ABSTRACT
Recent studies have shown the potential of broadly neutralizing antibodies (bnAbs) for HIV-1 treatment. One of the candidate antibodies moving into clinical trials is the bnAb PGDM1400. Here, we studied the therapeutic potency and escape pathways of bnAb PGDM1400 during monovalent therapy in human immune system (HIS) mice using the BG505, REJO, MJ4 and AMC008 virus isolates. PGDM1400 administered during chronic infection caused a modest decrease in viral load in the first week of administration in 7 out of 10 animals, which correlated with the in vitro neutralization sensitivity of the viruses to PGDM1400. As expected for monotherapy, viral loads rebounded after about a week and different viral escape pathways were observed, involving the deletion of glycans in the envelope glycoprotein at positions 130 or 160. (Pre)clinical trials should reveal whether PGDM1400 is a useful component of an antibody combination treatment or as part of a tri-specific antibody.
Introduction
Human immune deficiency virus (HIV)-1 has proven to be an extremely resilient virus, evading all efforts to discover vaccines or a cure. In the past 10 years, many broadly neutralizing antibodies (bnAbs) have been isolated from HIV-infected individuals using different techniques, including single B cell cloning.Citation1 When passively administered prior to challenge, bnAbs were found to block HIV-1 infection in both human immune system (HIS) mice and non-human primates.Citation2–8 The potent anti-viral activity of bnAbs implies that bnAbs could be an alternative for antiretroviral therapy (ART) and might serve as a component of HIV cure strategies. Therefore, several clinical studies have been conducted in which ART therapy of HIV-infected individuals was temporarily stopped and replaced with passive administration of bnAbs. The first clinical studies in which bnAbs were administered as monotherapy showed that viral rebound was delayed, but rapid viral escape was observed.Citation9–11 Various animal models showed that a combination of bnAbs targeting different epitopes on the envelope spike was most effective in suppressing viral replication.Citation7 Clinical trials, in which a combination of the bnAbs 3BNC117 and 10–1074 was administered, showed indeed a longer delay of viral rebound, because viral escape required mutations at two different sites on the envelope glycoprotein (Env).Citation12
PGDM1400, one of the most potent bnAb identified so far,Citation13 targets the apex of the Env trimer and is currently being used as part of a triple bnAb combination therapy consisting of N6-PGDM1400-10E8v4.Citation14 We and others have shown that PGDM1400 can protect against HIV-1 challenge in HIS miceCitation3 and simian-human immunodeficiency viruses (SHIV) challenge in non-human primates (NHPs).Citation8,Citation15 Furthermore, an engineered tri-specific antibody including PGDM1400 Fab as one of its arms showed great potency as prophylactic in animal models.Citation14 While the SHIV infection model in NHPs is considered to be the gold-standard in HIV-1 protection and therapeutic studies,Citation16 the various HIS mouse models offer a more easily accessible relevant alternative.Citation3,Citation7,Citation16 Furthermore, since HIS mice harbor only B and T cells of human origin, these mice do not produce anti-human antibodies that will affect the serum levels of the therapeutic antibody. Finally, HIS mice allow the use of HIV-1 for therapeutic and challenge studies as opposed to a chimeric SHIV.
Here, we used HIS mice to establish a multiclade infection model and used this model to study the therapeutic potential and viral escape pathways of PGDM1400. Our study illuminates the critical target residues of PGDM1400 and the diversity of neutralization escape mechanisms that can be employed by different HIV-1 isolates. The results should inform the design of combination therapies that include PGDM1400.
Results
To evaluate the viral suppressive capacity of PGDM1400 as an alternative for ART, a HIV-1 multiclade infection model was established. Four HIV-1 virus isolates from different clades were selected: BG505 from clade A; REJO and AMC008 from clade B and MJ4 from clade C. The sensitivity of each of these viruses to PGDM1400 was determined in vitro. All viruses were sensitive for PGDM1400 neutralization with IC50s below 1 μg/mL and IC90s below 10 μg/mL, with BG505 being the most sensitive (IC50 of 0.002 μg/ml and IC90 of 0.008 μg/ml) and AMC008 the most resistant (IC50 of 0.637 μg/ml and IC90 of 8.605 μg/ml) (). PGDM1400 is known to show suboptimal inhibition for some viruses with respect to maximum percent inhibition (incomplete neutralization) and slope of the neutralization curves in vitro.Citation17,Citation18 We observed <100% inhibition at high PGDM1400 concentrations for REJO, MJ4 and AMC008 and shallow neutralization curves for REJO and MJ4 viruses.
Figure 1. Therapeutic efficacy of bnAb PGDM1400 in HIS mice. (a) Neutralization sensitivity of BG505, REJO, MJ4 and AMC008 virus to bnAb PGDM1400 in vitro. IC50 and IC90 are indicated by the dotted lines and shown in the table in μg/ml. (b) Viral loads (RNA copies/ml) over time in the HIS mice infected with one of the four viruses. Each line represents one mouse and symbols reflect viral load measurements. The 4 weeks of PGDM1400 administration (20 mg/kg every 7 days) are shaded in gray and arrows for each infection of PGDM1400. (c) The PGDM1400 serum concentration (μg/ml) in each animal in gray with symbols reflecting measurements and the average per group indicated in color. (d) Changes in log10 viral load from start of antibody administration are indicated with each line representing one mouse and the average per group indicated in color
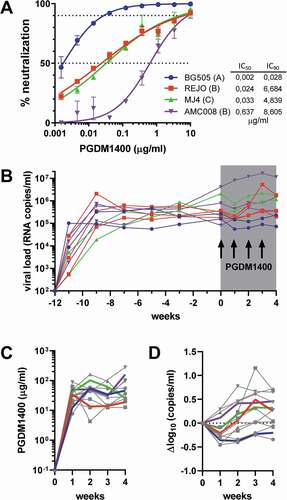
Next, HIS mice, n = 3 per group, were infected with one of the four virus isolates by intraperitoneal injection. Two mice were excluded from the experiment, as one MJ4-injected mouse was not infected and one BG505-infected mouse became severely anemic. The 10 remaining mice all developed a persistent viral infection after 3 weeks of infection, with loads between 105 and 107 viral RNA copies/ml. Viral loads were sustained throughout the experiment, up to 12 weeks after infection, with average viral loads of 2.1*105, 2.9*105, 1.1*106 and 1.7*106 at week 12 for BG505, REJO, MJ4 and AMC008, respectively ( and Supplemental Figure 1). Monitoring of CD4+ T cells (Supplemental Figure 2) showed that BG505 infection had none or only a very modest reductive effect on CD4+ T cell percentages. CD4+ T cells were moderately depleted in REJO and AMC008-infected mice, whereas a strong depletion of CD4+ T cells to below 25% of total T cells was observed in MJ4-infected mice.
After 12 weeks of stable HIV-1 infection, the animals were given 20 mg/kg of PGDM1400 every 7 days for 4 weeks. In our previous study,Citation3 we determined the t1/2 of PGDM1400 in HIS mice to be 3.3–5.4 days, and observed concentrations of the antibody in the blood after 7 days to be 19.5 μg/ml at a dose of 10 mg/kg. Therefore, we anticipated that a once a week injection at 20 mg/kg would be sufficient to reach plasma concentrations above 30 μg/ml. This translates to at least 50-fold the IC50 for the four virus strains used in study, a PDGM1400 level we previously reported to be protective in JRC-SF-challenged HIS mice.Citation3 The administration of the antibody was well tolerated; however, concentrations were somewhat lower than anticipated. Overall, the concentration of PGDM1400 did not drop below 25 μg/ml just before the next administration during the 4 weeks of antibody administration, except for the mice infected with REJO (), which reached at least concentrations of 13 μg/ml. The observed concentrations were at least 50-fold the IC50 for all viruses and at least twofold of the IC90 for REJO, threefold the IC90 for AMC008, fivefold the IC90 for MJ4 and 400-fold the IC90 for BG505.
In seven of the 10 mice, we observed a modest decrease in viral load 1 week after the start of PGDM1400 treatment, but this was not sustained throughout the treatment, except in the two animals infected with the BG505 virus (). The maximal decrease in viral load was 0.5 log10 after 1 week and all animals, except the BG505-infected mice, rebounded between weeks 1 and 2. The observed reduction in viral load was in proportion with the in vitro neutralization sensitivity of the virus to PGDM1400 and the achieved PGDM1400 concentrations, with BG505 the most sensitive virus showing the largest effect of the PGDM1400 treatment, and AMC008 the least sensitive. Two of three mice infected with REJO showed a modest reduction in viral load, whereas the viral load in the third mouse was nearly identical to pretreatment levels. Any therapeutic effect was less clear for MJ4, as one mouse showed an increased viral load 1 week after treatment and one mouse a 0.5log10 reduction.
Viral RNA was isolated from plasma throughout the course of the antibody treatment and the region encoding the variable regions 1 and 2 (V1V2) of Env, the target of PGDM1400, was sequenced. The sequences revealed that the glycan at position 160, which is the target of PGDM1400, was rapidly deleted by the BG505 and REJO viruses, but using different mutations (). A N160K substitution occurred in all three mice infected with REJO. One mouse infected with BG505 had a T162N substitution, while the second BG505-infected mouse revealed three different mutations at the subsequent timepoints, but with all leading to removal of the N160 glycan (T162A at week 2, T162N at week 3, and N160K at week 4).
Figure 2. Viral escape over time during PGDM1400 treatment. (a) Amino acid sequences of the HIV-1 envelope glycoprotein (amino acid 75–250) obtained from serum RNA per mouse per time point before and during PGDM1400 treatment. The original virus sequence is indicated above those found in the mice infected with that particular virus. The colors are similar to the colors used in as indicated in Figure 1A. The HXB2 sequence on which numbering is based, is included as reference. Known contact residues for PGDM1400Citation19 are indicated with *. The glycosylation site at position 130 is shaded in green and the one at position 160 in orange. Identical amino acids are indicated by – and gaps by ~. (b) Neutralization sensitivity of AMC008 virus and AMC008 N130D virus to bnAb PGDM1400. IC50 and IC90 are indicated by the dotted lines
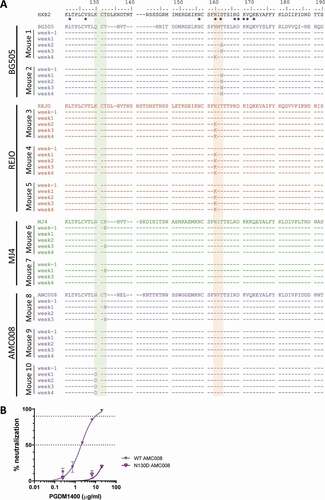
Strikingly, the AMC008 virus removed the glycan at position 130 again using different mutations (N130D in one mouse or T132N in another) in two of three animals. As glycan removal at this position has not been reported as an escape mechanism, we constructed an AMC008 virus lacking the N130 glycan and verified that the removal of the glycan at position 130 indeed renders the AMC008 virus resistant to PGDM1400 (). No mutations were found in the third AMC008-infected animal. We observed a K132E mutation in some of the MJ4 sequences from both MJ4-infected mice. However, this did not result in a loss of the glycan, as MJ4 does not have a glycan at position 130. Furthermore, in one animal the K132E was already present at the start of treatment, suggesting that the change might not be related to PGDM1400 escape. We did not observe any mutations at the N160 glycan in MJ4- and AMC0080-infected animals, nor did we observe any other mutations in the epitope of PGDM1400.
The one animal in which no viral mutations were observed during PGDM1400 administration was the animal with the highest viral load at the start of treatment. However, when all animals were taken into account, we did not observe a correlation between the viral load at the start of treatment and the decrease in viral load within the first week.
Discussion
Despite the success of ART, the burden of life-long treatment calls for alternatives. bnAbs have shown promise as HIV therapeutics in pre-clinical and clinical studies, causing decreased viral loads, delayed viral rebound and in some studies even a reduction of the viral reservoir. However, these studies have also shown that treatment with just one bnAb was only effective for a very short period, with viral escape decreasing the therapeutic efficacy rapidly.Citation7,Citation9–12,Citation20 Therefore, combinations of bnAbs will be necessary to obtain sustained suppression of HIV-1, calling for more bnAbs to be tested in therapeutic settings against multiple different viruses. Here, we studied the therapeutic efficacy of bnAb PGDM1400 in HIS mice infected with four viruses from different clades.
This study has shown that the HIS mice model can be used efficiently for antibody therapeutic studies against HIV-1 using different viral isolates from several clades. The benefits of this model are the low costs compared to non-human primates, as well as the ability to use HIV-1 and not chimeric SHIV viruses. In addition, the antibody half-life and antibody serum levels remained stable over time, suggesting that anti-drug antibodies were not elicited during the antibody treatment, which is often observed in similar studies performed in non-human primates.
Our data demonstrate that the bnAb PGDM1400 can moderately reduce the viral load in the majority of chronically infected animals and in proportion with the in vitro neutralization sensitivity of the virus to PGDM1400. However, the decline in viral load was small and short-lived due to rapid viral escape, as expected and observed in similar studies with other bnAbs as monotherapy.Citation7,Citation9–11 In a previous study, PG16, another bnAb targeting the Env trimer apex, showed a similarly modest and temporary decline in viral load (0.23 log10). PG16 neutralization sensitivity to the YU2 virus used in that study (IC50 of 0.29 μg/ml) was comparable to the sensitivities of the viruses used here for PGDM1400. They also observed escaped by the YU2-virus through the removal of the glycan at position 160 within the first week of PG16 treatment in all mice.Citation7 The glycan at position 160 has been shown to be essential for antibodies binding to the apex of the HIV-1 Env, together with residues 166, 169 and 171.Citation19,Citation21–25 In our study, we observed the same escape mechanism through the removal of the N160 glycan in five of 10 animals, all infected with BG505 or REJO. However, we also observed the removal of the N130 glycan in two of the five remaining animals. The glycan at position 130 has not been shown previously to be part of the target epitope of PGDM1400 or other apex targeting bnAbs. On the contrary, the N130 glycan was shown to hinder the binding of VRC38.Citation22 Also, in the high-resolution structure of the Env AMC011 SOSIP protein with PGT145, no direct contacts could be observed between the antibody and glycan 130.Citation26 To confirm the rather unexpected finding that N130 glycan removal confers PDGM1400 resistance, and is not just a bystander mutation, we constructed an AMC008 virus lacking the N130 glycan and observed that this mutation indeed renders the virus resistant. A possibly mechanistic explanation is that removing the N130 glycan changes the orientation or composition of the N160 glycan. The AMC008 virus was not very sensitive for PGDM1400 neutralization compared to BG505 or REJO, which could explain why the virus did not remove the glycan at 160. Removal of the glycan at position 130 might represent an easier alternative as the N130 glycan is less conserved and might therefore be less likely to cause viral fitness loss. In addition, the relative low sensitivity might also explain why the T132N mutation was detected in the viral population at weeks 1 and 3, but not at week two of treatment: PGDM1400 pressure on the AMC008-virus appears insufficient to outcompete non-mutated viruses completely. These results indicate that HIV-1 can escape neutralization through a diverse range of mutations in different viruses of clades A, B and C, which results in the removal of the glycan at position 160 or 130.
In this study, using multiple virus isolates, we did not observe a significant correlation between the reduction of viral load (treatment efficacy) and the in vitro PGDM1400 sensitivity (IC50 or IC90). Nor was a correlation found with viral load at the start of treatment, in contrast to previous observations.Citation11 In part, this is likely caused by the use of different viruses and small number of animals per virus, but also by the fact that the success of bnAb therapy depends on multiple factors, which might differ between isolates, including the maximum percent inhibition of the bNAbs.Citation18 For example, the REJO-infected mice, in which the PGDM1400 concentrations dropped to about twofold of the IC90, value, showed a viral load reduction and escape, while the mouse infected with AMC008, in which the antibody concentration remained over 10-fold above the IC90, showed no viral load reduction at all. Furthermore, while escape mutations were observed in two of the AMC008-infected mice, indicative of bNAb pressure, no such mutations were observed in the third mouse, while PGDM1400 serum concentrations were very similar between the three animals infected with AMC008. The lack of treatment efficacy in this animal could be due to the very high viral load at treatment initiation in this one animal. Furthermore, one of the MJ4-infected animals showed a decrease in viral load after a week of PGDM1400 treatment, but the other animal did not, even though no clear escape mutations were observed in either. The antibody concentration in serum for the responding animal was at least 10-fold above the IC90, whereas the antibody levels in the non-responding animal were at least sixfold above the IC90. Furthermore, incomplete neutralization could result in lower antibody treatment efficacy, and PGDM1400 was unable to reach a 100% inhibition at the highest concentration in vitro of three of the four viruses used here. However, again there was no direct correlation with the failure of treatment and incomplete neutralization, as REJO and MJ4 had very similar PGDM1400 IC50, IC90 values and similar levels of incomplete neutralization in vitro, but the MJ4-infected animals showed much lower responses to treatment and less selection pressure compared to the REJO-infected animals.
For BG505-infected mice, the reduction in viral load was most pronounced, but even in these mice, only a modest reduction of 0.46log10 and 0.26log10 was observed despite a serum level of more than 400x the in vitro IC90. Although the modest reduction is somewhat disappointing in light of the high neutralization sensitivity, it is not without precedent. In a study on VRCO1 administration in human subjects, it was shown that antibody infusion at concentrations 100x the in vitro IC80 did not yield a 0.5log10 reduction in viral load, and that instead 500x the in vitro IC80 was required to obtain more than 0.5log10 reduction.Citation27 The overall interpretation of the results from this study is that the antibody concentration most likely should be at least 10 to 500-fold above the IC90 in order to achieve a significant viral load reduction, which is in agreement with previous studies,Citation7,Citation11,Citation27 but that treatment success also depends on other factors that may weigh differently for each viral isolate, including the incomplete neutralization by the antibody and the consequences of the possible escape pathways on viral fitness. Additional animals and different viruses could increase the understanding of the different factors influencing the bnAb treatment; however, the main findings here that PGDM1400 treatment alone is not effective and causes rapid viral escape are already of significant value for future clinical trials which will include PGDM1400.
In conclusion, PGDM1400 caused a modest and temporal decline in viral load after administration in seven out of 10 mice, and the rapid emergence of viral escape variants resulted in viral rebound. PGDM1400, like any other bnAb, will have to be combined with other bnAbs, preferably targeting different epitopes, in cocktail or bi- and tri-specific antibody formats. The upcoming clinical studies with cocktails and tri-specific antibodies will reveal which combinations will have the largest effect on viral loads and with the least change of viral escape during prolonged treatment, and if PGDM1400 will be part of this.
Materials and methods
Antibody production
293 F cells (Invitrogen) were co-transfected with heavy and light chain plasmids, obtained previously,Citation13 using PEImax (Polysciences). Transfections were performed according to the manufacturer’s protocol and culture supernatants were harvested 5 days following transfection. Antibodies were further purified using a protein A/G column (ThermoFisher Scientific) and stored in phosphate-buffered saline (PBS) as described previously.Citation13
Virus production
Full-length viral genome plasmids pBG505/LAI, pREJO.c/2864 (NIH cat#11746), MJ4 (NIH cat# 6439) and pAMC008/LAI (BG505 and AMC008 envelope glycoprotein were cloned into the pLAI plasmidCitation28) and were used to transfect 293 T cells as described previously.Citation29 Viruses were harvested, frozen and titrated on TZM-bl cells as previously described.Citation29
HIS mice experiment
HIS mice were generated using non-obese diabetic (NOD), severe combined immunodeficiency (SCID), interleukin receptor common gamma chain knockout (IL2rγnull), termed NSG mice (The Jackson Laboratory). NSG newborns before 5 days were sublethally irradiated with 1 Gy using a Cs137 source and intrahepatically injected within 24 h with 5 × 104 CD4+CD38− fetal hematopoietic stem cells (hHSC). The humanization levels of the immune system in the peripheral blood were verified at week 12 and only mice with >20% human CD45+ cells, termed HIS-NSG mice, entered the experiments. At 13 weeks the HIS-NSG mice were infected with 10–100 ng CA-p24 of virus of the four different replication competent HIV virus isolates (3 mice per group) by intraperitoneal injection. Blood was collected every other week before PDGM1400 treatment and weekly during treatment. Humanization and CD4 + T cell dynamics were monitored by flow cytometry (LSR Fortessa, BD Biosciences) as described previously.Citation3 Viral RNA was extracted from plasma by QIAamp Viral RNA Mini Kit (Qiagen) and viral load was determined by quantitative RT-PCR as described previously.Citation3 Statistical analysis on the viral loads was performed using a Wilcoxon test (paired non-parametric t-test). From 12 weeks after infection onwards, PGDM1400 was administered in 50 µl PBS at a dose of 20 mg/kg via retro-orbital injection on a weekly basis for 4 weeks.
Antibody serum concentrations measurement
The PGDM1400 plasma concentrations were determined at weeks 1, 2, 3, and 4 after the start of treatment using a TZM-bl-based neutralization assay, as described previously.Citation3 Week 0 was taken as well for background measurements. Neutralization assays were done using three-fold serial dilutions of serum starting at a 1:30 dilution and PGDM1400 starting at 1.0 μg/ml and JRC-SF pseudovirus. Serum ID50 values and the PGDM1400 IC50 values were used to back-calculate PGDM1400 serum concentrations.
Viral sequences from serum
Part of the HIV-1 envelope glycoprotein (amino acid 75–250) was sequenced from serum viral RNA, obtained as per above, using a one-step RT-PCR (Jena Bioscience), followed by Sanger sequencing, performed according to the manufacturer’s protocol. The sequences were analyzed using BioEdit.
Abbreviations
ART: antiretroviral therapy
bnAb: broadly neutralizing antibody
Env: envelope glycoprotein
HIS: human immune system
IC50: half maximal inhibitory concentration
NGS: non-obese diabetic (NOD), interleukin receptor common gamma chain knockout (IL2rγnull), severe combined immunodeficiency (SCID)
NHP: non-human primate
HIV-1: Human immune deficiency virus 1
SHIV: simian-human immunodeficiency viruses
V1V2: variable regions 1 and 2
Author contributions statement
Y.U.V., J.V., and M.J.G. designed the study. Y.U.V. and J.V. set up the animal studies including ethical approval. Y.U.V., J.V., E.S.R., and C.A.L., performed the animal experiments and processed the samples from the animals. Y.U.V., M.A.V., E.S., and M.J.G. analyzed the samples from the animals. K.W., B.B., R.W.S. and M.J.G. acquired project funding and Y.U.V, R.W.S. and M.J.G. wrote the manuscript.
Supplemental Material
Download PDF (116.9 KB)Acknowledgments
Y.U.V. and R.W.S. are recipient of Veni (grant #016171112) and Vici grants (grant # 91818627), respectively, from the Netherlands Organization for Scientific Research (NWO). M.J.G. is a recipient of an American Foundation for AIDS Research (amfAR) Krim Fellowship (109514-61-RKVA), the 2017 AMC Fellowship and a ZonMW MKMD grant (#40-42600-98-705). Development of the HIS-NGS mouse model was supported by the Eurostar grant ResistAids (grant #E!10239). The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 MJ4 Infectious Molecular Clone (pMJ4) from Drs. Thumbi Ndung’u, Boris Renjifo and Max Essex and pREJO.c/2864 (cat# 11746) from Dr. John Kappes and Dr. Christina Ochsenbauer.
Disclosure statement
J.V. was employed by the company AIMM Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. M.J.G and R.W.S are listed on a patent application on PGDM1400.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- McCoy LE. The expanding array of HIV broadly neutralizing antibodies. Retrovirology. 2018;15(1):70. doi:https://doi.org/10.1186/s12977-018-0453-y.
- van Gils MJ, Sanders RW. In vivo protection by broadly neutralizing HIV antibodies. Trends Microbiol. 2014;22(10):550–7. doi:https://doi.org/10.1016/j.tim.2014.08.006.
- van der Velden YU, Villaudy J, Siteur-van Rijnstra E, van der Linden CA, Frankin E, Weijer K, Schermer E, Vink MA, Berkhout B, Sanders RW, et al. Short communication: protective efficacy of broadly neutralizing antibody PGDM1400 against HIV-1 challenge in humanized mice. AIDS Res Human Retroviruses. 2018;34(9):790–93. doi:https://doi.org/10.1089/aid.2018.0114.
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathogens. 2009;5(5):e1000433. doi:https://doi.org/10.1371/journal.ppat.1000433.
- Deruaz M, Moldt B, Le KM, Power KA, Vrbanac VD, Tanno S, Ghebremichael MS, Allen TM, Tager AM, Burton DR, et al. Protection of humanized mice from repeated intravaginal HIV challenge by passive immunization: a model for studying the efficacy of neutralizing antibodies in vivo. J Infect Dis. 2016;214(4):612–16. doi:https://doi.org/10.1093/infdis/jiw203.
- Stoddart CA, Galkina SA, Joshi P, Kosikova G, Long BR, Maidji E, Moreno ME, Rivera JM, Sanford UR, Sloan B, et al. Efficacy of broadly neutralizing monoclonal antibody PG16 in HIV-infected humanized mice. Virology. 2014;462-463:115–25. doi:https://doi.org/10.1016/j.virol.2014.05.036.
- Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–22. doi:https://doi.org/10.1038/nature11604.
- Julg B, Liu PT, Wagh K, Fischer WM, Abbink P, Mercado NB, Whitney JB, Nkolola JP, McMahan K, Tartaglia LJ, Borducchi EN, Khatiwada S, Kamath M, LeSuer JA, Seaman MS, Schmidt SD, Mascola JR, Burton DR, Korber BT, Barouch DH, et al. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Science Transationall Medicine. 2017;9(408). doi:https://doi.org/10.1126/scitranslmed.aao4235
- Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–91. doi:https://doi.org/10.1038/nature14411.
- Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, et al. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23(2):185–91. doi:https://doi.org/10.1038/nm.4268.
- Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. New Eng J Med. 2016;375(21):2037–50. doi:https://doi.org/10.1056/NEJMoa1608243.
- Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suárez I, Oliveira TY, Lorenzi JCC, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479–84. doi:https://doi.org/10.1038/s41586-018-0531-2.
- Sok D, van Gils MJ, Pauthner M, Julien J-P, Saye-Francisco KL, Hsueh J, Briney B, Lee JH, Le KM, Lee PS, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Nat Acad Sci. 2014;111(49):17624–29. doi:https://doi.org/10.1073/pnas.1415789111.
- Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, Lord DM, Wei RR, Deng G, Louder M, et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358(6359):85–90. doi:https://doi.org/10.1126/science.aan8630.
- Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, Sok D, Abbink P, Schmidt SD, Wang K, Chen X, Joyce MG, Georgiev IS, Choe M, Kwong PD, Doria-Rose NA, Le K, Louder MK, Bailer RT, Moore PL, Korber B, Seaman MS, Abdool Karim SS, Morris L, Koup RA, Mascola JR, Burton DR, Barouch DH, et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Trans Med. 2017;9(406). doi:https://doi.org/10.1126/scitranslmed.aal1321
- Hessell AJ, Haigwood NL. Animal models in HIV-1 protection and therapy. Curr Opinion HIV AIDS. 2015;10(3):170–76. doi:https://doi.org/10.1097/COH.0000000000000152.
- McCoy LE, Falkowska E, Doores KJ, Le K, Sok D, van Gils MJ, Euler Z, Burger JA, Seaman MS, Sanders RW, et al. Incomplete neutralization and deviation from sigmoidal neutralization curves for HIV broadly neutralizing monoclonal antibodies. PLoS Pathogens. 2015;11(8):e1005110. doi:https://doi.org/10.1371/journal.ppat.1005110.
- Webb NE, Montefiori DC, Lee B. Dose–response curve slope helps predict therapeutic potency and breadth of HIV broadly neutralizing antibodies. Nat Commun. 2015;6(1):8443. doi:https://doi.org/10.1038/ncomms9443.
- Lee JH, Andrabi R, Su C-Y, Yasmeen A, Julien J-P, Kong L, Wu NC, McBride R, Sok D, Pauthner M, et al. A broadly neutralizing antibody targets the dynamic HIV envelope trimer apex via a long, rigidified, and anionic β-hairpin structure. Immunity. 2017;46(4):690–702. doi:https://doi.org/10.1016/j.immuni.2017.03.017.
- Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu C-L, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556–60. doi:https://doi.org/10.1038/nature18929.
- Andrabi R, Voss JE, Liang C-H, Briney B, McCoy LE, Wu C-Y, Wong C-H, Poignard P, Burton D. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope V2 apex to facilitate vaccine design. Immunity. 2015;43(5):959–73. doi:https://doi.org/10.1016/j.immuni.2015.10.014.
- Cale EM, Gorman J, Radakovich NA, Crooks ET, Osawa K, Tong T, Li J, Nagarajan R, Ozorowski G, Ambrozak DR, Asokan M, Bailer RT, Bennici AK, Chen X, Doria-Rose NA, Druz A, Feng Y, Joyce MG, Louder MK, O'Dell S, Oliver C, Pancera M, Connors M, Hope TJ, Kepler TB, Wyatt RT, Ward AB, Georgiev IS, Kwong PD, Mascola JR, Binley JM, et al. Virus-like particles identify an HIV V1V2 apex-binding neutralizing antibody that lacks a protruding loop. Immunity. 2017;46:777–91 e10.
- Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509(7498):55–62. doi:https://doi.org/10.1038/nature13036.
- Doria-Rose NA, Georgiev I, O’Dell S, Chuang G-Y, Staupe RP, McLellan JS, Gorman J, Pancera M, Bonsignori M, Haynes BF, et al. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. J Virol. 2012;86(15):8319–23. doi:https://doi.org/10.1128/JVI.00696-12.
- Bricault CA, Yusim K, Seaman MS, Yoon H, Theiler J, Giorgi EE, Wagh K, Theiler M, Hraber P, Macke JP, Kreider EF, Learn GH, Hahn BH, Scheid JF, Kovacs JM, Shields JL, Lavine CL, Ghantous F, Rist M, Bayne MG, Neubauer GH, McMahan K, Peng H, Chéneau C, Jones JJ, Zeng J, Ochsenbauer C, Nkolola JP, Stephenson KE, Chen B, Gnanakaran S, Bonsignori M, Williams LD, Haynes BF, Doria-Rose N, Mascola JR, Montefiori DC, Barouch DH, Korber B, et al. HIV-1 neutralizing antibody signatures and application to epitope-targeted vaccine design. Cell Host Microbe. 2019;26:296. doi:https://doi.org/10.1016/j.chom.2019.07.016.
- Torrents de la Pena A, Rantalainen K, Cottrell CA, Allen JD, van Gils MJ, Torres JL, Crispin M, Sanders RW, Ward AB, et al. Similarities and differences between native HIV-1 envelope glycoprotein trimers and stabilized soluble trimer mimetics. PLoS Pathogens. 2019;15(7):e1007920. doi:https://doi.org/10.1371/journal.ppat.1007920.
- Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Trans Med. 2015;7:319r a206. doi:https://doi.org/10.1126/scitranslmed.aad5752.
- de Taeye SW, Ozorowski G, Torrents de la Pena A, Guttman M, Julien J-P, van den Kerkhof TGM, Burger J, Pritchard L, Pugach P, Yasmeen A, et al. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell. 2015;163(7):1702–15. doi:https://doi.org/10.1016/j.cell.2015.11.056.
- van den Kerkhof TL, de Taeye SW, Boeser-Nunnink BD, Burton DR, Kootstra NA, Schuitemaker H, Sanders RW, van Gils MJ. HIV-1 escapes from N332-directed antibody neutralization in an elite neutralizer by envelope glycoprotein elongation and introduction of unusual disulfide bonds. Retrovirology. 2016;13(1):48. doi:https://doi.org/10.1186/s12977-016-0279-4.
