ABSTRACT
Monoclonal antibody (mAb) therapy has been previously exploited for viral infections, such as respiratory syncytial virus pneumonia and Ebolavirus disease. In the ongoing COVID-19 pandemic, early signals of efficacy from convalescent plasma therapy have encouraged research and development of anti-SARS-CoV-2 mAbs. While many candidates are in preclinical development, we focus here on anti-SARS-CoV-2 neutralizing mAbs (or mAb cocktails) that represent the late-stage clinical pipeline, i.e., those currently in Phase 2 or Phase 3 clinical trials. We describe the structure, mechanism of action, and ongoing trials for VIR-7831, LY-CoV555, LY-CoV016, BGB-DXP593, REGN-COV2, and CT-P59. We speculate also on the next generation of these mAbs.
Background
In October 2020, the COVID-19 pandemic counts more than 40 million cases and over 1 million deaths. The rapid spreading of the disease has prompted an intense research activity to identify potential treatments, including investigations on existing drugs approved for other indications,Citation1 and the parallel de novo development of innovative treatments, including antiviral drugs or passive immune therapies.
In this scenario, the monoclonal antibody (mAb) arena for COVID-19 has been exploited through use of anti-inflammatory mAbs aimed at managing the cytokine storm,Citation2 with variable results so far for tocilizumab.Citation3 However, higher hopes have been raised from the efficacy signals obtained with convalescent plasma (CP) therapy,Citation4 where the active substances are assumed to be polyclonal neutralizing antibodies (nAb). The interpretation of trials on CP efficacy has been largely hampered by the lack of standardized doses and poor assessment of nAb with viral neutralization tests;Citation5 nevertheless, favorable preliminary results have led immediately to research and development of pharmaceutical-grade hyperimmune seraCitation6 and mAbs (). These mAbs have several obvious advantages over CP and immune sera (), given that the number of therapeutic antibodies is restricted to one or two per treatment: (a) selection of the most potent candidates (with IC50 or the more reliableCitation7,Citation8 IC100 parameter in the subnanomolar to low picomolar range); (b) better assessment of the therapeutic dose per body weight; c) exclusion of the so-called antibody-dependent enhancement (ADE) phenomenon.Citation9
Table 1. A comparison of antibody-based therapies for COVID-19
Figure 1. Characterization of antibody-based therapies for SARS-CoV-2
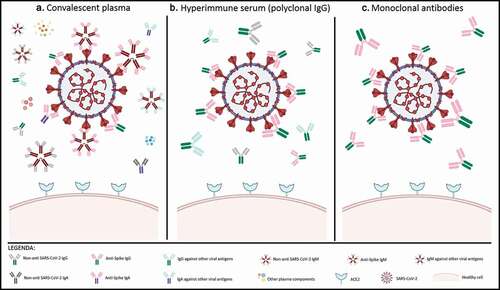
Neutralizing antiviral mAbs have been used successfully in clinical practice against respiratory syncytial virus (RSV) (e.g., palivizumab)Citation10 and Ebolavirus disease (EVD) (e.g., b114, REGN-EB3, and ZMapp).Citation11 Therefore, since the beginning of the SARS-CoV-2 pandemic, biopharmaceutical companies, and academic researchers cooperated to develop neutralizing mAbs derived from convalescent patients. Generally, the process involves several steps, including selection of peripheral blood mononuclear cells from CP donors, isolation of receptor binding domain (RBD)-specific single memory B lymphocytes, cloning, transfection, and finally mAbs production.Citation12 With this methodology, numerous mAbs with potential neutralizing activity against SARS-CoV-2 have been discovered, but only a few of them are being currently tested in clinical trials ().
Table 2. mAb-based therapeutics for COVID-19 in clinical trials
In this perspective, we discuss the anti-SARS-CoV-2 neutralizing mAbs that are currently in their most advanced phases of clinical development, and hence represent the most likely candidates for approval for clinical use. We do not discuss antiviral mAbs targeting viral receptors (i.e., the anti-CD147 meplazumab).
Methods
We searched the World Health Organization (https://www.who.int/ictrp/en/) and National Institutes of Health (https://clinicaltrials.gov/) databanks to identify clinical trials investigating antiviral mAbs as treatments for COVID-19 as of November 9, 2020. Among these, we focused on mAbs that had entered at least Phase 2 clinical studies. Investigational drugs were then selected and used as search terms in PubMed and Google Scholar. Agents were also cross-checked in the Chinese Antibody Society (https://chineseantibody.org/covid-19-track), and Antibody Society (https://www.antibodysociety.org/covid-19-biologics-tracker/) databanks.Citation13 We checked also in drug company webpages to access information provided in press releases. For each selected drug, we collected information about their mechanism of action, molecular target, and development status, including the main characteristics of the respective available trials. Data from preprints were also included.
Results
We identified 14 mAbs or mAb cocktails that entered clinical trials (). Five agents are currently involved in Phase 2/3 clinical trials, and these are the main subject of this perspective.
VIR-7831
VIR-7831 (also known as GSK4182136) is a fully human anti-SARS-CoV-2 mAb developed collaboratively by Vir Biotechnology and GSK, based on the identification and characterization of S309, an antibody identified from a convalescent patient, who recovered from SARS in 2003.Citation14 S309 was shown to neutralize SARS-CoV-2 as well. This antibody binds a highly conserved epitope shared by the two coronaviruses, thus indicating the unlikelihood of a mutational escape. The epitope is located on the spike protein, used by the virus to bind and enter human cells, leading to infection. VIR-7831 has been engineered starting from S309 to improve its pharmacokinetic features and obtain, in particular, an extended half-life. Notably, a second antibody, designated as VIR-7832, is under development with the same rationale. However, it has been engineered to work also as a T-cell vaccine, therefore, being able to recruit T-lymphocytes, which can then recognize and kill SARS-CoV-2-infected cells.Citation15
VIR-7831 is being currently investigated in patients with early-stage COVID-19 infection, who are at high risk for hospitalization (i.e., patients ≥55 y-old with existing lung or cardiovascular disease). The COMET-ICE trial (NCT04545060) has been designed to enroll 20 patients with early symptomatic COVID-19 in the lead-in phase and about 1300 patients in the expansion phase worldwide.Citation16 It is aimed at investigating the proportion of patients with COVID-19 progression at d 29, comparing VIR-7831 (500 mg intravenous (IV) infusion over a 14-d period) and placebo (efficacy endpoint). Secondary outcomes include the occurrence of adverse events and development (and titers) of anti-drug antibodies (ADA) to VIR-7831. The final report is scheduled for July 2021 but, should expectations be fulfilled, the drug should be available in the first quarter of 2021.
LY-CoV555 and LY-CoV016
LY-CoV555 (known also as LY3819253 and recently designated as bamlanivimab) is a recombinant, fully human neutralizing IgG1 mAb, developed by AbCellera and the US Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID),Citation17 in collaboration with Eli Lilly. In particular, this mAb was selected as the one with the best binding affinity for SARS-CoV-2 among over 500 antibodies,Citation18 and it targets the RBD of the spike protein.Citation13,Citation17–19 Another mAb, LY-CoV016 (known also as LY3832479 and recently designated as etesevimab), targeting a different spike epitope, has been developed by Eli Lilly with the aim of obtaining synergy with LY-CoV555.Citation20
In August 2020, the Phase 1 clinical trial of LY-CoV555 (NCT04411628), including 24 hospitalized patients with COVID-19 and evaluating safety, tolerability, pharmacokinetics, and pharmacodynamics upon intravenous administration, was completed.Citation21 The Phase 2 clinical trial BLAZE-1 (NCT04427501) is currently investigating the efficacy and safety of the combination of LY-CoV555 and LY-CoV016 in outpatients with diagnosis of mild to moderate COVID-19.Citation22 In this three-arm study, the additive effect of both antibodies in reducing the viral load was tested in comparison with LY-CoV555 monotherapy and placebo.Citation22 Preliminary results of the BLAZE-1 study, provided only for the comparison between LY-CoV555 monotherapy and placebo, showed a significant decrease in the viral load at d 11 (the primary outcome) in patients receiving the 2800-mg dose as compared the placebo group a reduction in the rate of hospitalizations (including emergency department admissions) on d 29 (1.6% in the LY-CoV555 group and 6.3% in the placebo group), a progressive reduction in symptom severity from d 2 to 11) and a good tolerability profile.Citation20,Citation23 Among the three doses tested (700 mg, 2800 mg, or 7000 mg) the one for which a statistical significant difference was observed is the intermediate (at d 11, difference −0.53 95% confidence interval, CI, −0.98 to −0.08), probably due to the size of the sample (about 100 per investigated group).Citation20,Citation24 However, the authors noted that this difference observed in the reduction of viral load at d 11 might not be clinically meaningful because it could be associated with the natural course of COVID-19. In this regard, the reduction of hospitalization or emergency department admission rates at d 29 represents a more relevant finding.Citation23,Citation24 Mostly based on this latter result, the request for Emergency Use Authorization (EUA) for the combination therapy in patients with mild-to-moderate COVID-19 was announced by Eli Lilly in October 2020, and the agreement with the US government for supplying vials in the first 2 months after authorization has been carried out.Citation25
The ongoing Phase 3 trial BLAZE-2 (NCT04497987), scheduled to enroll 2400 patients, evaluates the prevention of SARS-CoV-2 infection by administration of LY-CoV555 in skilled nursing, assisted living facility staff, and residents.Citation26 In addition, a Phase 2/3 trial versus placebo (ACTIV-2, NCT04518410) is ongoing in outpatients testing positive for COVID-19, and it evaluates whether LY-CoV555 is able to prevent the spread of infection and the disease progression toward the most serious conditions.Citation27 Finally, the ACTIV-3 study (NCT04501978), a Phase 3 trial including hospitalized patients, was investigating the effectiveness and safety of this mAb in comparison with remdesivir.Citation28 On October 13, 2020, this trial suspended the enrollment of patients upon recommendation of the independent Data Safety Monitoring Board, due to unspecified safety concerns that need to be investigated.Citation29 On October 26, 2020, the evaluation of ACTIV-3 data showed no significant differences in safety outcomes between groups, but the enrollment of new hospitalized patients was ended based on the absence of improvements in hospitalized COVID-19 patients with advanced disease.Citation25 On November 10, 2020, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for banlanivimab for the treatment of mild-to-moderate COVID-19 in adult and pediatric patients.Citation30 This is the first nAb authorized for clinical use.
BGB-DXP593
BGB-DXP593 was developed collaboratively by BeiGene and Singlomics Biopharmaceutical. The underlying mAb was identified by high-throughput single-cell sequencing of convalescent blood samples from recovered patients with COVID-19 at the Advanced Innovation Center for Genomics of Peking University.Citation31
The precise mechanism whereby BGB-DXP593 neutralizes SARS-CoV-2 is unknown. However, the observed similarity in RNA genomes of SARS-CoV and SARS-CoV-2 suggests that the crystal structure of SARS-CoV neutralizing mAb could be taken as reference to screen the SARS-CoV-2 antigen-binding clonotypes sharing similar CDR3H structures.Citation32 CDR3H is one of the complementary-determining regions of the mAb that binds the RBD of the virus.Citation33 Moreover, the investigators screened antigen-enriched B cells from 60 convalescent patients by high-throughput single-cell RNA and VDJ sequencing, and identified 14 potent neutralizing mAbs. Preclinical evidence showed that the structure identified when the most potent antibody (i.e., BD-368-2) binds the spike ectodomain trimer of the virus overlaps with the RBD-ACE2 complex structure. Thus, this antibody likely acts by inhibiting the entrance of the virus through this protein. On this basis, it could be hypothesized that BGB-DXP593 has a similar mechanism as BD-368-2 in neutralizing SARS-CoV-2.Citation32
BGB-DXP593 is being tested in a Phase 2, randomized, double-blind, placebo-controlled study (NCT04551898) to evaluate its efficacy and safety in patients with mild to moderate COVID-19, sponsored by BeiGene.Citation34 It will enroll about 180 participants (from 18 to 65 y), who experienced COVID-19 symptoms (e.g., fever, cough, shortness of breath, sore throat, diarrhea, dysgeusia, vomiting) for ≤7 d before assignment to treatment. The study consists of four treatment arms, three of which are designed to test three different doses of the antibody (low, medium and high dose) and one being the comparator arm with placebo. All the participants will receive the allocated treatment on d 1 and will be followed for safety up to 85 d. The primary objective is to investigate the safety and tolerability of BGB-DXP593 administrated intravenously as a single dose in patients with mild to moderate COVID-19; the primary outcome will assess the changes from baseline to d 8 of the virus diffusion, measured by reverse transcription-quantitative polymerase chain reaction in nasopharyngeal swabs. In this trial, eight secondary outcomes will be assessed also from baseline to d 15, 29, and 85.Citation35
REGN-COV2
REGN-COV2 is a novel cocktail of two mAbs (REGN10987 and REGN10933 recently named imdevimab and casirivimab, respectively) that is being investigated for both the treatment of patient with COVID-19 and the prevention of SARS-CoV-2 infection. This antiviral biologic was developed by Regeneron Pharmaceuticals with the same approach used to generate REGN-EB3, a triple antibody treatment for EVD.Citation36 The company evaluated a large and heterogeneous set of fully human antibodies obtained from both genetically engineered mice and B cells from convalescent patients. This allowed the expanded detection of rearrangements commonly present in the neutralizing SARS-CoV-2 mAb found in humans, and gave the possibility for a wide choice of selection.Citation37 Based on binding, neutralization, and structure characteristics, a pair of potent mAbs (REGN10987 and REGN10933) were identified and selected to generate REGN-COV2.Citation37
REGN10987 and REGN10933 bind, simultaneously and non-competitively, different epitopes of the RBD of SARS-CoV-2 spike, thus preventing the interaction of the viral protein with ACE2 and, and leading to the virus neutralization. The antigen-binding fragment of REGN10933 binds at the top of RBD, overlapping almost completely the region hosting the binding site for ACE2, while REGN10987 acts on the side of RBD endowed with low probability of interfering with ACE2.Citation37,Citation38 In the presence of the REGN-COV2 cocktail, escape mutant viruses failed to be generated efficiently, while this process was found to occur under exposure to either antibody.Citation38
REGN-COV2 is being currently evaluated in four late-stage clinical trials with ongoing recruitment. Two Phase 2/3 trials (NCT04425629 and NCT04426695) are investigating the efficacy of REGN-COV2, compared to placebo, in reducing the viral shedding in adult hospitalized and non-hospitalized COVID-19 patients, respectively.Citation39,Citation40 Both studies will be conducted in the U.S., Brazil, Mexico, and Chile, and are expected to enroll about 1850 hospitalized and 1050 non-hospitalized patients. A descriptive analysis of early data obtained from 275 non-hospitalized patients (randomized 1:1:1 to receive 8 grams of REGN-COV2, 2.4 grams of REGN-COV2 or placebo) showed that REGN-CoV2 lowered the viral load and reduced the time required to relieve symptoms.Citation41 Latest data obtained from additional 524 outpatients enrolled in the ongoing trial confirmed results of previous analysis.Citation42 In the additional patients with high viral load (classified as greater than 107 copies/mL) at baseline, REGN-COV2 showed a greater average daily reduction (0.68 log10 copies/mL) in viral load through d 7, compared to that observed in patients treated with placebo (combined dose groups; p < .0001). In particular, patients treated with REGN-COV2 had on average a 10-fold reduction in viral load compared to placebo by d 5. The viral load reduction observed in all patients with detectable virus at baseline was greater (0.36 log10 copies/mL) with REGN-COV2 compared to placebo (combined dose groups; p = .0003). Before receiving treatment, all patients (n = 799) were prospectively tested to assess if they had their own measurable antiviral antibodies (seropositive patients) or they did not (seronegative patients). About 38% of participants were seropositive, 51% were seronegative and 11% did not have confirmed serological status. Virologic results, together with those obtained from the previous analysis, showed that seronegative patients and/or patients with higher viral load at baseline derived greater benefit from the treatment with REGN-COV2. Data revealed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams). Furthermore, both doses were well tolerated. Clinical results of the overall population (n = 799) showed that the antibody cocktail also reduced the need for further medical attention, with a reduction of COVID-19-related medical visits by 57% through d 29 (2.8% combined dose groups; 6.5% placebo; p = .024) compared to placebo. Recruitment is still ongoing, and trials are estimated to be completed in December 2020. However, the REGN-COV2 trial conducted on hospitalized patients has been recently modified following REGN-COV2 Independent Data Monitoring Committee recommendations. In particular, the unfavorable benefit/risk profile and a potential safety signal led to the suspension of further enrollment of patients requiring high flow oxygen or mechanical ventilation until further information on already enrolled patients is available. On the contrary, hospitalized patients requiring either no or low-flow oxygen showed an acceptable risk/benefit ratio, allowing enrollment to continue.Citation43
REGN-COV2 is also being tested in the Phase 3 prevention trial (NCT04452318), conducted on uninfected subjects at high-risk of household exposure to a COVID-19 patient. This randomized double-blind study is evaluating the efficacy of REGN-COV2, compared to placebo, in preventing both symptomatic and asymptomatic SARS-CoV-2 infection in 2000 patients. The study started in July 2020 and is planned to be completed by August 2021.Citation44 Moreover, the open-label Phase 3 RECOVERY (NCT04381936) is evaluating REGN-COV2 at the University of Oxford. This large-scale randomized clinical trial was specifically designed to rapidly evaluate candidate drugs for SARS-CoV-2, including available promising investigational therapies. It investigates whether treatment with either lopinavir-ritonavir, hydroxychloroquine, corticosteroids, azithromycin, CP, synthetic neutralizing antibodies, or tocilizumab can prevent death in hospitalized patients with COVID-19. REGN-COV2 is the first specifically developed COVID-19 therapy included in this trial.Citation45 The RECOVERY study aims at investigating whether the addition of REGN-COV2 (8 grams) to the usual standard of care, versus the standard care alone, prevents all-cause mortality 28 d after randomization of hospitalized patients. Other outcomes include duration of hospital stay and composite endpoint of death or need for mechanical ventilation or extracorporeal membrane oxygenation.Citation46
Regeneron has shared all these results with the U.S. FDA, which recently approved the Emergency Use Authorization for the REGN-COV2 low dose in adults with mild-to-moderate COVID-19 who are at high risk for poor outcomes.Citation47
CT-P59
CT-P59 is a fully human anti-SARS-CoV-2 mAb developed by the Celltrion Group selected by the Korea Center for Disease Control.Citation48 Celltrion created a library of antibodies, identified by analyzing the blood of recovered Korean patients, including CT-P59.Citation48 CT-P59 binds to the RBD of the spike protein of the virus to inhibit its interaction with the receptor ACE2 and block the entrance of the virus. The binding orientation between CT-P59 and the RBD is different from other neutralizing mAbs. This suggest that CT-P59 can be a novel binder to the RBD and this docking could be considered for the development of new mAbs. Furthermore, CT-P59 has been proven to neutralize the D614G variant, one of the most infectious S protein’s mutations.Citation49,Citation50
In July 2020, the Phase 1 clinical trial (NCT04525079), including 32 healthy subjects, was started. It is a randomized, double-blind, placebo-controlled, parallel group trial to evaluate the safety, tolerability, and pharmacokinetics of CT-P59.Citation51 On September 11, 2020, the Celltrion Group announced positive interim results with no significant adverse drug events.Citation52 In August 2020, another Phase 1 clinical trial (NCT04593641),Citation53 including 18 patients with mild symptoms of SARS-CoV-2, was initiated. No results are available to date.Citation54
In September 2020, the Korean Ministry of Food and Drug Safety, based on the interim results of the Phase 1 clinical trial, approved a Phase 2/3 trial (NCT04602000) to evaluate the safety and efficacy of CT-P59 in patients with mild to moderate symptoms of COVID-19. It is a randomized, double-blind, placebo-controlled, parallel group trial. It will enroll about 1020 patients who will divide into three treatment arms, two with CT-P59 and one with placebo. The administered dosage of the mAb is not disclosed. The primary outcome is to evaluate the therapeutic efficacy from baseline to d 14 and 28. At d 14 the outcome will be measured as the proportion of negativized patients, the time required to the negative of nasopharyngeal swab, and the time to clinical recovery. At d 28 the outcome will be measured as the proportion of enrolled patients with symptoms that required hospitalization, oxygen therapy, or died.Citation55
Discussion
All the anti-SARS-CoV-2 mAbs currently under investigation in late-stage clinical trials are neutralizing mAbs targeted against the SARS-CoV-2 spike protein. These agents are all conventional full-length IgG. Nevertheless, IgM/IgA, IgY-based therapeutics, bi- or tri-specific antibodies, single-domain- derived from phage display libraries, fusion proteins, or other formats (e.g., DARPin, mRNA-encoding mAb, radiotherapeutics, IgM/IgA) are being developed and some of them will soon progress in clinical trials.
Apart from the antibody format, engineering has been accomplished in the natural isolates in different ways. For example, the LALA mutation was introduced into the Fc portion of CB6 to lower the risk of Fc-mediated acute lung injury.Citation56 Potential future developments include combining neutralizing mAb with the catalytic activity of Cas13, a fusion-protein targeting, and cutting the viral RNA (AntiBody And CAS fusion (ABACAS)), so that agents would exert both a prophylactic and anti-viral effect.Citation57,Citation58
Efficacy against mutant viral strains is also a relevant issue. The SARS-CoV-2 spike protein mutated after few months of viral circulation,Citation59 with one mutation outside the receptor-binding motif (23403A >G single nucleotide polymorphism, corresponding to D614G amino acid change), currently defining a dominant cladeCitation60 characterized by reduced S1 shedding and increased infectivity.Citation61 Althouh particular mutation increases the susceptibility to neutralization,Citation62,Citation63 only a few of these candidate drugs have been tested for their capability of neutralizing different strains of SARS-CoV-2. Antibody cocktails theoretically reduce the ability of mutant viruses to escape treatment and protect against spike variants that have already arisen in the human population.
The antibody isotype is also a critical point. Despite IgM, IgG and IgA are all capable of mediating neutralization, virus neutralization test titers correlated better with the binding levels of IgM and IgA1 than IgG.Citation64,Citation65 The lack of evidence about the efficacy of IgG formulations (either polyclonal or monoclonal) against any respiratory pathogenCitation66,Citation67 could theoretically be attributed to the lack of IgA. For this reason, Phase 3 trials testing IgG mAbs for COVID-19 prevention are eagerly awaited.
In this perspective, we did not include another relevant candidate, namely meplazumab (Ketantin®). Known also as HP6H8 and GTPL11026, meplazumab is a humanized monoclonal IgG2 anti-CD147 antibody, developed by Jiangsu Pacific Meinuoke Bio Pharmaceutical.Citation33 CD147 is both a cellular receptor for SARS-CoV-2Citation68 and a key factor of inflammation, making the mechanism of action of targeting drugs pleiotropic.
While numerous Phase 3 trials are still pending, mAb therapy has the requisites to make a difference in the management of COVID-19. Nevertheless, even if their efficacy will be proven and their safety will be acceptable, sufficient quantities may not be available to meet the expected large request, their price could make these drugs unaffordable for healthcare systems. Therefore, it will be a priority to identify those patients who could receive the highest benefit from these mAbs, thus maximizing the appropriate use of resources.
Abbreviations
| ADA | = | anti-drug antibodies |
| ADE | = | antibody-dependent phenomenon |
| CP | = | convalescent plasma |
| EVD | = | Ebola virus disease |
| mAb | = | monoclonal antibody |
| nAb | = | neutralizing antibody |
| RBD | = | receptor binding domain |
References
- Tuccori M, Convertino I, Ferraro S, Cappello E, Valdiserra G, Focosi D, Blandizzi C. The impact of the COVID-19 “Infodemic” on drug-utilization behaviors: implications for pharmacovigilance. Drug Saf. 2020;43(8):699–8. doi:https://doi.org/10.1007/s40264-020-00965-w.
- Convertino I, Tuccori M, Ferraro S, Valdiserra G, Cappello E, Focosi D, Blandizzi C. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit Care. 2020;24(1):331. doi:https://doi.org/10.1186/s13054-020-03020-3.
- Ramiro S, Mostard RLM, Magro-Checa C, van Dongen CMP, Dormans T, Buijs J, Gronenschild M, de Kruif MD, van Haren EHJ, van Kraaij T, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79(9):1143–51. doi:https://doi.org/10.1136/annrheumdis-2020-218479.
- Focosi D, Anderson AO, Tang JW, Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin Microbiol Rev. 2020;33(4). doi:https://doi.org/10.1128/CMR.00072-20.
- Focosi D, Maggi F, Mazzetti P, Pistello M. Viral infection neutralization tests: a focus on severe acute respiratory syndrome-coronavirus-2 with implications for convalescent plasma therapy. Rev Med Virol. 2020 Aug. doi:https://doi.org/10.1002/rmv.2170.
- Daniele F, Marco T, Guido A, Fabrizio M. What is the optimal usage of Covid-19 convalescent plasma donations? Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2020 Sep. doi:https://doi.org/10.1016/j.cmi.2020.09.036.
- Dong J, Huang B, Jia Z, Wang B, Gallolu Kankanamalage S, Titong A, Liu Y. Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerg Microbes Infect. 2020;9(1):1034–36. doi:https://doi.org/10.1080/22221751.2020.1768806.
- Kreer C, Zehner M, Weber T, Ercanoglu MS, Gieselmann L, Rohde C, Halwe S, Korenkov M, Schommers P, Vanshylla K, et al. Longitudinal isolation of potent near-germline SARS-CoV-2-neutralizing antibodies from COVID-19 patients. Cell. 2020;182(4):843–854.e12. doi:https://doi.org/10.1016/j.cell.2020.06.044.
- Karthik K, Senthilkumar TMA, Udhayavel S, Raj GD. Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Hum Vaccin Immunother. Aug 2020;1–6. doi:https://doi.org/10.1080/21645515.2020.1796425.
- Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013;(4)CD006602. doi:https://doi.org/10.1002/14651858.CD006602.pub4.
- Mulangu S, Dodd LE, Davey RTJ, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, et al. A randomized, controlled trial of ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–303. doi:https://doi.org/10.1056/NEJMoa1910993.
- Sajna KV, Kamat S. Antibodies at work in the time of SARS-CoV-2. Cytotherapy. 2020 Aug. doi:https://doi.org/10.1016/j.jcyt.2020.08.009.
- Yang L, Liu W, Yu X, Wu M, Reichert JM, Ho MCOVID-19. Antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antib Ther. 2020 Aug. doi:https://doi.org/10.1093/abt/tbaa020.
- Pinto D, Park Y-J, Beltramello M, Walls AC, Tortorici MA, Bianchi S, Jaconi S, Culap K, Zatta F, De Marco A, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–95. doi:https://doi.org/10.1038/s41586-020-2349-y.
- [no author listed]. Vir biotechnology and GSK start phase 2/3 study of COVID-19 antibody treatment. GSK website.
- [no author listed]. VIR-7831 for the early treatment of COVID-19 in outpatients (COMET-ICE). clinicaltrials.gov.
- Abbasi J. COVID-19 antibody trials have begun. JAMA. 2020;324(2):128. doi:https://doi.org/10.1001/jama.2020.11582.
- Yaragalla S, Narang P, Kadam KJ, Remedios, Kimberly C, Sahni S, Singh A, Khan N, Yadav S, et al. COVID-19 pandemic: a comprehensive updated review with an artificial intelligence (AI). [ Accessed 2020 Oct 1]. http://www.onlinescientificresearch.com/articles/covid19-pandemic-a-comprehensive-updated-review-with-an-artificial-intelligence-ai.pdf.
- Florindo HF, Kleiner R, Vaskovich-Koubi D, Acúrcio RC, Carreira B, Yeini E, Tiram G, Liubomirski Y, Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat Nanotechnol. 2020;15(8):630–45. doi:https://doi.org/10.1038/s41565-020-0732-3.
- [no author listed]. Lilly announces proof of concept data for neutralizing antibody LY-CoV555 in the COVID-19 outpatient setting. Eli Lilly webpage.
- A Study of LY3819253 (LY-CoV555) in Participants Hospitalized for COVID-19 - Full Text View - ClinicalTrials.gov. [Accessed 2020 Oct 1]. https://clinicaltrials.gov/ct2/show/NCT04411628?cond=NCT04411628&draw=2&rank=1.
- A Study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in Participants With Mild to Moderate COVID-19 Illness (BLAZE-1). clinicaltrials.gov.. [Accessed 2020 Nov 9].
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2020 Oct:NEJMoa2029849. doi:https://doi.org/10.1056/NEJMoa2029849.
- Kinch MS. Oh, the frustration of antibodies! ACS Pharmacol Transl Sci. Sep 2020;acsptsci.0c00138. doi:https://doi.org/10.1021/acsptsci.0c00138.
- Bamlanivimab COVID-19 Treatment — Precision Vaccinations. [Accessed 2020 Nov 4]. https://www.precisionvaccinations.com/vaccines/bamlanivimab-covid-19-treatment.
- [no author listed]. A study of LY3819253 (LY-CoV555) in preventing SARS-CoV-2 infection and COVID-19 in nursing home residents and staff (BLAZE-2). clinicaltrials.gov.
- [no author listed]. ACTIV-2: a study for outpatients with COVID-19. clinicaltrials.gov.
- [no author listed]. ACTIV-3: Therapeutics for Inpatients With COVID-19 (TICO). clinicaltrials.gov.
- Lovelace B, Farr C U.S. pauses Eli Lilly’s trial of a coronavirus antibody treatment over safety concerns. CNBC website. Published 2020 [Accessed 2020 Oct 15]. https://www.cnbc.com/2020/10/13/us-pauses-eli-lillys-trial-of-a-coronavirus-antibody-treatment-over-safety-concerns.html.
- [no author listed]. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. FDA Official Website. Published 2020 [Accessed 2020 Nov 10]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19.
- [no author listed]. A message from BeiGene regarding our COVID-19 (coronavirus) response. Beigene website.
- Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L, Zhu Q, Zhang X, Zheng Y, Geng C, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182(1):73–84.e16. doi:https://doi.org/10.1016/j.cell.2020.05.025.
- Prabakaran P, Gan J, Feng Y, Zhu Z, Choudhry V, Xiao X, Ji X, Dimitrov DS. Structure of severe acute respiratory syndrome coronavirus receptor-binding domain complexed with neutralizing antibody. J Biol Chem. 2006;281(23):15829–36. doi:https://doi.org/10.1074/jbc.M600697200.
- [no author listed]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody BGB-DXP593 in participants with mild-to-moderate coronavirus disease 2019 (COVID-19). clinicaltrials.gov.
- Prabakaran P, Gan J, Feng Y, Zhu Z, Choudhry V, Xiao X, Ji X, Dimitrov DS. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody BGB-DXP593 in participants with mild-to-moderate coronavirus disease 2019 (COVID-19) - full text view - clinicaltrials.gov. J Biol Chem. 2006. [Accessed 2020 Sep 29]. https://clinicaltrials.gov/ct2/show/study/NCT04551898?term=BGB-DXP593&draw=2&rank=1.
- Pascal KE, Dudgeon D, Trefry JC, Anantpadma M, Sakurai Y, Murin CD, Turner HL, Fairhurst J, Torres M, Rafique A, et al. Development of clinical-stage human monoclonal antibodies that treat advanced ebola virus disease in nonhuman primates. J Infect Dis. 2020;612(5):218. doi:https://doi.org/10.1093/infdis/jiy285.
- Hansen J, Baum A, Pascal KE, Russo V, Giordano S, Wloga E, Fulton BO, Yan Y, Koon K, Patel K, et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science (80-). 2020;369(6506):1010–14. doi:https://doi.org/10.1126/science.abd0827.
- Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, Giordano S, Lanza K, Negron N, Ni M, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science (80-). 2020;1018(August):1014–18.
- Safety, Tolerability, and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for Hospitalized Adult Patients With COVID-19. [ Accessed 2020 Oct 4]. https://clinicaltrials.gov/ct2/show/NCT04426695?term=REGN-10933&cond=COVID-19&phase=1235&draw=2&rank=2.
- [no author listed]. Safety, tolerability, and efficacy of anti-spike (S) SARS-CoV-2 monoclonal antibodies for the treatment of ambulatory adult patients with COVID-19. clinicaltrials.gov.
- [no author listed]. Regeneron’s REGN-COV2 antibody cocktail reduced viral levels and improved symptoms in non-hospitalized COVID-19 patients. Regeneron website.
- Regeneron Pharmaceuticals. Regeneron’ s COVID-19 Outpatient Trial Prospectively Demonstrates that REGN-COV2 Antibody Cocktail Significantly Reduced Virus Levels and Need for Further Medical Attention. Published online. PRNewswire Oct. 28, 2020 PRNewswire.
- Regeneron Pharmaceuticals. REGN-COV2 independent data monitoring committee recommends holding enrollment in hospitalized patients with high oxygen requirements and continuing enrollment in patients with low or no oxygen requirements. 2021.
- [no author listed]. Monoclonal antibodies study for prevention of SARS CoV-2 infection in healthy adults who are household contacts to an individual with a positive COVID-19 test. niaid.nih.gov.
- Mahase E. Covid-19: RECOVERY trial will evaluate “antiviral antibody cocktail”. Br Med J. 2020;370(m3584). doi:https://doi.org/10.1136/bmj.m3584.
- [no author listed]. Randomised Evaluation of COVID-19 Therapy (RECOVERY). clinicaltrials.gov.
- Casirivimab and Imdevimab. https://www.regeneron.com/casirivimab-imdevimab. [Accessed November 27, 2020]
- Celltrion accelerates development of COVID-19 antiviral treatment. [Accessed 2020 Nov 3]. https://www.celltrionhealthcare.com/en-us/board/newsdetail?modify_key=174&pagenumber=1&keyword=&keyword_type=.
- Soo-Young L, Cheolmin K, Dong-Kyun, Jihun L, Young-Il K, Ji-Min S, Yeon-Gil K, Jae-Hee J, Minsoo K, Jong-In K, et al. A Novel Neutralizing Antibody Targeting Receptor Binding Domain of SARS-CoV-2, 10 September 2020, PREPRINT (Version 1); Research Square. https://doi.org/10.21203/rs.3.rs–59639/v1
- Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, Zhao C, Zhang Q, Liu H, Nie L, et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182(5):1284–1294.e9. doi:https://doi.org/10.1016/j.cell.2020.07.012.
- To Evaluate the Safety, Tolerability and Pharmacokinetics of CT-P59 in Healthy Subjects - Full Text View - ClinicalTrials.gov. [Accessed 2020 Nov 3]. https://clinicaltrials.gov/ct2/show/NCT04525079?term=NCT04525079&draw=2&rank=1.
- Celltrion Announces Positive Interim Results From Phase I Trial of CT-P59. [Accessed 2020 Nov 3]. https://www.celltrionhealthcare.com/en-us/board/newsdetail?modify_key=364&keyword=&keyword_type=.
- This is a Phase 1 Study to Evaluate the Safety,Tolerability and Virology of CT P59 in Patients With Mild Symptoms of Symptoms of Coronavirus Disease (COVID-19) - Full Text View - ClinicalTrials.gov. [Accessed Nov 3]. https://clinicaltrials.gov/ct2/show/NCT04593641?term=NCT04593641&draw=2&rank=1.
- Celltrion receives Korean MFDS approval to initiate Phase I trial of CT-P59 in patients. [Accessed Nov 3]. https://www.celltrionhealthcare.com/en-us/board/newsdetail?modify_key=353&keyword=&keyword_type=.
- To Evaluate the Safety and Efficacy of CT-P59 in Patients With Mild to Moderate Syptoms of Severe Acute Respiratory Syndrome COVID-19 - Full Text View - ClinicalTrials.gov. [Accessed 2020 Nov 3]. https://clinicaltrials.gov/ct2/show/study/NCT04602000?term=CT-P59&draw=2&rank=2.
- Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, Song T, Bi X, Han C, Wu L, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584(7819):120–24. doi:https://doi.org/10.1038/s41586-020-2381-y.
- Chakraborty S. Neutralizing antibodies: viable treatment modality for COVID-19. Biotechnol Kiosk. 2020;2(6):10–16. doi:https://doi.org/10.37756/bk.20.2.6.2.
- Nalawansha DA, Samarasinghe KTG. Double-barreled CRISPR technology as a novel treatment strategy for COVID-19. ACS Pharmacol Transl Sci. 2020 Sep;3:790–800. doi:https://doi.org/10.1021/acsptsci.0c00071.
- Phelan J, Deelder W, Ward D, Campino S, Hibberd ML, Clark TG. Controlling the SARS-CoV-2 outbreak, insights from large scale whole genome sequences generated across the world. bioRxiv. 2020 Jan. doi:https://doi.org/10.1101/2020.04.28.066977.
- Koyama T, Weeraratne D, Snowdon JL, Parida L. Emergence of drift variants that may affect COVID-19 vaccine development and antibody treatment. Pathog (Basel, Switzerland). 2020;9(5). doi:https://doi.org/10.3390/pathogens9050324.
- Zhang L, Jackson CB, Mou H, Ojha A, Rangarajan E, Izard T, Farzan M, Choe H, et al. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv. 2020 Jan. doi:https://doi.org/10.1101/2020.06.12.148726.
- Weissman D, Alameh MG, de Silva T, Collini P, Hornsby H, Brown R, LaBranche CC, Edwards RJ, Sutherland L, Santra S, et al. D614G spike mutation increases SARS CoV-2 susceptibility to neutralization. medRxiv. 2020 Jan. doi:https://doi.org/10.1101/2020.07.22.20159905.
- Lee CY-P, Amrun SN, Chee RS-L, Goh Y S, Mak T-M, Octavia S, Yeo N K-W, Chang ZW, Tay MZ, Torres-Ruesta A, et al. Neutralizing antibodies from early cases of SARS-CoV-2 infection offer cross-protection against the SARS-CoV-2 D614G variant. bioRxiv. 2020 Jan. doi:https://doi.org/10.1101/2020.10.08.332544.
- Klingler J, Weiss S, Itri V, Liu, Xiaomei, Oguntuyo KY, Stevens C, Ikegame S, Hung C-T, Enyindah-Asonye G, Amanat F, et al. Role of IgM and IgA antibodies to the neutralization of SARS-CoV-2. medRxiv. 2020 Jan. doi:https://doi.org/10.1101/2020.08.18.20177303.
- Gasser R, Cloutier M, Prévost J, Fink C, Ducas E, Ding S, Dussault N, Landry P, Tremblay T, Laforce-Lavoie A, et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. bioRxiv. 2020 Jan. doi:https://doi.org/10.1101/2020.10.09.333278.
- Subbarao K, Mordant F, Rudraraju R. Convalescent plasma treatment for COVID-19: tempering expectations with the influenza experience. Eur J Immunol. 2020;50(10):1447–53. doi:https://doi.org/10.1002/eji.202048723.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam JS, Beck CR, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi:https://doi.org/10.1093/infdis/jiu396.
- Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, Gong L, Zhang Y, Cui H-Y, Geng J-J, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 Mar. doi:https://doi.org/10.1101/2020.03.14.988345.
