ABSTRACT
Early humoral immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are dominated by IgM and IgA antibodies, which greatly contribute to virus neutralization at mucosal sites. Given the essential roles of IgM and IgA in the control and elimination of SARS-CoV-2 infection, the mucosal immunity could be exploited for therapeutic and prophylactic purposes. However, almost all neutralizing antibodies that are authorized for emergency use and under clinical development are IgG antibodies, and no vaccine has been developed to boost mucosal immunity for SARS-CoV-2 infection. In addition to IgM and IgA, bispecific antibodies (bsAbs) combine specificities of two antibodies in one molecule, representing an important alternative to monoclonal antibody cocktails. Here, we summarize the latest advances in studies on IgM, IgA and bsAbs against SARS-CoV-2. The current challenges and future directions in vaccine design and antibody-based therapeutics are also discussed.
Introduction
Coronavirus disease-2019 (COVID-19) is a global threat induced by a newly emerged virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The rapid spread of COVID-19 not only prompts the development of effective vaccines at an unprecedented pace but also expedites the development of novel therapies, including therapeutic SARS-CoV-2-neutralizing antibodies and the reuse of existing antibodies approved for other indications.
Antibodies are a versatile and important component of the human immune system, of which the monoclonal antibody (mAb) represents a new frontier for the treatment of infectious diseases due to its specificity and potency. As predicted by William Haseltine, a biologist in Harvard, mAbs would be the first therapy specifically developed to target SARS-CoV-2.Citation1 To date, more than 10 mAbs have been granted Emergency Use Authorization (EUA) by the United States or approved by other countries to treat COVID-19, and over 70 mAbs are being evaluated in clinical trials in different therapeutic settings. These trials will be essential for the development of novel COVID-19 treatments in the very near future.
In patients with COVID-19, the severity of the disease correlates to high viral load in the respiratory tract, the primary site of SARS-CoV-2 infection and shedding.Citation2 Analysis of antibody responses has shown that SARS-CoV-2 induces specific antibodies mediated by three major immunoglobulin (Ig) isotypes, IgM, IgA, and IgG.Citation3,Citation4 Among them, specific IgM and IgA are the early antibody responses that start and peak within 7 days, whereas specific IgG antibodies develop more than a week (10–18 days) after infection and persist for months ().Citation4–6 However, almost all neutralizing mAbs in clinical use are the IgG isotype. No IgM or IgA mAbs are currently marketed. Moreover, these IgG mAbs are mostly administered via intravenous (i.v.) infusion. The concentration of IgG antibodies is 200–500 times lower in the lungs than in serum, highlighting that i.v. administration could not induce effective mucosal immune responses.Citation7 What is worse, many potent IgG mAbs, including those with EUAs and some in clinical trials, do not neutralize the emerging SARS-CoV-2 variants of concern (VOCs).Citation8–11 Thus, there is an urgent need for the development of more potent antibody-based therapies against the virus.
Figure 1. Antibody responses to SARS-CoV-2 infection. (a) Antibody responses of IgM, IgA, and IgG upon SARS-CoV-2 infection.Citation6 w, week. (b) The structures of pentameric IgM (PDB code 6KXS),Citation12 dimeric sIgA (PDB code 3CHN),Citation13 monomeric IgA (PDB code 1 R70),Citation14 and IgG (PDB code 1HZH)Citation15 are shown, with the specific domains in different colors. Fab, antigen-binding fragment; J-chain, joining chain; SC, secretory component.
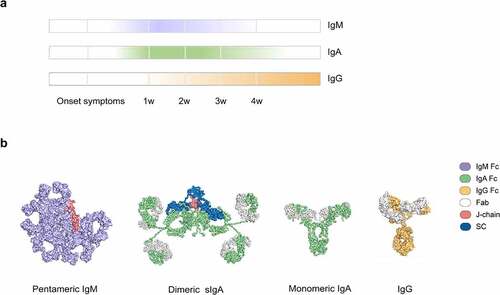
Upon SARS-CoV-2 infection, viruses first affect the upper respiratory tract. Therefore, the mucous membrane is the first line of immune system defense. IgM and IgA are mucosal antibodies in the early stages of immune response against mucosal pathogens. IgM typically assembles into pentamers that contain 10 antigen-binding sites and the joining chain (J-chain) (). The J-chain of pentameric IgM enables its binding to the polymeric Ig receptor (pIgR) on cells, allowing the transcytosis of IgM from the circulation to the mucosal surfaces.Citation16 In contrast, IgA exists in monomeric form (mIgA) in serum but is present as dimers (dIgA) at mucosal surface, termed secretory IgA (sIgA), which contains two IgA molecules with a J-chain and a secretory component (SC) (). In respiratory and gastrointestinal tracts, IgM and sIgA serve as the main mediator of mucosal immunity. These features make the intranasal delivery of IgM or IgA neutralizing antibodies feasible for the treatment of COVID-19. Meanwhile, these characteristics also raise questions as to whether SARS-CoV-2-induced IgM or IgA neutralizing antibodies exert more potent effects than IgG, and whether IgM or IgA neutralizing antibodies are superior to IgG in covering escape variants of SARS-CoV-2. If so, more data are needed to show how we can improve the current vaccines or develop novel immunization methods to boost early and mucosal immune response in COVID-19.
Given these considerations, we provide here an overview of IgM and IgA therapeutic antibodies for COVID-19, focusing on those that target SARS-CoV-2. In addition, we also summarize the anti-SARS-CoV-2 bispecific antibodies (bsAbs), which are an important alternative to monoclonal antibody cocktails.
SARS-CoV-2 and conventional IgG mAbs
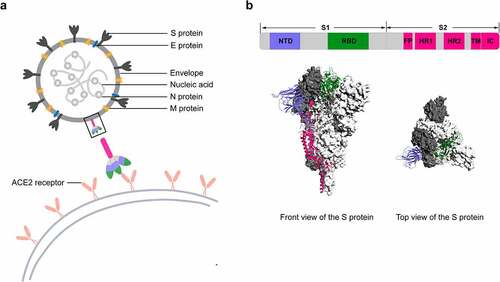
SARS-CoV-2 is an enveloped RNA virus that causes COVID-19, and the spike glycoprotein (S protein) on its surface is a transmembrane homotrimer and the target of neutralizing antibodies (). The S protein has two functional subunits (S1 and S2), of which the S1 subunit facilitates viral attachment to the surface of host cells. The S1 subunit further includes the N-terminal domain (NTD) and receptor-binding domain (RBD), which represent the key targets for neutralizing mAbs and potential therapies ().Citation17 Since the outbreak of the pandemic, neutralizing IgG mAbs against RBD or NTD have been the focus of investigation and development efforts. Of interest, all mAbs authorized or in clinical trials target the RBD, which interacts with the angiotensin-converting enzyme 2 (ACE2) receptor ().Citation18 While most mAbs recognize different epitopes fully or partially overlapping with the ACE2-binding sites, some mAbs target sites close or distal to the ACE2-binding sites. Although none of the NTD-directed mAbs are under clinical testing, the NTD is an essential and promising target for neutralizing mAbs.Citation8,Citation19–22 However, the neutralization mechanism of NTD-binding mAbs remains unclear. One possible mechanism is that the NTD-specific mAbs may neutralize SARS-CoV-2 by retraining the conformational changes of the S protein.Citation19 Another study suggested that the anti-NTD mAbs may inhibit SARS-CoV-2 infection at a post-attachment phase and block subsequent virus entry or fusion steps.Citation21
Figure 2. Schematic diagram of SARS-CoV-2 particle and the S protein.
Therapeutic IgG mAbs against SARS-CoV-2 and the existing antibodies against non-SARS-CoV-2 antigens in COVID-19 have been extensively discussed in several detailed reviews.Citation23–27 We thus do not focus on them here, but summarize all the therapeutic IgG antibodies for COVID-19 that we identified in , including their origin, development platform, target, features, and the current status of clinical trials. The targets are varied, and include SARS-CoV-2, cytokine and chemokine, and complement. Given the emergence of SARS-CoV-2 variants, we also summarize the neutralization of SARS-CoV-2 VOCs by the existing IgG antibodies with EUAs or in clinical development (). The summarized VOCs include B.1.1.7 (Alpha, first identified in the United Kingdom), B.1.351 (Beta, first identified in South Africa), P.1 (Gamma, first identified in Brazil), B.1.617.2 (Delta, first identified in India), B.1.617.1 (Kappa, first identified in India), and B.1.427/B.1.429 (Epsilon, first identified in USA), as well as two pseudoviruses containing multiple mutations. Although many existing mAbs are resistant to the emerging SARS-CoV-2 VOCs, a global consortium study recently provided a detailed epitope landscape on the SARS-CoV-2 S protein and offered a framework for selecting antibody treatment.Citation115 The result of this effort not only helps us understand how viral variants might affect antibody-based therapeutics but also guides both treatment and prevention.
Table 1. Overview of IgG antibodies evaluated as possible COVID-19 treatments
Table 2. Neutralization of SARS-CoV-2 variants by the IgG antibodies with EUA or in clinical development
Therapeutic IgM antibodies against SARS-CoV-2
So far, specific IgM antibodies have been largely developed for SARS-CoV-2 serological testing. Thus, the investigation of therapeutic IgM antibodies against SARS-CoV-2 is very limited. In previous studies, reduced IgM levels have been observed in patients with severe pandemic influenza.Citation116 As a result, the treatment with IgM-enriched preparations has emerged. Indeed, the clinical trials that evaluate the passive immunotherapy with COVID-19 convalescent plasma (CCP) have rapidly grown owing to the absence of specific antiviral therapy. In CCP, specific antibodies (IgG/IgM/IgA) against SARS-CoV-2 are regarded as active components, since all isotypes display neutralizing activities.Citation117 However, numerous non-antibody proteins and chemical factors in CCP may drive detrimental outcomes in patients.Citation118 CCP therapy also raises a flurry of ethical questions.Citation119 As such, the quality, efficacy and safety of CCP against COVID-19 need to be further investigated and determined.
Instead of CCP, the preparation of polyvalent antibody for COVID-19 is another therapeutic choice. Trimodulin, a polyvalent antibody preparation derived from human plasma, contains IgM (~23%), IgA (~21%) and IgG (~56%).Citation120 In COVID-19 cell models, addition of trimodulin reduced inflammation and induced stronger immunomodulation compared to intravenous Ig preparation (IVIG).Citation121 Hence, trimodulin is currently being tested in a Phase 2 clinical trial for COVID-19 (NCT04576728) (). Nonetheless, the IgM component of trimodulin is of minor importance for Fc receptor (FcR)-mediated effector functions, so the beneficial immunomodulatory effects of trimodulin might be attributed to the IgA component, a neglected but critical part of SARS-CoV-2 infectionCitation121 discussed in the following section.
Table 3. Overview of IgM and IgA antibodies against SARS-CoV-2
In addition to the polyvalent antibody preparation, recombinant mAbs of IgG, IgM and IgA isotypes sharing the same antigen-binding fragment (Fab) against S protein were developed.Citation126 Remarkably, the neutralizing ability of IgM and IgA mAbs was dramatically higher than IgG mAbs, suggesting a strategy for developing effective therapies of IgM and IgA instead of IgG for COVID-19.Citation126 One explanation for the efficient neutralization conferred by IgM and IgA might be their capacity to bind multiple virions. Recently, an elegant work reported six engineered IgM antibodies that exhibit higher binding and neutralizing activities than their parental IgG1 antibodies. Among them, one IgM antibody (IgM-14), engineered from a previously isolated mAb (CoV2-14) by phage display,Citation70 showed over 230-fold potency in neutralizing SARS-CoV-2 compared to its corresponding IgG version (IgG-14) (). Strikingly, IgM-14 was more potent than IgG-14 in neutralizing SARS-CoV-2 VOCs, including Alpha, Beta and Gamma variants, as well as 21 other RBD mutants, indicating that IgM-14 is superior to IgG-14 in covering viral escape mutations. In mice, IgM-14 not only conferred potent therapeutic protection against different variants but also displayed desirable pharmacokinetics and safety profiles when administered intranasally.Citation122 Therefore, two Phase 1 clinical trials of IgM-14 (also known as IGM-6268) were started very recently in healthy volunteers and patients with mild-to-moderate COVID-19.
Therapeutic IgA antibodies against SARS-CoV-2
Mucosal immune system is by far the largest component of the entire human immune system. Most viruses invade via mucosal sites (e.g., respiratory tracts) where sIgA plays an important role. For years, sIgA has been described as the predominant antibody and the first barrier against pathogens at mucosal sites. Importantly, IgA has been shown to exert neutralizing activities on multiple viruses, such as human immunodeficiency virus (HIV),Citation127 and influenza virus.Citation128 In addition, IgA also contributes to virus neutralization to a greater extent than IgG in COVID-19, and the neutralizing IgA remains detectable in saliva for a longer time,Citation129 suggesting a critical role of IgA during the early phase of SARS-CoV-2 infection.
It should be noted that the circulating IgA, even in polymeric form, cannot have the same protective effect as mucosal sIgA to limit infections. Indeed, dIgA derived from COVID-19 convalescent donors is more potent than mIgA and the corresponding IgG against the same target,Citation123 suggesting that dIgA is a more potent neutralizer than IgG. The same holds true in another in vitro setting ().Citation124 Nevertheless, more studies are urgently required to assess the safety and therapeutic effects of IgA-enriched products in preventing SARS-CoV-2 infection.
In 2020, the first human IgA mAb against SARS-CoV-2, named mAb362, was developed.Citation125 In particular, mAb362 showed cross-reactivity against the RBD of both SARS-CoV-1 and SARS-CoV-2, and competitively blocked ACE2 receptor binding. Notably, mAb362 as mIgA, dIgA and sIgA showed significantly enhanced potency in neutralizing SARS-CoV-2 pseudovirus compared to the IgG isotype. The most potent mAb362 sIgA also neutralized authentic SARS-CoV-2, whereas the IgG isotype did not, indicating effective mucosal immunity of sIgA antibodies against SARS-CoV-2 (). Interestingly, in patients with Selective IgA Deficiency (SID), the lack of neutralizing anti-SARS-CoV-2 IgA and sIgA antibodies represents a possible cause of COVID-19 severity, vaccine failure and prolonged viral shedding,Citation130 emphasizing the importance of IgA antibodies in mucosal immune responses upon SARS-CoV-2 infection.
Therapeutic bsAbs against SARS-CoV-2
Combining multiple IgG mAbs has been known to have a synergistic effect on neutralizing SARS-CoV-2 by targeting different epitopes of the RBD. For example, the combination of casirivimab and imdevimab has been granted EUA to treat mild-to-moderate symptoms of COVID-19 in high-risk patients. However, effects similar to those of mAb combinations can be achieved by a single bsAb, which have two distinct specificities and may have reduced time-consuming and expensive development (), as well as increased potency due to enhanced functional affinity. Use of bsAb may also decrease the likelihood of viral escape.Citation135,Citation136
Figure 3. Structural models of bsAb formats used in SARS-CoV-2 infection.
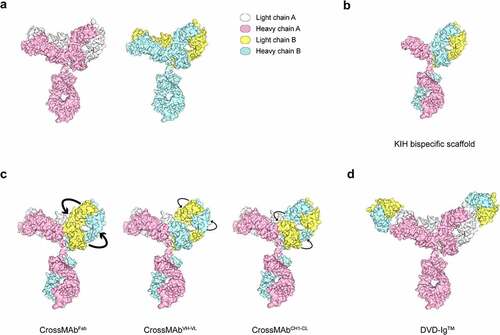
The first bsAb against SARS-CoV-2 was constructed by linking non-neutralizing binders to neutralizing binders in a bispecific scaffold.Citation132 Specifically, the authors first identified Fabs that bind to the RBD but do not block ACE2 binding by phage display, and then they assembled them into a knob-in-hole (KIH) bispecific IgG scaffold with human-derived variable heavy (VH) binders that block ACE2, resulting in a VH/Fab bsAb (). Remarkably, these bsAbs showed 20- to 25-fold more potency in neutralizing pseudotyped and authentic SARS-CoV-2 than the mono-specific bivalent VH-Fc or IgG alone or even as a cocktail. The study was an attempt to target multiple epitopes, both neutralizing and non-neutralizing, within a single therapeutic molecule, providing a promising and rapid engineering strategy to improve the potency of SARS-CoV-2 antibodies.
Soon afterward, another study reported a human bispecific IgG1-like molecule CoV-X2 in a CrossMAb format () on the basis of two neutralizing mAbs (C121 and C135) derived from convalescent COVID-19 patients.Citation38 CoV-X2 could simultaneously bind two non-overlapping RBD epitopes, and showed a broader coverage of SARS-CoV-2 variants, including the escape mutants generated by the parental mAbs; in a mouse model, CoV-X2 also protected mice from disease and suppressed viral escape.Citation135
Very recently, Cho et al. reported five ultrapotent DVD-Ig bsAbs () by combining non-overlapping specificities.Citation136 Of all the bsAbs that could neutralize authentic SARS-CoV-2, one bsAb, CV1206_521_GS, neutralized SARS-CoV-2 with more than 100-fold higher potency than a cocktail of its constituent antibodies. Further analysis revealed that CV1206_521_GS crosslinked NTD and RBD in adjacent S proteins, a mode of action that is unavailable to conventional mAbs even when used in combination. In addition, two other bsAbs showed the ability to neutralize SARS-CoV-2 VOCs, including Alpha, Beta, Gamma and Delta variants, at near wild-type potency. More importantly, one potent bsAb was effective against SARS-CoV-2 carrying a key variant mutation of E484K in the hamster model.Citation136 This finding provided a novel design of bsAb by targeting different epitopes to improve the potency in neutralizing SARS-CoV-2 variants.
Although antibody cocktails that target different regions of the S protein are still the main format for the treatment of SARS-CoV-2, the newly explored bsAbs can exert potent effects via distinct mechanisms of action that cannot be achieved by conventional mAbs. The details of the design and format of the above bsAbs are summarized in .
Table 4. Overview of bsAbs against SARS-CoV-2
Challenges and future perspectives
The COVID-19 pandemic has caused unprecedented health and economic crises worldwide. Historically, it has also triggered unprecedented efforts to develop vaccines and efficacious treatments for the disease. Although several COVID-19 vaccines are being used, all of them are administered intramuscularly or subcutaneously, which might not always induce an effective mucosal immune response.Citation137–139 So far, no vaccine to boost mucosal immunity has been developed for SARS-CoV-2 infection. Therefore, the current challenge in vaccine design is to induce long-lasting systemic and mucosal protection against all SARS-CoV-2 variants, and the same is true for antibody-based therapies. In this case, intranasal administration of selected high-affinity poly-reactive IgM or sIgA might be a promising approach for COVID-19.
Traditionally, IgM antibodies have proven difficult to express and purify due to their large size and complexity. Thanks to advances in manufacturing, engineered IgM antibodies such as IgM-1433 can be produced with good quality, and it will be administered by intranasal and intraoral spray in clinical trials. In fact, several engineered IgM antibodies are being investigated in oncology clinical trials, and more than half of these IgM target antigens that are poorly immunogenic, which makes it difficult to generate IgG mAbs.Citation16 However, multivalent antibodies, like IgM, might have an off-target effects, resulting in low affinity, less specificity and unexpected toxicities. Nonetheless, the use of IgM is anticipated as an essential approach to defend against complex pathogen infections, especially viruses that are difficult to target.
In addition to IgM, specific IgA response has been considered for vaccine design since the 1960s. The rotavirus vaccine is recognized as a model system for the therapeutic potential of intestinal IgA in digestive viral infections.Citation140 Another example is the oral poliovirus vaccine, which induces strong specific IgA responses to neutralize distinct serotypes.Citation141 Apart from an oral route, nasal administration is another strategy to induce sIgA in respiratory tracts. For example, intranasal administration of influenza vaccines induces strong IgA responses in nasal mucus, which correlate with vaccine efficacy.Citation142,Citation143 A very recent study also reported a single intranasal dose of SARS-CoV-2 vaccine candidate that induces potent IgA responses in hamsters.Citation144 Although vaccine-induced IgA responses have been largely considered, the development of neutralizing IgA antibodies in preventing viral infections is very limited compared to IgG mAbs. It is noteworthy that IgA antibodies have been reported to have anti-inflammatory roles by inhibiting complement activation mediated by IgM or IgG. In this case, intranasal immunization should be an effective means to generate sIgA responses in respiratory tracts where SARS-CoV-2 could be eliminated without inducing dysregulated inflammatory consequences.
Last, but not least, exploration of novel engineered bsAbs may offer great potential as a versatile alternative to conventional mAbs. In addition, single-domain antibodies (sdAbs) derived from variable heavy homodimer (VHH) domains of antibodies in camels or llamas will become a trend for the next-generation of antibody-based therapeutics in the future. sdAbs are typically a peptide consisting of only heavy chains that retain the full antigen-binding capacity as conventional antibodies.Citation145 The small size (~15 kDa) of sdAbs allows them to reach antigens that conventional mAbs cannot.Citation146 Other benefits of sdAbs include flexible formatting, rapid and low-cost development, high production efficiency, and easy administration via nebulized inhalation.Citation146,Citation147 Although the small size of sdAbs leads to a rapid renal clearance, strategies to extend their half-life, such as conjugation to the Fc domain of a conventional antibody, have been used.Citation146 Humanized sdAbs targeting the RBD exhibit potent neutralization activity against both pseudotyped and authentic SARS-CoV-2, and fusion of the human IgG1 Fc to sdAbs further improves their neutralization activity by up to 10 times.Citation148 Reformatting sdAbs into multivalent constructsCitation149 or a bispecific formatCitation150 makes them more potent to broadly neutralize SARS-CoV-2 variants. In this regard, sdAb represents a promising therapeutic agent for passive immunization against SARS-CoV-2.
In summary, a comprehensive understanding of all immune processes involved in SARS-CoV-2 infection will be required to fully control the pandemic. Future vaccine development should aim at inducing rapid and mucosal immune responses via different routes of administration, including but not limited to intranasal delivery, which may achieve desirable results beyond those with conventional vaccine administrations. In terms of antibody-based therapeutics, efforts should be made to develop IgM and IgA antibodies, as well as engineered bsAbs or cross-isotype moleculesCitation151 against SARS-CoV-2.
Abbreviations
ACE2, angiotensin-converting enzyme 2; bsAb(s), bispecific antibody(ies); CCP, COVID-19 convalescent plasma; CDR, complementarity-determining region; CH, constant heavy; CL, constant light; COVID-19, coronavirus disease 2019; dIgA, dimeric IgA; EUA, Emergency Use Authorization; Fab(s), antigen-binding fragment(s); FcR, Fc receptor; HIV, human immunodeficiency virusIg, immunoglobulin; i.v., intravenous; IVIG, intravenous Ig preparation; J-chain, joining chain; KIH, knob-in-holem; Ab, monoclonal antibody; mIgA, monomeric IgA; NTD, N-terminal domain; PDB, protein data bank; pIgR, polymeric Ig receptor; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SC, secretory componentsd; Abs, single-domain antibodies; SID, Selective IgA Deficiency; sIgA, secretory IgAS protein, spike glycoprotein; VH, variable heavy; VHH, variable heavy homodimer; VL, variable light; VOCs, variants of concern
Contributions
JZ and HZ wrote the paper and made the figures and tables. HZ and LS designed the review and made corrections.
Acknowledgments
This work was supported by the Shenzhen Science and Technology Innovation Commission (No. JCYJ20190807155011406, KQTD20180411143323605, JSGG20200225152008136) given to LS; and the Program of Medical Discipline Leader in Yunnan Health System (No. D-2019027) to HZ.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Simoneaux R, S SL. Monoclonal antibody therapies in COVID-19. ASA Monitor. 2021;85:1–17. 10.1097/01.ASM.0000742616.84770.00.
- Pujadas E, Chaudhry F, McBride R, Richter F, Zhao S, Wajnberg A, Nadkarni G, Glicksberg BS, Houldsworth J, Cordon-Cardo C. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020 Sep;8(9):e70. PMID: 32771081. doi:10.1016/S2213-2600(20)30354-4.
- Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai XF, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020 Jun;26(6):845–48. 10.1038/s41591-020-0897-1: PMID: 32350462
- Nuccetelli M, Pieri M, Gisone F, Bernardini S. Combined anti-SARS-CoV-2 IgA, IgG, and IgM detection as a better strategy to prevent second infection spreading waves. Immunol Invest. 2020 Sep;18:1–13. PMID: 32945214. doi:10.1080/08820139.2020.1823407.
- Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020 Dec 4;370(6521):1227–30. 10.1126/science.abd7728. PMID: 33115920.
- Guo L, Ren L, Yang S, Xiao M, Yang CF, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Xiao Y, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020 Jul 28;71(15):778–85. 10.1093/cid/ciaa310. PMID: 32198501.
- DeFrancesco L. COVID-19 antibodies on trial. Nat Biotechnol. 2020 Nov;38(11):1242–52. PMID: 33087898. doi:10.1038/s41587-020-0732-8.
- Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021 May;593(7857):130–35. 10.1038/s41586-021-03398-2: PMID: 33684923
- McCallum M, Bassi J, De Marco A, Chen A, Walls AC, Di Iulio J, Tortorici MA, Navarro MJ, Silacci-Fregni C, Saliba C, et al. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science. 2021 Aug 6;373(6555):648–54. 10.1126/science.abi7994. PMID: 34210893.
- Chen RE, Winkler ES, Case JB, Aziati ID, Bricker TL, Joshi A, Darling TL, Ying B, Errico JM, Shrihari S, et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature. 2021 Aug;596(7870):103–08. 10.1038/s41586-021-03720-y: PMID: 34153975
- Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid MA, Agostini ML, Guarino B, Di Iulio J, Rosen LE, Tucker H, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv, Preprint. August 6, 2021. 10.1101/2021.03.09.434607
- Perkins SJ, Nealis AS, Sutton BJ, Feinstein A. Solution structure of human and mouse immunoglobulin M by synchrotron X-ray scattering and molecular graphics modelling. A possible mechanism for complement activation. J Mol Biol. 1991 Oct 20;221(4):1345–66. doi:10.1016/0022-2836(91)90937-2. PMID: 1942055.
- Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. Location of secretory component on the Fc edge of dimeric IgA1 reveals insight into the role of secretory IgA1 in mucosal immunity. Mucosal Immunol. 2009 Jan;2(1):74–84. PMID: 19079336. doi:10.1038/mi.2008.68.
- Furtado PB, Whitty PW, Robertson A, Eaton JT, Almogren A, Kerr MA, Woof JM, Perkins SJ. Solution structure determination of monomeric human IgA2 by X-ray and neutron scattering, analytical ultracentrifugation and constrained modelling: a comparison with monomeric human IgA1. J Mol Biol. 2004 May 14;338(5):921–41. doi:10.1016/j.jmb.2004.03.007. PMID: 15111057.
- Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001 Aug 10;293(5532):1155–59. doi:10.1126/science.1061692. PMID: 11498595.
- Keyt BA, Baliga R, Sinclair AM, Carroll SF, Peterson MS. Structure function, and therapeutic use of IgM antibodies. Antibodies (Basel). 2020 Oct 13;9(4). 10.3390/antib9040053. PMID: 33066119.
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 Mar 13;367(6483):1260–63. doi:10.1126/science.abb2507. PMID: 32075877.
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr 16;181(2):271–280 e8. 10.1016/j.cell.2020.02.052. PMID: 32142651.
- Chi X, Yan R, Zhang J, Zhang G, Zhang Y, Hao M, Zhang Z, Fan P, Dong Y, Yang Y, et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020 Aug 7;369(6504):650–55. 10.1126/science.abc6952. PMID: 32571838.
- Liu L, Wang P, Nair MS, Yu J, Rapp M, Wang Q, Luo Y, Chan JF, Sahi V, Figueroa A, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020 Aug;584(7821):450–56. 10.1038/s41586-020-2571-7: PMID: 32698192
- Suryadevara N, Shrihari S, Gilchuk P, VanBlargan LA, Binshtein E, Zost SJ, Nargi RS, Sutton RE, Winkler ES, Chen EC, et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell. 2021 Apr 29;184(9):2316–2331 e15. 10.1016/j.cell.2021.03.029. PMID: 33773105.
- Voss WN, Hou YJ, Johnson NV, Delidakis G, Kim JE, Javanmardi K, Horton AP, Bartzoka F, Paresi CJ, Tanno Y, et al. Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science. 2021 Jun 4;372(6546):1108–12. 10.1126/science.abg5268. PMID: 33947773.
- Yu F, Xiang R, Deng X, Wang L, Yu Z, Tian S, Liang R, Li Y, Ying T, Jiang S. Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2. Signal Transduct Target Ther. 2020 Sep 22;5(1):212. doi:10.1038/s41392-020-00318-0. PMID: 32963228.
- Carrillo J, Izquierdo-Useros N, Avila-Nieto C, Pradenas E, Clotet B, Blanco J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem Biophys Res Commun. 2021 Jan 29;538. 187–91. 10.1016/j.bbrc.2020.10.108. PMID: 33187644
- Pum A, Ennemoser M, Adage T, Kungl AJ. Cytokines and chemokines in sars-COV-2 infections-therapeutic strategies targeting cytokine storm. Biomolecules. 2021 Jan 12;11(1):91. doi:10.3390/biom11010091. PMID: 33445810.
- Bayat M, Asemani Y, Mohammadi MR, Sanaei M, Namvarpour M, Eftekhari R. An overview of some potential immunotherapeutic options against COVID-19. Int Immunopharmacol. 2021 Jun;95:107516. PMID: 33765610. doi:10.1016/j.intimp.2021.107516.
- Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021 Jun 10;184(12):3086–108. doi:10.1016/j.cell.2021.05.005. PMID: 34087172.
- Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, Jaconi S, Culap K, Zatta F, De Marco A, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020 Jul;583(7815):290–95. 10.1038/s41586-020-2349-y: PMID: 32422645
- Tuccori M, Ferraro S, Convertino I, Cappello E, Valdiserra G, Blandizzi C, Maggi F, Focosi D. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline. MAbs. 2020 Jan-Dec;12(1):1854149. PMID: 33319649. doi:10.1080/19420862.2020.1854149.
- Hansen J, Baum A, Pascal KE, Russo V, Giordano S, Wloga E, Fulton BO, Yan Y, Koon K, Patel K, et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020 Aug 21;369(6506):1010–14. 10.1126/science.abd0827. PMID: 32540901.
- Jones BE, Brown-Augsburger PL, Corbett KS, Westendorf K, Davies J, Cujec TP, Wiethoff CM, Blackbourne JL, Heinz BA, Foster D, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021 May 12;13(593). 10.1126/scitranslmed.abf1906. PMID: 33820835.
- Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B, et al. Bamlanivimab plus Etesevimab in mild or moderate COVID-19. N Engl J Med. 2021 Jul 14;385(15):1382–92. 10.1056/NEJMoa2102685. PMID: 34260849
- Zost SJ, Gilchuk P, Case JB, Binshtein E, Chen RE, Nkolola JP, Schafer A, Reidy JX, Trivette A, Nargi RS, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020 Aug;584(7821):443–49. 10.1038/s41586-020-2548-6: PMID: 32668443
- Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, Yu J, Shan S, Zhou B, Song S, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 Aug;584(7819):115–19. 10.1038/s41586-020-2380-z: PMID: 32454513
- Wang R, Zhang Q, Ge J, Ren W, Zhang R, Lan J, Ju B, Su B, Yu F, Chen P, et al. Analysis of SARS-CoV-2 variant mutations reveals neutralization escape mechanisms and the ability to use ACE2 receptors from additional species. Immunity. 2021 Jul 13;54(7):1611–1621 e5. 10.1016/j.immuni.2021.06.003. PMID: 34166623.
- Kim C, Ryu DK, Lee J, Kim YI, Seo JM, Kim YG, Jeong JH, Kim M, Kim JI, Kim P, et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021 Jan 12;12(1):288. 10.1038/s41467-020-20602-5. PMID: 33436577.
- Lv Z, Deng YQ, Ye Q, Cao L, Sun CY, Fan C, Huang W, Sun S, Sun Y, Zhu L, et al. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020 Sep 18;369(6510):1505–09. PMID: 32703908. doi:10.1126/science.abc5881
- Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, Agudelo M, Barnes CO, Gazumyan A, Finkin S, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 Aug;584(7821):437–42. 10.1038/s41586-020-2456-9: PMID: 32555388
- Schafer A, Muecksch F, Lorenzi JCC, Leist SR, Cipolla M, Bournazos S, Schmidt F, Maison RM, Gazumyan A, Martinez DR, et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J Exp Med. 2021 Mar 1;218(3). 10.1084/jem.20201993. PMID: 33211088.
- Andreano E, Nicastri E, Paciello I, Pileri P, Manganaro N, Piccini G, Manenti A, Pantano E, Kabanova A, Troisi M, et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell. 2021 Apr 1;184(7):1821–1835 e16. 10.1016/j.cell.2021.02.035. PMID: 33667349.
- Lanini S, Milleri S, Andreano E, Nosari S, Paciello I, Piccini G, Gentili A. A single intramuscular injection of monoclonal antibody MAD0004J08 induces in healthy adults SARS-CoV-2 neutralising antibody titres exceeding those induced by infection and vaccination. medRxiv. August, 04, 2021. preprint 10.1101/2021.08.03.21261441
- Ning L, Abagna HB, Jiang Q, Liu S, Huang J. Development and application of therapeutic antibodies against COVID-19. Int J Biol Sci. 2021;17(6):1486–96. doi:10.7150/ijbs.59149. PMID: 33907512
- Zylberman V, Sanguineti S, Pontoriero AV, Higa SV, Cerutti ML, Morrone Seijo SM, Pardo R, Munoz L, Acuna Intrieri ME, Alzogaray VA, et al. Development of a hyperimmune equine serum therapy for COVID-19 in Argentina. Medicina (B Aires). 2020;80(Suppl 3):1–6. PMID: 32658841.
- Lopardo G, Belloso WH, Nannini E, Colonna M, Sanguineti S, Zylberman V, Munoz L, Dobarro M, Lebersztein G, Farina J, et al. RBD-specific polyclonal F(ab)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: a randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. EClinicalMedicine. 2021 Apr;34:100843. doi:10.1016/j.eclinm.2021.100843. PMID: 33870149.
- Vanhove B, Duvaux O, Rousse J, Royer PJ, Evanno G, Ciron C, Lheriteau E, Vacher L, Gervois N, Oger R, et al. High neutralizing potency of swine glyco-humanized polyclonal antibodies against SARS-CoV-2. Eur J Immunol. 2021 Jun;51(6):1412–22. 10.1002/eji.202049072: PMID: 33576494
- Yang L, Liu W, Yu X, Wu M, Reichert JM, Ho M. COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antib Ther. 2020 Jul;3(3):205–12. PMID: 33215063. doi:10.1093/abt/tbaa020.
- Bertoglio F, Fuhner V, Ruschig M, Heine PA, Abassi L, Klunemann T, Rand U, Meier D, Langreder N, Steinke S, et al. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021 Jul 27;36(4):109433. 10.1016/j.celrep.2021.109433. PMID: 34273271.
- Wang S, Peng Y, Wang R, Jiao S, Wang M, Huang W, Shan C, Jiang W, Li Z, Gu C, et al. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat Commun. 2020 Nov 13;11(1):5752. 10.1038/s41467-020-19568-1. PMID: 33188207.
- Meng X, Wang P, Xiong Y, Wu Y, Lin X, Lu S, Li R, Zhao B, Liu J, Zeng S, et al. Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: a Phase 1 clinical study of the SARS-CoV-2 RBD-targeting monoclonal antibody. Emerg Microbes Infect. 2021 Dec;10(1):1638–48. 10.1080/22221751.2021.1960900: PMID: 34346827
- Westendorf K, Zentelis S, Foster D, Vaillancourt P, Wiggin M, Lovett E, Hendle J, Pustilnik A, Sauder JM, Kraft L, et al. LY-CoV1404 potently neutralizes SARS-CoV-2 variants. bioRxiv. 2021 May 4. 10.1101/2021.04.30.442182. PMID: 33972947.
- Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus A, van Kuppeveld FJM, Haagmans BL, Grosveld F, Bosch BJ. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020 May 4;11(1):2251. doi:10.1038/s41467-020-16256-y. PMID: 32366817.
- Alsoussi WB, Turner JS, Case JB, Zhao H, Schmitz AJ, Zhou JQ, Chen RE, Lei T, Rizk AA, McIntire KM, et al. a potently neutralizing antibody protects mice against SARS-CoV-2 infection. J Immunol. 2020 Aug 15;205(4):915–22. 10.4049/jimmunol.2000583. PMID: 32591393.
- Guo Y, Huang L, Zhang G, Yao Y, Zhou H, Shen S, Shen B, Li B, Li X, Zhang Q, et al. A SARS-CoV-2 neutralizing antibody with extensive Spike binding coverage and modified for optimal therapeutic outcomes. Nat Commun. 2021 May 11;12(1):2623. 10.1038/s41467-021-22926-2. PMID: 33976198.
- Liu Z, Wu H, Egland KA, Gilliland TC, Dunn MD, Luke TC, Sullivan EJ, Klimstra WB, Bausch CL, Whelan SPJ. Human immunoglobulin from transchromosomic bovines hyperimmunized with SARS-CoV-2 spike antigen efficiently neutralizes viral variants. Hum Vaccin Immunother. 2021 Jul;6:1–10. PMID: 34228597. doi:10.1080/21645515.2021.1940652.
- Song D, Wang W, Dong C, Ning Z, Liu X, Liu C, Du G, Sha C, Wang K, Lu J, et al. Structure and function analysis of a potent human neutralizing antibody CA521(FALA) against SARS-CoV-2. Commun Biol. 2021 Apr 23;4(1):500. 10.1038/s42003-021-02029-w. PMID: 33893388.
- Zhang Q, Zhou R, Yang J, Dou C, Gan T, Liu F, Hu B, Song D, Lu C, Hu W. A randomized, double-blind, placebo-controlled, first-in-human clinical trial to assess safety, tolerability, and pharmacokinetics of ly-covmab, a potent human neutralizing antibody against SARS-CoV-2. Infect Dis Ther. 2021 Dec 8. doi: 10.1007/s40121-021-00572-x. PMID: 34878625.
- Gu C, Cao X, Wang Z, Hu X, Yao Y, Zhou Y, Liu P, Liu X, Gao G, Hu X, et al. A human antibody of potent efficacy against SARS-CoV-2 in rhesus macaques showed strong blocking activity to B.1.351. MAbs. 2021 Jan-Dec;13(1):1930636. 10.1080/19420862.2021.1930636: PMID: 34097570
- Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9(1):382–85. PMID: 32065055. doi:10.1080/22221751.2020.1729069.
- Zeng X, Li L, Lin J, Li X, Liu B, Kong Y, Zeng S, Du J, Xiao H, Zhang T, et al. Isolation of a human monoclonal antibody specific for the receptor binding domain of SARS-CoV-2 using a competitive phage biopanning strategy. Antib Ther. 2020 Apr;3(2):95–100. 10.1093/abt/tbaa008: PMID: 33912790
- Chen X, Li R, Pan Z, Qian C, Yang Y, You R, Zhao J, Liu P, Gao L, Li Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020 Jun;17(6):647–49. 10.1038/s41423-020-0426-7: PMID: 32313207
- Wu Y, Wang F, Shen C, Peng W, Li D, Zhao C, Li Z, Li S, Bi Y, Yang Y, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020 Jun 12;368(6496):1274–78. 10.1126/science.abc2241. PMID: 32404477.
- Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L, Zhu Q, Zhang X, Zheng Y, Geng C, et al. Potent neutralizing antibodies against sars-COV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020 Jul 9;182(1):73–84 e16. 10.1016/j.cell.2020.05.025. PMID: 32425270.
- Shi R, Shan C, Duan X, Chen Z, Liu P, Song J, Song T, Bi X, Han C, Wu L, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020 Aug;584(7819):120–24. 10.1038/s41586-020-2381-y: PMID: 32454512
- Wec AZ, Wrapp D, Herbert AS, Maurer DP, Haslwanter D, Sakharkar M, Jangra RK, Dieterle ME, Lilov A, Huang D, et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020 Aug 7;369(6504):731–36. 10.1126/science.abc7424. PMID: 32540900.
- Brouwer PJM, Caniels TG, van der Straten K, Snitselaar JL, Aldon Y, Bangaru S, Torres JL, Okba NMA, Claireaux M, Kerster G, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020 Aug 7;369(6504):643–50. 10.1126/science.abc5902. PMID: 32540902.
- Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He WT, Limbo O, Smith C, Song G, Woehl J, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020 Aug 21;369(6506):956–63. 10.1126/science.abc7520. PMID: 32540903.
- Zhou D, Duyvesteyn HME, Chen CP, Huang CG, Chen TH, Shih SR, Lin YC, Cheng CY, Cheng SH, Huang YC, et al. Structural basis for the neutralization of SARS-CoV-2 by an antibody from a convalescent patient. Nat Struct Mol Biol. 2020 Oct;27(10):950–58. 10.1038/s41594-020-0480-y: PMID: 32737466
- Tortorici MA, Beltramello M, Lempp FA, Pinto D, Dang HV, Rosen LE, McCallum M, Bowen J, Minola A, Jaconi S, et al. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science. 2020 Nov 20;370(6519):950–57. 10.1126/science.abe3354. PMID: 32972994.
- Li W, Chen C, Drelich A, Martinez DR, Gralinski LE, Sun Z, Schafer A, Kulkarni SS, Liu X, Leist SR, et al. Rapid identification of a human antibody with high prophylactic and therapeutic efficacy in three animal models of SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29832–38. 10.1073/pnas.2010197117. PMID: 33139569.
- Ku Z, Xie X, Davidson E, Ye X, Su H, Menachery VD, Li Y, Yuan Z, Zhang X, Muruato AE, et al. Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape. Nat Commun. 2021 Jan 20;12(1):469. 10.1038/s41467-020-20789-7. PMID: 33473140.
- Rappazzo CG, Tse LV, Kaku CI, Wrapp D, Sakharkar M, Huang D, Deveau LM, Yockachonis TJ, Herbert AS, Battles MB, et al. Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science. 2021 Feb 19;371(6531):823–29. 10.1126/science.abf4830. PMID: 33495307.
- Halwe S, Kupke A, Vanshylla K, Liberta F, Gruell H, Zehner M, Rohde C, Krahling V, Gellhorn Serra M, Kreer C, et al. Intranasal administration of a monoclonal neutralizing antibody protects mice against SARS-CoV-2 infection. Viruses. 2021 Jul 29;13(8):1498. 10.3390/v13081498. PMID: 34452363.
- Kaku Y, Kuwata T, Zahid HM, Hashiguchi T, Noda T, Kuramoto N, Biswas S, Matsumoto K, Shimizu M, Kawanami Y, et al. Resistance of SARS-CoV-2 variants to neutralization by antibodies induced in convalescent patients with COVID-19. Cell Rep. 2021 Jul 13;36(2):109385. 10.1016/j.celrep.2021.109385. PMID: 34237284.
- Du Y, Shi R, Zhang Y, Duan X, Li L, Zhang J, Wang F, Zhang R, Shen H, Wang Y, et al. A broadly neutralizing humanized ACE2-targeting antibody against SARS-CoV-2 variants. Nat Commun. 2021 Aug 17;12(1):5000. 10.1038/s41467-021-25331-x. PMID: 34404805.
- Dussupt V, Sankhala RS, Mendez-Rivera L, Townsley SM, Schmidt F, Wieczorek L, Lal KG, Donofrio GC, Tran U, Jackson ND, et al. Low-dose in vivo protection and neutralization across SARS-CoV-2 variants by monoclonal antibody combinations. Nat Immunol. 2021 Oct 29;22(12):1503–14. 10.1038/s41590-021-01068-z. PMID: 34716452
- Banach BB, Cerutti G, Fahad AS, Shen CH, Oliveira De Souza M, Katsamba PS, Tsybovsky Y, Wang P, Nair MS, Huang Y, et al. Paired heavy- and light-chain signatures contribute to potent SARS-CoV-2 neutralization in public antibody responses. Cell Rep. 2021 Oct 5;37(1):109771. 10.1016/j.celrep.2021.109771. PMID: 34587480.
- VanBlargan LA, Adams LJ, Liu Z, Chen RE, Gilchuk P, Raju S, Smith BK, Zhao H, Case JB, Winkler ES, et al. A potently neutralizing SARS-CoV-2 antibody inhibits variants of concern by utilizing unique binding residues in a highly conserved epitope. Immunity. 2021 Oct 12;54(10):2399–2416 e6. 10.1016/j.immuni.2021.08.016. PMID: 34481543.
- Wang P, Casner RG, Nair MS, Yu J, Guo Y, Wang M, Chan JF, Cerutti G, Iketani S, Liu L, et al. A monoclonal antibody that neutralizes SARS-CoV-2 variants, SARS-CoV, and other sarbecoviruses. Emerg Microbes Infect. 2021 Nov;26:1–34. doi:10.1080/22221751.2021.2011623. PMID: 34836485.
- Li T, Han X, Gu C, Guo H, Zhang H, Wang Y, Hu C, Wang K, Liu F, Luo F, et al. Potent SARS-CoV-2 neutralizing antibodies with protective efficacy against newly emerged mutational variants. Nat Commun. 2021 Nov 2;12(1):6304. 10.1038/s41467-021-26539-7. PMID: 34728625.
- Jiang W, Wang J, Jiao S, Gu C, Xu W, Chen B, Wang R, Chen H, Xie Y, Wang A, et al. Characterization of MW06, a human monoclonal antibody with cross-neutralization activity against both SARS-CoV-2 and SARS-CoV. MAbs. 2021 Jan-Dec;13(1):1953683. 10.1080/19420862.2021.1953683: PMID: 34313527
- Chen Y, Zhu L, Huang W, Tong X, Wu H, Tao Y, Tong B, Huang H, Chen J, Zhao X, et al. Potent RBD-specific neutralizing rabbit monoclonal antibodies recognize emerging SARS-CoV-2 variants elicited by DNA prime-protein boost vaccination. Emerg Microbes Infect. 2021 Dec;10(1):1390–403. 10.1080/22221751.2021.1942227: PMID: 34120577
- Yadav PD, Mendiratta SK, Mohandas S, Singh AK, Abraham P, Shete A, Bandyopadhyay S, Kumar S, Parikh A, Kalita P, et al. ZRC3308 monoclonal antibody cocktail shows protective efficacy in Syrian hamsters against SARS-CoV-2 infection. Viruses. 2021 Dec 3;13(12):2424. 10.3390/v13122424. PMID: 34960695.
- Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, Zhou N, Petty LA, Baang JH, Dillman NO, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2021 Jul 15;73(2):e445–e454. 10.1093/cid/ciaa954. PMID: 32651997.
- Kaplon H, Reichert JM. Antibodies to watch in 2021. MAbs. 2021 Jan-Dec;13(1):1860476. PMID: 33459118. doi:10.1080/19420862.2020.1860476.
- Alfinito E, Beccaria M, Ciccarese M. Biosensing cytokine IL-6: a comparative analysis of natural and synthetic receptors. Biosensors (Basel). 2020 Aug 24;10(9). 10.3390/bios10090106. PMID: 32847008.
- Palanques-Pastor T, Lopez-Briz E, Poveda Andres JL. Involvement of interleukin 6 in SARS-CoV-2 infection: siltuximab as a therapeutic option against COVID-19. Eur J Hosp Pharm. 2020 Sep;27(5):297–98. PMID: 32499314. doi:10.1136/ejhpharm-2020-002322.
- Vaidya G, Czer LSC, Kobashigawa J, Kittleson M, Patel J, Chang D, Kransdorf E, Shikhare A, Tran H, Vo A, et al. Successful treatment of severe COVID-19 pneumonia with clazakizumab in a heart transplant recipient: a case report. Transplant Proc. 2020 Nov;52(9):2711–14. 10.1016/j.transproceed.2020.06.003: PMID: 32563584
- Gremese E, Cingolani A, Bosello SL, Alivernini S, Tolusso B, Perniola S, Landi F, Pompili M, Murri R, Santoliquido A, et al. Sarilumab use in severe SARS-CoV-2 pneumonia. EClinicalMedicine. 2020 Oct;27:100553. doi:10.1016/j.eclinm.2020.100553. PMID: 33043284.
- Wilkinson T, Dixon R, Page C, Carroll M, Griffiths G, Ho LP, De Soyza A, Felton T, Lewis KE, Phekoo K, et al. ACCORD: a Multicentre, seamless, phase 2 adaptive randomisation platform study to assess the efficacy and safety of multiple candidate agents for the treatment of COVID-19 in hospitalised patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020 Jul 31;21(1):691. 10.1186/s13063-020-04584-9. PMID: 32736596.
- Mareev VY, Orlova YA, Pavlikova EP, Akopyan ZA, Matskeplishvili ST, Plisyk AG, Seredenina EM, Potapenko AV, Malakhov PS, Samokhodskaya LM, et al. Proactive anti-inflammatory and anticoagulant therapy in the treatment of advanced stages of novel coronavirus infection (COVID-19). Case series and study design: cOLchicine versus ruxolitinib and secukinumab in open prospective randomIzed trial (COLORIT). Kardiologiia. 2020 Oct 5;60(9):4–21. 10.18087/cardio.2020.9.n1338. PMID: 33131470.
- Polat Ekinci A, Pehlivan G, Gokalp MO. Surveillance of psoriatic patients on biologic treatment during the COVID-19 pandemic: a single-center experience. Dermatol Ther. 2021 Jan;34(1):e14700. PMID: 33369063. doi:10.1111/dth.14700.
- Landi L, Ravaglia C, Russo E, Cataleta P, Fusari M, Boschi A, Giannarelli D, Facondini F, Valentini I, Panzini I, et al. Blockage of interleukin-1beta with canakinumab in patients with Covid-19. Sci Rep. 2020 Dec 11;10(1):21775. 10.1038/s41598-020-78492-y. PMID: 33311551.
- Wang CJ, Truong AK. COVID-19 infection on IL-23 inhibition. Dermatol Ther. 2020 Nov;33(6):e13893. PMID: 32584451. doi:10.1111/dth.13893.
- Bonaventura A, Vecchie A, Wang TS, Lee E, Cremer PC, Carey B, Rajendram P, Hudock KM, Korbee L, Van Tassell BW, et al. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front Immunol. 2020;11:1625. PMID: 32719685. doi:10.3389/fimmu.2020.01625.
- De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, Boffini N, Tentori S, Mette F, Farina N, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2020 Aug;2(8):e465–e473. 10.1016/S2665-9913(20)30170-3: PMID: 32835256
- Temesgen Z, Assi M, Shweta FNU, Vergidis P, Rizza SA, Bauer PR, Pickering BW, Razonable RR, Libertin CR, Burger CD, et al. GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin Proc. 2020 Nov;95(11):2382–94. 10.1016/j.mayocp.2020.08.038: PMID: 33153629
- Abdel-Haq N, Asmar BI, Deza Leon MP, McGrath EJ, Arora HS, Cashen K, Tilford B, Charaf Eddine A, Sethuraman U, Ang JY. SARS-CoV-2-associated multisystem inflammatory syndrome in children: clinical manifestations and the role of infliximab treatment. Eur J Pediatr. 2021 May;180(5):1581–91. PMID: 33452570. doi:10.1007/s00431-021-03935-1.
- Pang J, Xu F, Aondio G, Li Y, Fumagalli A, Lu M, Valmadre G, Wei J, Bian Y, Canesi M, et al. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nat Commun. 2021 Feb 5;12(1):814. 10.1038/s41467-021-21085-8. PMID: 33547300.
- Cure E, Kucuk A, Cure MC. Can emapalumab be life saving for refractory, recurrent, and progressive cytokine storm caused by COVID-19, which is resistant to anakinra, tocilizumab, and Janus kinase inhibitors. Indian J Pharmacol. 2021 May-Jun;53(3):226–28. PMID: 34169908. doi:10.4103/ijp.IJP_615_20.
- Elneil S, Lalezari JP, Pourhassan NZ. Case study of a critically ill person with COVID-19 on ECMO successfully treated with leronlimab. J Transl Autoimmun. 2021;4:100097. PMID: 33778462. doi:10.1016/j.jtauto.2021.100097.
- Smith K, Pace A, Ortiz S, Kazani S, Rottinghaus S. A phase 3 open-label, randomized, controlled study to evaluate the efficacy and safety of intravenously administered ravulizumab compared with best supportive care in patients with covid-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020 Jul 13;21(1):639. doi:10.1186/s13063-020-04548-z. PMID: 32660611.
- Trimarchi H, Gianserra R, Lampo M, Monkowski M, Lodolo J. Eculizumab, SARS-CoV-2 and atypical hemolytic uremic syndrome. Clin Kidney J. 2020 Oct;13(5):739–41. PMID: 33117528. doi:10.1093/ckj/sfaa166.
- Vlaar APJ, de Bruin S, Busch M, Timmermans S, van Zeggeren IE, Koning R, Ter Horst L, Bulle EB, van Baarle F, van de Poll MCG, et al. Anti-C5a antibody IFX-1 (vilobelimab) treatment versus best supportive care for patients with severe COVID-19 (PANAMO): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. 2020 Dec;2(12):e764–e773. 10.1016/S2665-9913(20)30341-6: PMID: 33015643
- Carvelli J, Demaria O, Vely F, Batista L, Chouaki Benmansour N, Fares J, Carpentier S, Thibult ML, Morel A, Remark R, et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020 Dec;588(7836):146–50. 10.1038/s41586-020-2600-6: PMID: 32726800
- Loganathan S, Athalye SN, Joshi SR. Itolizumab, an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications. Expert Opin Biol Ther. 2020 Sep;20(9):1025–31. PMID: 32700604. doi:10.1080/14712598.2020.1798399.
- Drozdzal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, Ghavami S, Los MJ. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat. 2020 Dec;53:100719. PMID: 32717568. doi:10.1016/j.drup.2020.100719.
- Willingham SB, Criner G, Hill C, Hu S, Rudnick JA, Daine-Matsuoka B, Hsieh J, Mashhedi H, Hotson AN, Brody J, et al. Characterization and phase 1 trial of a b cell activating anti-CD73 antibody for the immunotherapy of COVID-19. medRxiv. 2020 September 15. preprint. doi:10.1101/2020.09.10.20191486
- Yaqinuddin A, Kashir J. Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Med Hypotheses. 2020 Apr 22;140. 109777. 10.1016/j.mehy.2020.109777. PMID: 32344314
- Artigas C, Lemort M, Mestrez F, Gil T, Flamen P. COVID-19 pneumonia mimicking immunotherapy-induced pneumonitis on 18F-fdg pet/ct in a patient under treatment with nivolumab. Clin Nucl Med. 2020 Aug;45(8):e381–e382. PMID: 32520508. doi:10.1097/RLU.0000000000003152.
- Wang S, Qiu Z, Hou Y, Deng X, Xu W, Zheng T, Wu P, Xie S, Bian W, Zhang C, et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021 Feb;31(2):126–40. 10.1038/s41422-020-00460-y: PMID: 33420426
- Agrati C, Bordoni V, Sacchi A, Petrosillo N, Nicastri E, Del Nonno F, D’Offizi G, Palmieri F, Marchioni L, Capobianchi MR, et al. Elevated P-selectin in severe covid-19: considerations for therapeutic options. Mediterr J Hematol Infect Dis. 2021;13(1):e2021016. PMID: 33747397. doi:10.4084/MJHID.2021.016.
- Kang CK, Choe PG, Park S, Kim TS, Seong MW, Kim NJ, Oh MD, Park WB, Kim YW. Compassionate use of hzVSF-v13 in two patients with severe COVID-19. J Med Virol. 2020 Nov;92(11):2371–73. PMID: 32458425. doi:10.1002/jmv.26063.
- Colarusso C, Terlizzi M, Pinto A, Sorrentino R. A lesson from a saboteur: high-MW kininogen impact in coronavirus-induced disease 2019. Br J Pharmacol. 2020 Nov;177(21):4866–72. PMID: 32497257. doi:10.1111/bph.15154.
- Farmani AR, Mahdavinezhad F, Moslemi R, Mehrabi Z, Noori A, Kouhestani M, Noroozi Z, Ai J, Rezaei N. Anti-IgE monoclonal antibodies as potential treatment in COVID-19. Immunopharmacol Immunotoxicol. 2021 Jun;43(3):259–64. PMID: 34018464. doi:10.1080/08923973.2021.1925906.
- Hastie KM, Li H, Bedinger D, Schendel SL, Dennison SM, Li K, Rayaprolu V, Yu X, Mann C, Zandonatti M, et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science. 2021 Oct 22;374(6566):472–78. 10.1126/science.abh2315. PMID: 34554826.
- Justel M, Socias L, Almansa R, Ramirez P, Gallegos MC, Fernandez V, Gordon M, Andaluz-Ojeda D, Nogales L, Rojo S, et al. IgM levels in plasma predict outcome in severe pandemic influenza. J Clin Virol. 2013 Nov;58(3):564–67. 10.1016/j.jcv.2013.09.006: PMID: 24076102
- Klingler J, Weiss S, Itri V, Liu X, Oguntuyo KY, Stevens C, Ikegame S, Hung CT, Enyindah-Asonye G, Amanat F, et al. Role of immunoglobulin M and A Antibodies in the neutralization of severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2021 Mar 29;223(6):957–70. 10.1093/infdis/jiaa784. PMID: 33367897.
- Focosi D, Franchini M, Pirofski LA, Burnouf T, Fairweather D, Joyner MJ, Casadevall A. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors. Viruses. 2021 Aug 11;13(8):1594. doi:10.3390/v13081594. PMID: 34452459.
- Garraud O. Passive immunotherapy with convalescent plasma against COVID-19? What about the evidence base and clinical trials? Transfus Apher Sci. 2020 Aug;59(4):102858. PMID: 32631501. doi:10.1016/j.transci.2020.102858.
- Welte T, Dellinger RP, Ebelt H, Ferrer M, Opal SM, Singer M, Vincent JL, Werdan K, Martin-Loeches I, Almirall J, et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med. 2018 Apr;44(4):438–48. 10.1007/s00134-018-5143-7: PMID: 29632995
- Bohlander F, Riehl D, Weissmuller S, Gutscher M, Schuttrumpf J, Faust S. Immunomodulation: immunoglobulin preparations suppress hyperinflammation in a COVID-19 model via FcgammaRIIA and FcalphaRI. Front Immunol. 2021;12:700429. PMID: 34177967. doi:10.3389/fimmu.2021.700429.
- Ku Z, Xie X, Hinton PR, Liu X, Ye X, Muruato AE, Ng DC, Biswas S, Zou J, Liu Y, et al. Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature. 2021 Jul;595(7869):718–23. 10.1038/s41586-021-03673-2: PMID: 34082438
- Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, Cipolla M, Hoffmann HH, Oliveira TY, Oren DA, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021 Jan 20;13(577). 10.1126/scitranslmed.abf1555. PMID: 33288661.
- Sun L, Kallolimath S, Palt R, Stiasny K, Mayrhofer P, Maresch D, Eidenberger L, Steinkellner H. Increased in vitro neutralizing activity of SARS-CoV-2 IgA1 dimers compared to monomers and IgG. Proc Natl Acad Sci U S A. 2021 Nov 2;118(44):e2107148118. doi:10.1073/pnas.2107148118. PMID: 34702738.
- Ejemel M, Li Q, Hou S, Schiller ZA, Tree JA, Wallace A, Amcheslavsky A, Kurt Yilmaz N, Buttigieg KR, Elmore MJ, et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat Commun. 2020 Aug 21;11(1):4198. 10.1038/s41467-020-18058-8. PMID: 32826914.
- Pisil Y, Yazici Z, Shida H, Miura T. Is SARS-CoV-2 neutralized more effectively by IgM and IgA than IgG having the same fab region? Pathogens. 2021 Jun 13;10(6):751. doi:10.3390/pathogens10060751. PMID: 34199224.
- Wills S, Hwang KK, Liu P, Dennison SM, Tay MZ, Shen X, Pollara J, Lucas JT, Parks R, Rerks-Ngarm S, et al. HIV-1-specific iga monoclonal antibodies from an HIV-1 vaccinee mediate galactosylceramide blocking and phagocytosis. J Virol. 2018 Apr 1;92(7). 10.1128/JVI.01552-17. PMID: 29321320.
- Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995 Feb;69(2):1339–43. PMID: 7815518. doi:10.1128/JVI.69.2.1339-1343.1995.
- Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021 Jan 20;13(577). 10.1126/scitranslmed.abd2223. PMID: 33288662.
- Quinti I, Mortari EP, Fernandez Salinas A, Milito C, Carsetti R. IgA antibodies and Iga deficiency in SARS-CoV-2 infection. Front Cell Infect Microbiol. 2021;11:655896. PMID: 33889552. doi:10.3389/fcimb.2021.655896.
- Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM Jr., Rawson S, Rits-Volloch S, Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020 Sep 25;369(6511):1586–92. doi:10.1126/science.abd4251. PMID: 32694201.
- Lim SA, Gramespacher JA, Pance K, Rettko NJ, Solomon P, Jin J, Lui I, Elledge SK, Liu J, Bracken CJ, et al. Bispecific VH/Fab antibodies targeting neutralizing and non-neutralizing Spike epitopes demonstrate enhanced potency against SARS-CoV-2. MAbs. 2021 Jan-Dec;13(1):1893426. 10.1080/19420862.2021.1893426: PMID: 33666135
- Husain B, Ellerman D. Expanding the boundaries of biotherapeutics with bispecific antibodies. BioDrugs. 2018 Oct;32(5):441–64. PMID: 30132211. doi:10.1007/s40259-018-0299-9.
- Digiammarino EL, Harlan JE, Walter KA, Ladror US, Edalji RP, Hutchins CW, Lake MR, Greischar AJ, Liu J, Ghayur T, et al. Ligand association rates to the inner-variable-domain of a dual-variable-domain immunoglobulin are significantly impacted by linker design. MAbs. 2011 Sep-Oct;3(5):487–94. 10.4161/mabs.3.5.16326: PMID: 21814039
- De Gasparo R, Pedotti M, Simonelli L, Nickl P, Muecksch F, Cassaniti I, Percivalle E, Lorenzi JCC, Mazzola F, Magri D, et al. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice. Nature. 2021 May;593(7859):424–28. 10.1038/s41586-021-03461-y: PMID: 33767445
- Cho H, Gonzales-Wartz KK, Huang D, Yuan M, Peterson M, Liang J, Beutler N, Torres JL, Cong Y, Postnikova E, et al. Bispecific antibodies targeting distinct regions of the spike protein potently neutralize SARS-CoV-2 variants of concern. Sci Transl Med. 2021 Sep;14(616):eabj5413. 10.1126/scitranslmed.abj5413: PMID: 34519517
- van Riet E, Ainai A, Suzuki T, Hasegawa H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine. 2012 Aug 31;30(40):5893–900. doi:10.1016/j.vaccine.2012.04.109. PMID: 22835738.
- Bagga B, Cehelsky JE, Vaishnaw A, Wilkinson T, Meyers R, Harrison LM, Roddam PL, Walsh EE, DeVincenzo JP. Effect of preexisting serum and mucosal antibody on experimental Respiratory Syncytial Virus (RSV) challenge and infection of adults. J Infect Dis. 2015 Dec 1;212(11):1719–25. doi:10.1093/infdis/jiv281. PMID: 25977264.
- Chan RWY, Liu S, Cheung JY, Tsun JGS, Chan KC, Chan KYY, Fung GPG, Li AM, Lam HS. The mucosal and serological immune responses to the novel coronavirus (SARS-CoV-2) vaccines. Front Immunol. 2021;12:744887. PMID: 34712232. doi:10.3389/fimmu.2021.744887.
- Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis. 2013 Jul 15;208(2):284–94. doi:10.1093/infdis/jit166. PMID: 23596320.
- Hird TR, Grassly NC, Andino R. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8(4):e1002599. doi:10.1371/journal.ppat.1002599. PMID: 22532797
- Ainai A, Tamura S, Suzuki T, van Riet E, Ito R, Odagiri T, Tashiro M, Kurata T, Hasegawa H. Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults. Hum Vaccin Immunother. 2013 Sep;9(9):1962–70. PMID: 23896606. doi:10.4161/hv.25458.
- Suzuki T, Kawaguchi A, Ainai A, Tamura S, Ito R, Multihartina P, Setiawaty V, Pangesti KN, Odagiri T, Tashiro M, et al. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7809–14. 10.1073/pnas.1503885112. PMID: 26056267.
- Liu X, Luongo C, Matsuoka Y, Park HS, Santos C, Yang L, Moore IN, Afroz S, Johnson RF, Lafont BAP, et al. A single intranasal dose of a live-attenuated parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in hamsters. Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2109744118. 10.1073/pnas.2109744118. PMID: 34876520.
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993 Jun 3;363(6428):446–48. doi:10.1038/363446a0. PMID: 8502296.
- Steeland S, Vandenbroucke RE, Libert C. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today. 2016 Jul;21(7):1076–113. PMID: 27080147. doi:10.1016/j.drudis.2016.04.003.
- Ingram JR, Schmidt FI, Ploegh HL. Exploiting nanobodies’ singular traits. Annu Rev Immunol. 2018 Apr 26;36(1):695–715. doi:10.1146/annurev-immunol-042617-053327. PMID: 29490163.
- Chi X, Liu X, Wang C, Zhang X, Li X, Hou J, Ren L, Jin Q, Wang J, Yang W. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat Commun. 2020 Sep 10;11(1):4528. doi:10.1038/s41467-020-18387-8. PMID: 32913273.
- Koenig PA, Das H, Liu H, Kummerer BM, Gohr FN, Jenster LM, Schiffelers LDJ, Tesfamariam YM, Uchima M, Wuerth JD, et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science. 2021 Feb 12;371(6530). 10.1126/science.abe6230. PMID: 33436526.
- Wu X, Cheng L, Fu M, Huang B, Zhu L, Xu S, Shi H, Zhang D, Yuan H, Nawaz W, et al. A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration. Cell Rep. 2021 Oct 19;37(3):109869. 10.1016/j.celrep.2021.109869. PMID: 34644535.
- Kelton W, Mehta N, Charab W, Lee J, Lee CH, Kojima T, Kang TH, Georgiou G. IgGA: a “cross-isotype” engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol. 2014 Dec 18;21(12):1603–09. doi:10.1016/j.chembiol.2014.10.017. PMID: 25500223.
