ABSTRACT
The food additive nitrite (E249, E250) is commonly used in meat curing as a food preservation method. Because of potential negative health effects of nitrite, its use is strictly regulated. In an earlier study we have shown that the calculated intake of nitrite in children can exceed the acceptable daily intake (ADI) when conversion from dietary nitrate to nitrite is included. This study examined time-dependent changes in nitrite levels in four Swedish meat products frequently eaten by children: pork/beef sausage, liver paté and two types of chicken sausage, and how the production process, storage and also boiling (e.g., simmering in salted water) and frying affect the initial added nitrite level. The results showed a steep decrease in nitrite level between the point of addition to the product and the first sampling of the product 24 h later. After this time, residual nitrite levels continued to decrease, but much more slowly, until the recommended use-by date. Interestingly, this continuing decrease in nitrite was much smaller in the chicken products than in the pork/beef products. In a pilot study on pork/beef sausage, we found no effects of boiling on residual nitrite levels, but frying decreased nitrite levels by 50%. In scenarios of time-dependent depletion of nitrite using the data obtained for sausages to represent all cured meat products and including conversion from dietary nitrate, calculated nitrite intake in 4-year-old children generally exceeded the ADI. Moreover, the actual intake of nitrite from cured meat is dependent on the type of meat source, with a higher residual nitrite levels in chicken products compared with pork/beef products. This may result in increased nitrite exposure among consumers shifting their consumption pattern of processed meats from red to white meat products.
Introduction
Nitrite (E249, E250) and nitrate (E251, E252) are approved food additives in the European Union (EU Regulation No. 1129/2011/EC Citation2011) and are widely used in meat preservation. The amount of nitrite permitted for use as a food additive in cured meat is currently 150 mg kg−1 (expressed as NaNO2), except for somewhat higher levels in some traditional cured products. Nitrite has long been a widely used curing agent for meat products owing to its favourable properties, but during the 1970s the debate on the formation of carcinogenic nitrosamines resulted in strong pressure to decrease the use of nitrite for curing in order to reduce the risk of nitrosamine formation and thereby the potential health risks (Sindelar & Milkowski Citation2012). However, opinion differs within the EU regarding the need to use nitrite in meat processing. Thus, Denmark still maintains national legislation specifying a maximum amount of 60 mg kg−1, instead of 150 mg kg−1 according to EU legislation. The Danish authorities state that the necessary preservative effect and microbiological safety can be achieved at the lower maximum levels of nitrite in the Danish regulations, while at the same time reducing the risk of nitrosamine formation (EU Commission Decision Citation2010/561/EU 2010; Herrmann Citation2014).
A well-known health effect of nitrite in humans is methaemoglobinaemia, which is the binding of nitrite transformation products to haemoglobin with resulting impairment of oxygen transport capacity. However, the acceptable daily intake (ADI) of nitrite is not based on nitrosamines or methaemoglobinaemia. The ADI for nitrite is 0.07 mg kg−1 body weight (b.w.) and is based on adverse effects on the lung and cardiovascular system in rodents (Joint FAO/WHO Expert Committee on Food Authorities Citation2003). According to the Scientific Committee for Food (SCF) (Citation1997), this ADI is applicable to all sources of dietary exposure. There are high levels of nitrate/nitrite also in other foodstuffs than meat products (Larsson et al. Citation2011; Iammarino et al. Citation2013). However, based on the possible formation of carcinogenic nitroso compounds, the SCF recommends that the levels of nitrite added to food be lowered to the minimum required to achieve the necessary preservative effect and ensure microbiological safety.
Due to the potential health effects of nitrite, it is of the utmost importance to determine total exposure from all food sources. Even if meat products are the single most important nitrite-containing food for the average consumer, nitrite formation in the body from dietary nitrate (vegetables and water) has also to be considered. It has been estimated that about 25% of ingested nitrate is secreted in human saliva, of which about 20% is reduced to nitrite, i.e., about 5% of the overall dose of nitrate, clearly establishing saliva as a major site of nitrite production in the body (Walker Citation1990). Using this as the basis for calculation, a Swedish study on nitrate/nitrite intake in children, based on a dietary survey from 2003, showed that about 70% of nitrite exposure originated from estimated in vivo transformation of dietary nitrate to nitrite (Larsson et al. Citation2011). Another potential nitrite source is endogenous production in the body, but this source was not included in our calculation of nitrite exposure. Thus, lower nitrite exposure may be beneficial for human health, but lower nitrite content in cured meat could also increase the health risks arising from microbial contamination.
Different independent meta-analyses of epidemiological studies demonstrate a significantly increased risk of development of colorectal cancer associated with higher consumption of red meat, especially processed red meat (World Cancer Research Fund [WCRF] Citation2007). There is evidence from the literature that haem–Fe is involved in this epidemiological association and that it may play a central role in colon carcinogenesis associated with red meat intake (Bastide et al. Citation2015; Hammerling et al. Forthcoming). Poultry, which contains lower amounts of haem–Fe than pork and beef, has not been associated with an increase in colorectal cancer. Nitrite per se is not carcinogenic, but under conditions that result in endogenous nitrosation, the possibility that nitrite is involved in the carcinogenic process cannot be excluded (Habermeyer et al. Citation2015). Inorganic nitrite, through intestinal conversion of precursor compounds to N-nitrosation compounds, has been proposed as one of several potential causative agents in food-borne risks of contracting colorectal cancer.
Several previous studies have reported that the level of added nitrite in processed meat products decreases over time from the moment of addition to the point of consumption (Hill et al. Citation1973; Pérez-Rodríguez et al. Citation1996). This will influence dietary exposure assessments and highlights the importance of reliable analytical measurements of these products at the consumer level. Therefore, the aim of this study was to monitor the nitrite levels in some processed meat products frequently consumed by Swedish children, namely red (pork/beef) and white (chicken) meat-based sausages and liver paté. The specific objective was to determine how nitrite levels change time-dependently from the time point of addition at production stage to the use-by date. In addition, scenario calculations were made on nitrite intake among children using the residual nitrite levels determined in processed meat and estimated conversion of nitrate to nitrite from other foodstuffs. The estimated intakes obtained were compared with the ADI values established for nitrite.
Materials and methods
Sampling and sample preparation
Four Swedish cured meat products were used in this study. The formulations fell within the following specifications: pork/beef sausage (pork and beef meat 61%, fat 19%, protein 12%, E250, E300, carbohydrates 11%), chicken lunch sausages (poultry meat 54%, fat 14%, E250), chicken grill sausages (poultry meat 59%, fat 14%, E250) and liver paté (pork liver 31%, pork meat 16%, fat 23%, protein 12%, carbohydrate 9%, E471, salt 2.3%, E250, E330, E300). These products are frequently consumed in Sweden, especially by children (Enghardt Barbieri et al. Citation2003).
All cured samples were prepared by three Swedish manufacturers following their standard recipe and commercial method of manufacturing (grinding, mixing, curing, stuffing and thermal processing). The amount of sodium nitrite added to pork/beef sausage (grill type), lunch chicken sausages, grill chicken sausages and liver paté (106, 118, 112 and 119 mg kg−1, respectively, expressed as NaNO2) was below the maximum permitted (150 mg kg−1) by EU regulations (EU Directive Citation2006/52/EC 2006). Samples were dispatched from the producers to the National Food Agency (NFA) laboratory within 24 h of production and stored in a refrigerator at 2–3°C until analysis. Thereafter the products were analysed for various lengths of time () from processing to the use-by date of each product, and even after that date. The initial nitrite levels at the time of food production were determined by using data of the actual quantities added during formulation of the products, whereas nitrite levels were determined by chemical analyses at all other time points. On each analysis occasion, two packs of pork/beef sausages (10 sausages, 500 g), chicken lunch sausage (10 sausages, 600 g), chicken grill sausage (10 sausages, 400 g) and liver paté (500 g) were homogenised separately in a blender.
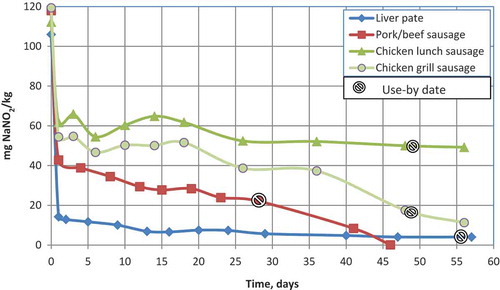
Figure 1. (colour online) Measured changes in nitrite content (mg NaNO2 kg–1) in samples of pork/beef sausage, chicken sausages and liver paté from production date to use-by date. Day 0 is the day on which a calculated initial amount of nitrite was added by the manufacturer. The beginning of the storage period is designated as day 1. The storage temperature was 2–3°C.
Cooking procedures
Two additional experiments were conducted in a pilot study to determine the effect of boiling (simmering in salted water to serving temperature) and frying on residual nitrite levels in the sausage products. Five pork/beef grill sausages were heated in water for 10 min at 75 ± 1°C. After simmering, all samples were blotted to remove excess water, homogenised and analysed. Six pork/beef grill sausages from another pack were fried in maize oil. After frying, the samples were allowed to cool, drained on absorbent paper, homogenised and analysed for nitrite. As control samples, two packages of pork/beef sausage were homogenised separately and analysed for nitrite. The tap water used in the boiling study was cooled and the nitrite content before and after cooking was also determined.
Chemical analysis
In order to determine the effect of depletion of nitrite on exposure estimates, all samples were analysed for nitrite using a spectrophotometric method based on the sensitive and widely used diazotisation-coupling Griess reaction (NMKL Citation2013). In this, nitrite is determined by diazotising with sulphanilamide and coupling with N-(1-naphthyl)-ethylenediamine di-hydrochloride to form a highly coloured azo dye that is measured at 540 nm. It has been demonstrated in our laboratory at NFA that the use of the Griess reaction provides more reliable results in determination of low concentrations of nitrate and nitrite ions in meat products than the available HPLC techniques (European Committee for Standardization Citation2005). Thus, several analyses in parallel of meat products showed that the HPLC-UV method was not able to detect or measure lower concentrations of residual nitrate/nitrite than the spectrophotometric method (Merino Citation2009).
Duplicate analyses were carried out to estimate the within- and between-sample precision of the results. The combined relative uncertainty for meat products, calculated as the sum of intermediate precision and the uncertainty of recovery within the validation study, was 2.6%. The study followed the recommended internal quality control procedure, including successful participation in proficiency testing.
Intake calculations
The intake scenario in this paper is based on NFA’s earlier Swedish national dietary survey on children, performed in 2003 (Enghardt Barbieri et al. Citation2003; Larsson et al. Citation2011). As our aim was to study the most sensitive consumer group we used data on 4-year-old children. Data from that survey (n = 590; mean weight = 18.2 kg), and specifically their individual intake of nitrite-containing processed meat, were used. However, we replaced actual consumption of all types of processed meat with either pork/beef sausage consumption, or chicken sausage consumption, in both cases using the levels measured in the present study. Moreover, different factors (0%, 5% and 20%) for endogenous conversion of nitrate to nitrite (from vegetables, fruit and drinking water) were also added to the children’s total nitrite intakes, which were given as median and 95th percentile values.
Results
The decrease in nitrite levels as a function of time after addition of NaNO2 to the meat products studied is shown in . Already at 24 h after the addition of nitrite to lunch chicken sausage, grill chicken sausage, pork/beef sausage and liver paté, the nitrite levels had decreased to approximately 55%, 45%, 35% and 15% of the initial level, respectively. However, the decline in added nitrite was less pronounced in chicken sausage than in pork/beef sausage and liver paté, so that at the use-by date the nitrite levels were still approximately 40% in chicken lunch sausage and 15% in chicken grill sausage (). A noteworthy feature was that the accepted storage period (until use-by date is reached) was almost twice as long for chicken sausage (48 days) as for pork/beef sausage (28 days). In fact, on applying the accepted period for chicken sausage, there was almost no nitrite left in the pork/beef sausage after 48 days.
The results of the pilot study showed that boiling did not alter the residual nitrite level at all (data not shown). However, frying decreased the mean nitrite level from 11 to 5.6 mg kg–1, i.e., to approximately 50% of the initial level. This decrease did not appear to be related to the weight loss of the fried samples. Hence, it could be attributable to formation of unknown nitrogen-containing compounds, an issue that would require further research. The residual nitrite level in the assays was 11 mg kg−1 or less. Consequently, even though frying significantly affected the level of residual nitrite, it had a minor influence on the estimated total dietary intake of nitrite.
Intake scenarios on the calculated nitrite intake in 4-year-old Swedish children were also formulated (), combining basal consumption data from an earlier intake study (Enghardt Barbieri et al. Citation2003; Larsson et al. Citation2011) with the nitrite levels determined in pork/beef and chicken sausages in the present study () to represent the levels in all processed meats registered in the survey. The intake calculations used median and 95th percentile values and, depending on the nitrate–nitrite conversion factor applied, this resulted in different exposure levels. Also, these estimated intake data clearly showed the influence of storage time on actual exposure levels. With this method of calculation, it is obvious that not many alternatives resulted in nitrite intake below the ADI of 0.07 mg kg−1 b.w. (). Note that in nitrite levels are given as NaNO2 (mg kg−1 product) but in the scenarios for intake calculations (mg kg−1 b.w. day–1) the nitrite ion was used.
Table 1. Basal data used in the scenario calculations (see ). The nitrite levels are taken from data points representing samples analysed or extrapolated from adjacent data points.
Table 2. Intake scenarios for nitrite ion in 4-year-old Swedish children based on a Swedish consumption survey (Enghardt Barbieri et al. Citation2003).
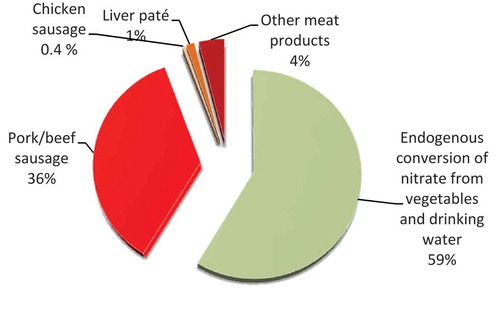
The calculated total dietary intake of nitrite among 4-year-old children () was based on our measured data on nitrite in various cured meat products, estimated conversion (5%) of nitrate in vegetables and fruit to nitrite, and previously published food consumption data (Larsson et al. Citation2011). This clearly showed that nitrate to nitrite conversion from vegetables and fruit was the major source of nitrite exposure. However, a significant amount also originated from beef/pork sausage (36%). Even if intake from chicken sausage is currently low, our finding of considerably higher residual nitrite levels in chicken sausage compared with beef/pork sausage suggests that a switch in consumption pattern to cured chicken products may result in a significant increase in total nitrite exposure.
Discussion
Our analysis of commonly consumed processed meat products on the Swedish market showed that during the early product life, there was a rapid initial decrease in nitrite from the point of sodium nitrite addition. The nitrite decrease continued in later stages of product life, but at a much slower rate, and at the use-by date the residual level of nitrite in the products was 5–19% of the amount initially added, depending on the food product. After the manufacturing process, which included a dry heating step, by their use-by date chicken sausages contained consistently higher nitrite levels (approximately 50% higher) than liver paté and pork/beef sausage (4% and 20% of added nitrite, respectively). These levels were based on analysis of processed products that are not cooked before consumption. Cooking may further decrease nitrite levels. For example, our pilot study showed that frying, but not boiling, decreased the nitrite content in sausages. Whether this was associated with a change in nitrosamine formation is not known (Josefsson & Nygren Citation1981).
In our scenarios, calculated nitrite intake in children based on the nitrite levels measured in sausage was within the ADI when based only on processed meat consumption, but exceeded the ADI when nitrite formed from dietary nitrate was included ().
Earlier studies have presented results on the complex chemistry of NO3/NO2/NO in various meat-based systems. A review by Skibsted (Citation2011) describes in detail nitric oxide chemistry in muscle-based foods, including the chemical reaction behind the colour of cured meat. Cassens et al. (Citation1979) has shown that nitrite added to meat homogenates is partly transformed to other nitrogen-containing compounds and bound to myoglobin and other meat constituents, e.g., lipids and proteins. The formation of nitrate from nitrite may also have to be considered (Pegg & Honikel Citation2015). In the present study, as well as in several earlier studies, it has been shown that nitrite depletion increases with time in meat-based systems (Pérez-Rodríguez et al. Citation1996; Armenteros et al. Citation2012). In addition to heating during the initial processing phase, factors that have been shown to influence the nitrite levels in meat products include pH, temperature and ascorbate addition (Gibson et al. Citation1984). Honikel (Citation2008) estimated that the decline in nitrite levels due to heating during manufacturing is about 35% of the added level, and thereafter there is a continuing decrease in nitrite levels during storage. Special features of poultry meat regarding nitrite levels during storage have also been described (Kilic et al. Citation2001, Citation2002), with a difference between nitrite levels in poultry and pork/beef meat possibly due to pH differences between the products. However, owing to the much higher abundance of haem in red meat compared with chicken, it is also possible that nitrite-induced formation of nitrosylhaem causes greater and faster loss of added nitrite in red meat products (Hammerling et al. Forthcoming).
In our scenarios of time-dependent depletion of nitrite, the calculated nitrite intake in children in most cases exceeded the ADI for nitrite when conversion from dietary nitrate was included and when the nitrite levels recorded in sausages in the present study were used to represent all processed meat. However, actual intake of nitrite via cured meat is highly dependent on time after production start and type of meat. The current trend of increased consumption of meat raises concerns, as well as the potential increase in nitrite exposure among consumers as a consequence of a shift to white meat cured products. The recommendation made by WCRF (Citation2007) in its report to limit intake of red meat and avoid processed meat and the growing Islamic population in Western countries will probably result in increased sales and consumption of different white meat-based cured products, bringing about a change in the consumption pattern of meat mainly from red meat to chicken and turkey. Consequently, it is reasonable to assume that there will be a future increase in nitrite intake among consumers, but it is difficult to estimate the possible health risk from this higher intake. In addition, it is likely that most (59%) of the nitrite originates from conversion of nitrate in other food items, especially vegetables () (European Food Safety Authority Citation2013). Thus, if conversion is considered, this difference in nitrite content between chicken and pork/beef meat may have a limited impact on total nitrite exposure.
Increased consumption of vegetables is widely recommended because of their generally recognised beneficial health effects. WCRF (Citation2007) rates the evidence as ‘convincing to probable’ that diets high in vegetables and/or fruits protect against a variety of cancers, but it is not clear whether this effect is related to their high nitrate content. Other beneficial effects of nitrate have been described in experimental and human intervention studies and in epidemiology regarding effects on blood pressure, myocardial infarction and stroke (Habermeyer et al. Citation2015). Within the body, nitrate and nitrite may function as an alternative source of nitric oxide, an important and multifaceted physiological signalling molecule (Weitzberg & Lundberg Citation2013). The biological activities of nitric oxide related to secondary products may include pharmacological effects, e.g., on blood vessels and blood pressure and on the induction of oxidative stress/inflammation. However, it is concluded in the literature that available evidence of these effects is inadequate for comprehensive and reliable assessment of positive or negative health effects of nitrate/nitrate, especially long-term effects (Habermeyer et al. Citation2015).
The conversion of nitrate to nitrite in the body was a crucial factor in estimation of nitrite exposure in this study. However, the usual conversion factor of 5% is an approximate figure and it has been estimated to be as high as 20% for some individuals, so both values were included here. As also pointed out by several other expert groups (Thomson et al. Citation2007; Leth et al. Citation2008; Menard et al. Citation2008), for consistency the conversion factor should be better defined to achieve more reliable nitrite exposure estimates. At the same time, it is obvious that the scientific basis for establishing the conversion factor must be improved.
To conclude, the present study examined time-dependent depletion of nitrite in various cured meat products and how this affects nitrite intake estimations. We selected food products on the Swedish market that are especially popular among children, i.e., sausages based on pork/beef or chicken and liver paté. We found a strong initial decrease in added nitrite (during processing), followed by a gradual decline during subsequent storage and cooking, a decrease that was higher in pork/beef than in chicken products. These findings, together with a factor for conversion of dietary nitrate, were used in scenarios on estimated nitrite dietary exposure in children. In these scenarios, the intake in 4-year-old children exceeded in most cases ADI for nitrite. The findings could be used in a more general perspective as an argument for improving sources of uncertainty affecting dietary exposure assessments but also for a discussion on the basis and methods for risk assessment of nitrate and nitrite.
Conclusions
A rapid decrease in nitrite levels occurred initially after the addition of nitrite during production of the actual meat products.
The depletion of nitrite depended on time and the type of cured meat product, with higher residual nitrite levels in cured chicken products than in cured pork/beef products.
Nitrite intake from all dietary sources, including nitrate–nitrite conversion, led to the ADI being exceeded in all scenarios calculated. This suggests that an approach to estimating the ADI not accounting for conversion of dietary nitrate causes an underestimation of the true nitrite intake.
The WCRF recommendation on limiting consumption of red/processed meat could result in increased nitrite exposure among consumers as a consequence of a shift in consumption pattern towards white meat cured food products.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors wish to thank Andersson & Tillman AB, Atria Skandinavia and Sörensens Livs AB for the kind support with meat products to be used in the study. Eva Warensjö-Lemming is acknowledged for assistance with food data handling.
References
- Armenteros M, Aristoy M, Toldrá F. 2012. Evolution of nitrate and nitrite during the processing of dry-cured ham with partial replacement of NaCl by other chloride salts. Meat Sci. 91:378–381.
- Bastide NM, Chenni F, Audebert M, Santarelli RL, Tache S, Naud N, Baradat M, Jouanin I, Surya R, Hobbs DA, et al. 2015. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 75:870–879.
- Cassens RG, Greaser ML, Ito T, Lee M. 1979. Reactions of nitrite in meat. Symposium proceedings: alternatives to nitrite. Food Technol. 33:46–56.
- Enghardt Barbieri H, Pearson M, Becker W Riksmaten – barn. 2003. Livsmedels- och näringsstatus bland barn i Sverige. Livsmedelsverket, Uppsala (Sweden): Ord & Form.
- EU Commission Decision 2010/561/EU. 2010. Concerning national provisions notified by Denmark on the addition of nitrite on certain meat products. Off J. L.247:55.
- EU Commission Regulation (EC) 1129/2011 [Internet]. 2011. [cited 2015 Dec 12]. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011R1129
- EU Directive 2006/52/EC [Internet]. 2006. [cited 2015 Dec]. Available from http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006L0052&from=EN
- European Committee for Standardization. 2005. European Standard EN 12014-Part 4. IC method for the determination of nitrate content of meat product. Brussels.
- European Food Safety Authority. 2013. External Scientific Report: Study on the influence of food processing on nitrate levels in vegetables. Maribor: EFSA supporting publication EN-514.
- Gibson A, Roberts T, Robinson A. 1984. Factors controlling the growth of Clostridium botulim types A and B in pasteurized meats VI. Nitrite monitoring during storage of pasteurized pork slurries. J Food Technol. 19:29–44.
- Habermeyer M, Roth A, Guth S, Diel P, Engel K-H, Epe B, Fürst P, Heinz V, Humpf H-U, Joost H-G, et al. 2015. Nitrate and nitrite in the diet: how to assess their benefit and risk for human health. Mol Nutr Food Res. 59:106–128.
- Hammerling U, Bergman Laurila J, Grafström R, Ilbäck N-G. Forthcoming. Consumption of red/processed meat and colorectal cancer: possible mechanisms underlying the significant association. Crit Rev Food Sci Nutr. 56.
- Herrmann SS. 2014. N-nitrosamines in processed meat products-analysis, occurrence, formation, mitigation and exposure [ PhD thesis]. Söborg: National Food Institute-Technical University of Denmark
- Hill LH, Webb NB, Moncol LD, Adams AT. 1973. Changes in residual nitrite in sausage and luncheon meat products during storage. J Milk Food Technol. 36:515–519.
- Honikel KO. 2008. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 78:68–76.
- Iammarino M, Di Taranto A, Cristino M. 2013. Endogenous levels of nitrites and nitrates in wide consumption foodstuffs: results of five years of official controls and monitoring. Food Chem. 140:763–771.
- Joint FAO/WHO Expert Committee on Food Additives. 2003. Nitrite (and potential endogenous formation of N-nitroso compounds) [Internet]. WHO Food Addit Ser. 50; [cited 2015 Dec]. Available from: http://www.inchem.org/documents/jecfa/jecmono/v50je05.htm
- Josefsson E, Nygren S. 1981. Volatile N-nitroso compounds in foods in Sweden. Vår Föda. 33:147–165.
- Kilic B, Cassens R, Borchert L. 2001. Influence of turkey meat on residual nitrite in cured meat products. J Food Protec. 64:235–239.
- Kilic B, Cassens R, Borchert L. 2002. Effect of turkey meat, phosphate, sodium lactate, carrageenan, and konjac on residual nitrite in cured meats. J Food Sci. 67:29–31.
- Larsson K, Darnerud PO, Ilbäck N-G, Merino L. 2011. Estimated dietary intake of nitrite and nitrate in Swedish children. Food Addit Contamin Part A. 28:659–666.
- Leth T, Fagt S, Nielsen S, Andersen R. 2008. Nitrite and nitrate content in meat products and estimated intake in Denmark from 1998 to 2006. Food Addit Contamin Part A. 25:1237–1245.
- Menard C, Heraud F, Volatier L, Leblanc J-C. 2008. Assessment of dietary exposure of nitrate and nitrite in France. Food Addit Contamin Part A. 25:971–988.
- Merino L. 2009. Development and validation of a method for determination of residual nitrite/nitrate in foodstuffs and water after zinc reduction. Food Anal Methods. 2:212–220.
- Nordic Committee on Food Analysis. 2013. Determination of nitrate and/or nitrite in foodstuffs and water by spectrophotometry after zinc reduction and Griess reaction. NMKL No. 194; Oslo.
- Pegg RB, Honikel OK. 2015. Principles of curing. Handbook of fermented meat and poultry. 2nd ed. Edited by Fidel Toldrá. Chichester: John Wiley & Sons, Ltd.
- Pérez-Rodríguez ML, Bosch-Bosch N, Garciá-Mata M. 1996. Monitoring nitrite and nitrate residues in frankfurters during processing and storage. Meat Sci. 44:65–73.
- [SCF] Scientific Committee for Food. 1997. Reports of the scientific committee for food (38th series). Opinions of the scientific committee for food on: nitrates and nitrite. Opinion on nitrates and nitrite expressed on 22 September 1995, report dated 1997. Brussels: European Commission.
- Sindelar JJ, Milkowski AL. 2012. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide. 26:259–266.
- Skibsted LH. 2011. Nitric oxide and quality and safety of muscle based foods. Nitric Oxide. 24:176–183.
- Thomson BM, Nokes CJ, Cressey PJ. 2007. Intake and risk assessment of nitrate and nitrite from New Zealand foods and drinking water. Food Addit Contamin. 24:113–121.
- Walker R. 1990. Nitrates, nitrites and N-nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food Addit Contamin. 7:717–768.
- Weitzberg E, Lundberg O. 2013. Novel aspects of dietary nitrate and human health. Annu Rev Nutr. 33:129–159.
- [WCRF] World Cancer Research Fund/American Institute for Cancer Research. 2007. Food, nutrition and the prevention of cancer: a global perspective. Washington (DC): World Cancer Research Fund/American Institute for Cancer Research; 517 pp.