration of a theoretical blood sample at that time. Inter-individual variability in pharmacokinetics was described by an exponential error model, and the residual error was modeled as an additive error. Initial (T½α = ln(2)/λ1) and terminal (T½ß = ln(2)/λ2) half-lives are derived parameters, where λ1 and λ2 are rate constants for the initial and terminal phases derived from CL, Q (intercompartmental clearance), Vc (central volume), and Vp (peripheral volume).
Disclosure of Potential Conflicts of Interest
The authors have no relevant conflicts of interest to disclose.
Supplemental Material
Download MS Word (31 KB)Acknowledgments
The authors are deeply appreciative of the patients and families who participated in this study. We additionally thank Shane Cross, Barry Shulkin, Catherine Billups, and Amy Sanders for their roles in conducting the trial, management of data, and collection of materials for this analysis.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–8. doi:https://doi.org/10.1056/NEJMoa0911123.
- Mueller I, Ehlert K, Endres S, Pill L, Siebert N, Kietz S, Brock P, Garaventa A, Valteau-Couanet D, Janzek E, et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD2 antibody ch14.18/CHO. MAbs. 2018;10:55–61. doi:https://doi.org/10.1080/19420862.2017.1402997.
- Sorkin LS, Otto M, Baldwin WM 3rd, Vail E, Gillies SD, Handgretinger R, Barfield RC, Yu HM, Yu AL. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149:135–42. doi:https://doi.org/10.1016/j.pain.2010.01.024.
- Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, Matsushima K, Ueda R, Hanai N, Shitara K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64(6):2127–33. doi:https://doi.org/10.1158/0008-5472.CAN-03-2068.
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73. doi:https://doi.org/10.1074/jbc.M210665200.
- Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, Gan J, Hutson P, Seo S, Kim K, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32(14):1445–52. doi:https://doi.org/10.1200/JCO.2013.50.4423.
- Heiner JP, Miraldi F, Kallick S, Makley J, Neely J, Smith-Mensah WH, Cheung NK. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res. 1987;47:5377–81.
- Murray JL, Cunningham JE, Brewer H, Mujoo K, Zukiwski AA, Podoloff DA, Kasi LP, Bhadkamkar V, Fritsche HA, Benjamin RS. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. J Clin Oncol. 1994;12:184–93. doi:https://doi.org/10.1200/JCO.1994.12.1.184.
- Frost JD, Hank JA, Reaman GH, Frierdich S, Seeger RC, Gan J, Anderson PM, Ettinger LJ, Cairo MS, Blazar BR, et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children’s Cancer Group. Cancer. 1997;80(2):317–33. doi:https://doi.org/10.1002/(SICI)1097-0142(19970715)80:2<317::AID-CNCR21>3.0.CO;2-W.
- McDowell KA. Putting natural killers to work, translational studies. University of Wisconsin-Madison Graduate School. Madison: University of Wisconsin; 2013.
- Anghelescu DL, Goldberg JL, Faughnan LG, Wu J, Mao S, Furman WL, Santana VM, Navid F. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr Blood Cancer. 2015;62(2):224–28. doi:https://doi.org/10.1002/pbc.25280.
- Furman WL, Federico SM, McCarville MB, Shulkin BL, Davidoff AM, Krasin MJ, Sahr N, Sykes A, Wu J, Brennan RC. A Phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res. 2019;25(21):6320–28. doi:https://doi.org/10.1158/1078-0432.CCR-19-1452.
- Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, Parisi MT, Servaes SEN, Diccianni MB, Sondel PM, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18:946–57. doi:https://doi.org/10.1016/S1470-2045(17)30355-8.
- Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, Meagher M, Shafer A, Ng CY, Leung W, et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–49. doi:https://doi.org/10.1158/1078-0432.CCR-17-0379.
- Ladenstein RL, Poetschger U, Valteau-Couanet D, Gray J, Luksch R, Balwierz W, Castel V, Ash S, Beck Popovic M, Laureys G, et al. Randomization of dose-reduced subcutaneous interleukin-2 (scIL2) in maintenance immunotherapy (IT) with anti-GD2 antibody dinutuximab beta (DB) long-term infusion (LTI) in front–line high-risk neuroblastoma patients: early results from the HR-NBL1/SIOPEN trial. J Clin Oncol. 2019;37:10013–10013.
- Ladenstein R, Potschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, Laureys G, Brock P, Michon JM, Owens C, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1617–29. doi:https://doi.org/10.1016/S1470-2045(18)30578-3.
- Roth M, Linkowski M, Tarim J, Piperdi S, Sowers R, Geller D, Gill J, Gorlick R. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. 2014;120(4):548–54. doi:https://doi.org/10.1002/cncr.28461.
- Poon VI, Roth M, Piperdi S, Geller D, Gill J, Rudzinski ER, Hawkins DS, Gorlick R. Ganglioside GD2 expression is maintained upon recurrence in patients with osteosarcoma. Clin Sarcoma Res. 2015;5:4. doi:https://doi.org/10.1186/s13569-014-0020-9.
- Butch ER, Mead PE, Amador Diaz V, Tillman H, Stewart E, Mishra JK, Kim J, Bahrami A, Dearling JLJ, Packard AB. Positron emission tomography detects in vivo expression of disialoganglioside GD2 in mouse models of primary and metastatic osteosarcoma. Cancer Res. 2019;79(12):3112–24. doi:https://doi.org/10.1158/0008-5472.CAN-18-3340.
- Program CTE. Common terminology criteria for adverse events, Version 3.0 (CTCAE).
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi:https://doi.org/10.1093/jnci/92.3.205.
- Ady N, Zucker JM, Asselain B, Edeline V, Bonnin F, Michon J, Gongora R, Manil L. A new 123I-MIBG whole body scan scoring method–application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A:256–61. doi:https://doi.org/10.1016/0959-8049(94)00509-4.
- Messina JA, Cheng SC, Franc BL, Charron M, Shulkin B, To B, Maris JM, Yanik G, Hawkins RA, Matthay KK. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer. 2006;47:865–74. doi:https://doi.org/10.1002/pbc.20777.
- Albertini MR, Gan J, Jaeger P, Hank JA, Storer B, Schell K, Rivest T, Surfus J, Reisfeld RA, Schiller JH, et al. Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J Immunother Emphasis Tumor Immunol. 1996;19(4):278–95. doi:https://doi.org/10.1097/00002371-199607000-00004.
- Albertini MR, Hank JA, Schiller JH, Khorsand M, Borchert AA, Gan J, Bechhofer R, Storer B, Reisfeld RA, Sondel PM, et al. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3:1277–88.
- Hank JA, Surfus JE, Gan J, Ostendorf A, Gillies SD, Sondel PM. Determination of peak serum levels and immune response to the humanized anti-ganglioside antibody-interleukin-2 immunocytokine. Methods Mol Med. 2003;85:123–31. doi:https://doi.org/10.1385/1-59259-380-1:123.
- Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user’s guides (1989-2011). Ellicott City (MD): Icon Development Solutions; 2011.