ABSTRACT
While the potential therapeutic utility of angiotensin-converting enzyme 2 (ACE2) is well established, the clinical development of ACE2 drugs has been limited, likely due in part to the short half-life of the protein. In contrast, Ig-like ACE2 fusion proteins have exhibited greatly extended plasma half-life in vivo, and they have been shown to have a potent neutralization effect against SARS-CoV-2. Clinical investigation of Ig-like ACE2 fusion proteins as COVID-19 interventions is thus warranted.
The emergence of Coronavirus Disease 2019, which is caused by a previously unknown coronavirus and responsible for unexpected worldwide infections and deaths, highlights two scientific issues in emerging infectious diseases. First, it demonstrates that novel highly infectious and pathogenic coronaviruses have the capacity to repeatedly evolve from the reservoir of severe acute respiratory syndrome (SARS)-related coronaviruses in nature (perhaps from bats or pangolins) and cause fatal diseases in human. Second, the development of broad-spectrum antiviral drugs that can combat future new SARS-like coronaviruses is urgently needed.
Table 1. Recombinant and Fc-fused ACE2 therapeutics.
Angiotensin-converting enzyme 2 (ACE2), which was shown to be a functional receptor for SARS-CoV,Citation1 is also suggested to be a functional receptor for SARS-CoV-2 in cell line models.Citation2 Although angiotensin-converting enzyme (ACE) was discovered more than 60 y ago, ACE2 is an enzyme component of the renin-angiotensin system that was only discovered in 2000. Unlike ACE, ACE2 hydrolysis of angiotensin II (AT II) into angiotensin1–7 (AT1–7) has a much higher efficiency (~400-fold) than that for angiotensin I (AT I) to angiotensin1–9 (AT1–9). Nearly all tissues express ACE2 mRNA, with the highest expression in the intestinal epithelium.Citation3 In lung, ACE2 co-localizes with cholesterol and sphingolipid-rich lipid raft microdomains in the plasma membrane of pneumocytes, and its expression level is positively correlated to the state of airway epithelial differentiation.Citation4
The life-threatening lung injury caused by coronaviruses does not result solely from the binding of the viral spike protein to ACE2. The NL63 coronavirus, which is a ubiquitous human pathogen and is not generally associated with diffuse alveolar damage (DAD), also binds to ACE2.Citation5 DAD, the histological change associated with acute respiratory distress syndrome (ARDS), is a common reaction to pneumocyte damage and may particularly be initiated by the SARS-CoV-2Citation6 and SARS-CoV.Citation7 Moreover, DAD has a high mortality rate and, other than supportive clinical care, there are few specific therapeutic options that have proven beneficial to patients.
The potential therapeutic utility of recombinant ACE2 (rACE2) for acute lung injury resulting from viruses and other causes has been known for decades,Citation8 as it is well established that ACE2 is not only a functional receptor, but it provides a protective effect by restricting activation of the local renin–angiotensin system during acute lung injury. It is now well established that during acute lung injury, ACE converts AT I to AT II, which binds to either angiotensin II receptor 1a (AT1aR), leading to tissue damage and lung edema, or to angiotensin II receptor 2 (AT2 R), reducing tissue damage. ACE2 in turn converts the potent AT II to the less-damaging AT1–7 (). SARS binding, lipopolysaccharide, sepsis and acid treatment all result in ACE2 downregulation in lung.Citation8 Interestingly, catalytically active but not mutant, catalytically inactive rACE2 protein alleviates the symptoms of acute lung injury in ARDS animal models induced by acid aspiration and sepsis, suggesting functional ACE2 protects the lung from acute injury.Citation9 In an endotoxin infusion-induced ARDS model, active ACE2 protein, supplied through intravenous administration, significantly improved the outcome of respiratory failure by its ability to increase the oxygen levels of ARDS by almost 40% in pigs.Citation10
Figure 1. Schematic representation of the role of ACE2-Ig regulation of acute lung injury.
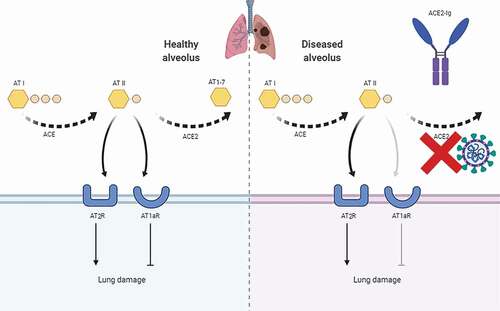
Although the possibility that rACE2 might have therapeutic utility has been known for decades, the clinical development of such drugs has been limited, likely due in part to the short half-life of rACE2 in humans.Citation11 Fusing recombinant receptor extracellular domains with the crystallizable fragment (Fc) of human immunoglobulin G (IgG) to increase their in vivo stability is a well-studied genetic method used for therapeutic proteins.Citation12 Previously, we connected the extracellular domain of T cell immunoreceptor with Ig and ITIM domains (TIGIT) to an IgG Fc region and characterized the resulting molecule, TIGIT-Ig, in a murine lupus model.Citation13 A pharmacokinetic study demonstrated TIGIT-Ig had very high stability both in vitro and in vitro, and its possible role of balancing immune tolerance at the feto-maternal interface is under investigation.Citation14 Furthermore, we recently showed that Fc fusions of human ACE2 proteins also have good pharmacokinetic profiles in vivo,Citation15 which was consistent with results from an earlier study showing that fusion proteins containing the extracellular domain of murine ACE2 fused to the Fc region of IgG had full peptidase activity and greatly extended plasma half-life in mice. We tested the coronavirus neutralization effect of ACE2-Ig in vitro. ACE2-Ig shows even more potent neutralization of SARS-CoV-2 than SARS-CoV, reflecting its higher S protein affinity.Citation15
Very recently, other groups of investigators have also initiated the development of ACE2 protein-based therapeutics (). Sorrento Therapeutics, Inc. announced the development of STI-4398,Citation16 an ACE2 IgG-like fusion protein, and STI-4920,Citation17 a bispecific fusion protein constructed with a fully human anti-spike antibody and a truncated ACE2 protein that binds to a different epitope of the spike protein. Both drugs are now under in vitro cell studies for SARS-CoV-2 virus infection and neutralization. Additionally, APN-1, a recombinant human ACE2, is under investigation in a Phase 2 trial in which patients are administered APN01 intravenously twice daily (NCT04335136).
Although ACE2 did not attract the attention of the pharmaceutical industry when it was first reported 15 y ago,Citation8 the new outbreak of coronavirus has led us to refocus on the potential therapeutic utility of ACE2 fusion proteins. This is particularly relevant as we prepare to confront another potential SARS-like coronavirus pandemic beyond SARS-CoV-2 in the future, armed with only a limited number of therapeutic options.
Conflicts of interest
The author declares no competing interests.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant no: 82041012).
Additional information
Funding
References
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–3. doi:https://doi.org/10.1038/nm1267.
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–73. doi:https://doi.org/10.1038/s41586-020-2012-7.
- Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1-7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–12. doi:https://doi.org/10.1161/01.CIR.0000094734.67990.99.
- Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–48. doi:https://doi.org/10.1097/SHK.0000000000000633.
- Hofmann H, Pyrc K, van der Hoek L, Geier M, Berkhout B, Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A. 2005;102:7988–93. doi:https://doi.org/10.1073/pnas.0409465102.
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–22. doi:https://doi.org/10.1016/S2213-2600(20)30076-X.
- Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–78. doi:https://doi.org/10.1016/S0140-6736(03)13413-7.
- Nicholls J, Peiris M. Good ACE, bad ACE do battle in lung injury, SARS. Nat Med. 2005;11:821–22. doi:https://doi.org/10.1038/nm0805-821.
- Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–16. doi:https://doi.org/10.1038/nature03712.
- Treml B, Neu N, Kleinsasser A, Gritsch C, Finsterwalder T, Geiger R, Schuster M, Janzek E, Loibner H, Penninger J, et al. Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit Care Med. 2010;38:596–601. doi:https://doi.org/10.1097/CCM.0b013e3181c03009.
- Haschke M, Schuster M, Poglitsch M, Loibner H, Salzberg M, Bruggisser M, Penninger J, Krähenbühl S. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52(9):783–92. doi:https://doi.org/10.1007/s40262-013-0072-7.
- Jafari R, Zolbanin NM, Rafatpanah H, Majidi J, Kazemi T. Fc-fusion proteins in therapy: an updated view. Curr Med Chem. 2017;24:1228–37. doi:https://doi.org/10.2174/0929867324666170113112759.
- Liu S, Sun L, Wang C, Cui Y, Ling Y, Li T, Lin F, Fu W, Ding M, Zhang S, et al. Treatment of murine lupus with TIGIT-Ig. Clin Immunol. 2019;203:72–80. doi:https://doi.org/10.1016/j.clim.2019.04.007.
- Fu W, Ma Z, Lei C, Ding M, Hu S. TIGIT-Fc promote immune tolerance at the feto-maternal interface. bioRxiv. 2019;819243.
- Lei C, Qian K, Li T, Zhang S, Fu W, Ding M, Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat Commun. 2020;11:2070. doi:https://doi.org/10.1038/s41467-020-16048-4.
- Sorrento therapeutics press release. March 20, 2020. https://investors.sorrentotherapeutics.com/news-releases/news-release-details/sorrento-develops-sti-4398-covidtraptm-protein-potential. Web 25 May 2020.
- Sorrento Therapeutics press release. March 24, 2020. https://investors.sorrentotherapeutics.com/news-releases/news-release-details/sorrento-collaborates-mabpharm-development-and-commercialization.Web 25 May 2020
