ABSTRACT
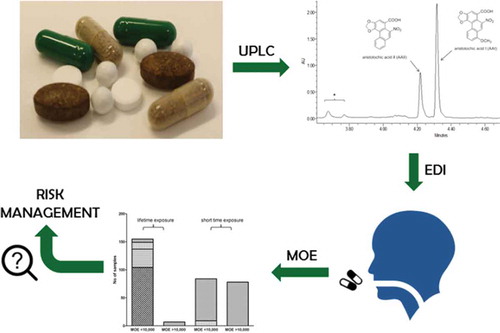
After the incidences of induction of aristolochic acid nephropathy after consumption of herbal weight loss preparations that accidentally contained aristolochic acids (AAs), several countries defined national restrictions on the presence of AAs in food, including plant food supplements (PFS) and herbal products. This study investigates whether the risks associated with exposure to AAs via PFS and herbal products are at present indeed negligible. Data reported in literature on AA levels in PFS and other herbal products and also obtained from a new series of PFS in the present study were used to calculate the estimated daily intakes (EDIs) and corresponding margins of exposure (MOEs). Available literature data revealed that 206 out of 573 samples were found to contain aristolochic acid I (AAI) and/or aristolochic acid II (AAII). The results obtained from recently collected PFS revealed that both AAI and AAII were detected in three out of 18 analysed PFS at levels up to 594.8 and 235.3 µg g–1, respectively, being in line with the levels reported in literature. The EDIs resulting from intake of these PFS resulted in MOEs that were generally below 10,000, corroborating the priority for risk management. Although these results refer to PFS collected by targeted sampling strategies, the data reveal that AA-containing PFS are still freely available. When considering that the use of these samples may be limited to shorter periods of time, the EDIs might be lower, but MOE values would still be lower than 10,000 for more than 50% of the AA-containing PFS and herbal products. In conclusion, the presence of AAs in PFS and herbal products even several years after instalment of the legal restrictions still raises concern, especially for people who frequently use the respective PFS and herbal products.
GRAPHICAL ABSTRACT
Introduction
Plant food supplements (PFS) and other herbal products are widely consumed for their perceived health benefits. It is important to note that most of these traditional botanical products have never been the subject of thorough pre-marketing toxicological safety assessment as required, for example, for modern pharmaceuticals or food additives (Schilter et al. Citation2003; Speijers et al. Citation2010). Based on their traditional use for long periods of time, these botanical preparations are often assumed to be safe. However, this is not always the case. A recent inventory on the possible presence of botanical ingredients that may be a safety concern because they are genotoxic and carcinogenic revealed concerns over especially alkenylbenzenes, pyrrolizidine alkaloids and aristolochic acids (AAs) (Van den Berg et al. Citation2011), the latter group being the topic of the present study. AAs have been proven to cause nephrotoxicity, genotoxicity and carcinogenicity and have been classified by the IARC in group 1, meaning that there is sufficient evidence that they cause tumours in humans (IARC Citation2002).
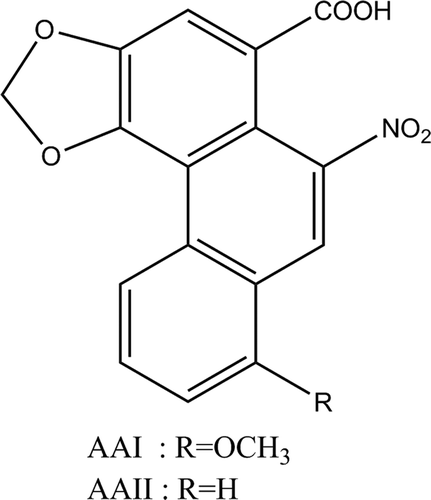
AAs are found in plants from the Aristolochiaceae family, which are also known to be one of the oldest traditional remedy systems used worldwide (Zhang et al. Citation2004). AAs derived from Aristolochia species occur as a mixture of two structurally related nitrophenanthrene carboxylic acids, including aristolochic acid I (AAI) and aristolochic acid II (AAII) () (IARC Citation2002). Aristolochia herbs have been used in obstetrics and for the treatment of snake bites (Arlt et al. Citation2002), as a therapy for arthritis, gout, rheumatism, and for festering wounds (Stiborová et al. Citation2008). Aristolochia species are also commonly used in Chinese traditional medicines. The dry fruits of Aristolochia contorta Bge. and Aristolochia debilis Seib. et Zucc., named Madouling in the Chinese Pharmacopoeia, are used to treat respiratory diseases, while the herb parts known as Tianxianteng are used as an anti-rheumatic agent (EMEA Citation2000).
Due to the anti-inflammatory properties of AAs, AA-containing preparations were developed as pharmaceutical preparations in Germany (Stiborová et al. Citation2008) until studies proved that AAs were carcinogenic in rats (Mengs et al. Citation1982). Studies conducted over the years have associated AAs with Chinese herb nephropathy and Balkan endemic nephropathy, later known as aristolochic acid nephropathy (AAN) (Grollman et al. Citation2009). In 1991, a unique form of nephropathy was reported in Belgium. Over 100 young women suffered from kidney damage leading to kidney transplantation (Stiborová et al. Citation2008), with the lesions developing into renal and bladder cancer in several patients (Vanherweghem et al. Citation1993; Vanhaelen et al. Citation1994). It was recognised that these adverse effects were the result of prolonged intake of a Chinese herb-based weight loss preparation which contained Aristolochia fangchi instead of Stephania tetranda because both plants are known under the same name, ‘Fang Ji’, in Chinese folk medicine (Vanherweghem et al. Citation1993).
After the occurrence of this dramatic episode, similar cases were reported in other European countries such as France, Spain, Germany, the UK, and also in the United States (Debelle et al. Citation2008). The responsible botanical preparations were marketed and sold in different forms: as dietary products in the form of pills or herbal infusions, for medicinal purposes such as reducing eczema, curing hepatitis B, arthritis and rheumatisms, and as pain relievers (Debelle et al. Citation2008). Subsequent to the Belgian incident, the European Agency for the Evaluation of Medicinal Products issued a position paper in October 2000 warning European Union member states ‘to take steps to ensure that the public is protected from exposure to AAs arising from the deliberate use of Aristolochia species or as a result of confusion with other botanical ingredients’ (EMEA Citation2000, p. 9). Subsequently, most member states have restricted the use of Aristolochia as well as of Stephania species in botanical products (EMEA Citation2000). In the Netherlands, AAs became regulated as herbal preparations in the Commodities Act ‘Herbal preparations’. Within this act, the presence of AAs and their derivatives have been prohibited from being marketed since 2001 (Martena et al. Citation2007). In 2001, the USFDA also advised consumers to stop using products containing AAs (USFDA Citation2001). The sale of AA-containing botanical products was also prohibited in several other countries such as Australia, Canada and New Zealand, and in many Asian countries (e.g., Japan) (IARC Citation2002; Medsafe Citation2003; Martena et al. Citation2007). In spite of this, PFS and traditional medicines containing AAs appear to be still available in markets (Lee et al. Citation2002; Schaneberg & Khan Citation2004; Chan et al. Citation2007; Martena et al. Citation2007; Vaclavik et al. Citation2014).
In the present study, the data on AA levels in PFS and other herbal products were collected from the literature and we provide an update on the presence and level of AAs in PFS purchased via online markets. The aim was to investigate whether after the Belgium incident and the subsequent regulatory awareness and measures taken, the risks associated with exposure to AAs via PFS and herbal products are indeed negligible. Based on the AA levels thus obtained, estimated daily intakes (EDIs) were established enabling risk assessment by the MOE approach to evaluate the risk from exposure to AAs from PFS purchased online from all over the world. Including the levels of AAs in PFS and other herbal products reported since 1990 was of interest given that the respective papers did not perform a risk assessment.
Materials and methods
Literature search for food samples containing AAs
Data from literature studies included in the present study were selected based on the following criteria: (1) the study reported on products that were collected from the early 1990s until 2016 and included analyses of AAs in herbal products, Chinese medicinal products or food supplements for oral use; and (2) the tested products represented commercially available products obtained from the local market, products ordered online or supplements taken by patients with AAN. Based on the collected data, the average percentage of samples that tested positive for the presence of AAI and/or AAII was calculated for each study over the years.
Collection of samples for analysis
A total of 18 PFS from different brands were purchased from different online sources. A targeted sampling approach was applied collecting samples containing botanicals of concern. Product information and the respective botanical ingredients of concern as indicated on the label of each product are summarised in .
Table 1. Product description of the PFS analysed in the present study.
Chemicals
AAI and AAII were purchased from Sigma-Aldrich (Zwijndrecht, the Netherlands). Dimethyl sulfoxide (DMSO) (> 99.9%) was obtained from Acros Organics (Geel, Belgium). Methanol and acetonitrile (ULC/MS grade) were obtained from Biosolve (Valkenswaard, the Netherlands). Trifluoroacetic acid (TFA) (> 99.8%) was purchased from Merck (Darmstadt, Germany).
Methanol extraction
To quantify the AA content in the PFS samples, methanol extracts were prepared by adding 1 g of sample to 10 ml of methanol followed by sonication for 20 min at RT. The methanol extracts were centrifuged for 5 min at 50,000 rpm and the supernatants collected for ultra-performance liquid chromatography (UPLC) analysis of AA levels. The extraction efficiency was evaluated by spiking 1 g of samples S2 and S9 () with different concentrations of AAI and AAII before starting the whole extraction procedure as described above. The average percentage of recovery was used to correct the levels of AAI and AAII in the PFS.
UPLC analysis
To quantify the presence of AAs, 3.5 µl of undiluted samples were analysed by UPLC (Waters Acquity) equipped with a Waters BEH C18 1.7 µm column, 2.1 × 50 mm (Waters Ireland) as described previously (Abdullah et al. Citation2016) with minor modifications. In short, a gradient was made with ultra-pure water containing 0.1% (v/v) TFA as solvent A and acetonitrile as solvent B. The flow rate was set to 0.6 ml min–1. The starting condition was 80:20 (A:B), changing to 75:25 from 1 to 3 min, then to 20:80 from 3 to 5 min, and keeping the gradient at this condition for 1 min. Then the gradient was modified to 0:100 from 6 to 7.3 min and retained for another 0.2 min after which the starting condition was reset from 7.5 to 8 min and kept at that level for another 1 min to equilibrate the column. Detection was carried out with a photodiode array detector (Waters, Milford, MA, USA) and chromatograms were analysed at 240 nm.
Estimated daily intakes (EDIs) of AAs resulting from the use of PFS and herbal products
The exposure estimation of AAs from current PFS was based on the recommended daily intake of the PFS as indicated by the suppliers (). EDIs were estimated using a body weight of 70 kg, the default value for adult body weight as proposed by EFSA (Citation2012). Since the PFS samples that tested positive appeared to contain both AAI and AAII, a combined exposure assessment and risk assessment was performed. Based on the similarity in the mode of action and target organ toxicity for both AAs, the combined exposure by dose addition was calculated based on the direct addition for AAI and AAII by assuming an equal potency for both AAs. For PFS and other herbal products for which AA levels were reported in the literature, EDIs were calculated using the same approach assuming consumption of 0.25 g of AA-containing products, three times a day (Vanherweghem et al. Citation1993) and 70 kg bw.
Calculation of margin of exposure (MOE) values
Considering the fact that AAs are genotoxic and carcinogenic, a harmonised approach called the margin of exposure (MOE) is recommended to judge if risk management actions are required (EFSA Citation2005). The MOE is a dimensionless ratio between the BMDL10 (lower confidence bound of the benchmark dose giving 10% extra cancer incidence) and the EDI. The BMDL10 values were calculated from data on the induction of kidney tumours by AAs in rats (Mengs et al. Citation1982) using all models for dichotomous data using the Environmental Protection Agency’s (EPA) Benchmark Dose Software (BMDS) version 2.5. The doses and the duration of treatment were adjusted to the standard lifespan (104 weeks), as discussed by Paini et al. (Citation2011). Only models that met the requirements for acceptance of the model fit were considered for the determination of BMDL10 values. The MOE-based risk assessment was performed for the PFS and herbal products containing AAs in the newly collected samples and the samples for which data were found in literature.
Results
Literature search for PFS and other herbal products containing AAs
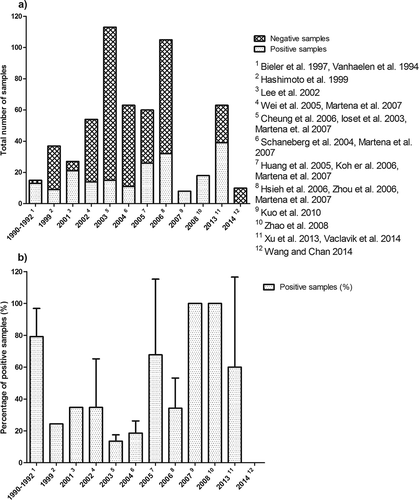
presents an overview of published data on levels of AAs in botanical samples collected from 1990 to 2016 (Vanhaelen et al. Citation1994; Bieler et al. Citation1997; Hashimoto et al. Citation1999; Ioset et al. Citation2003; Schaneberg & Khan Citation2004; Huang et al. Citation2005; Wei et al. Citation2005; Cheung et al. Citation2006; Hsieh et al. Citation2006; Koh et al. Citation2006; Zhou et al. Citation2006; Martena et al. Citation2007; Zhao et al. Citation2008; Kuo et al. Citation2010; Lee et al. Citation2002; Xu et al. Citation2013; Vaclavik et al. Citation2014; Wang & Chan Citation2014). In total, 206 out of 573 (36.0%) samples were positive for the presence of AAI, AAII or both AAs (see the supplemental data online). From 573 samples, 55 were purchased via online markets (Schaneberg & Khan Citation2004; Vaclavik et al. Citation2014), of which 12 samples (22%) tested positive for the presence of AAI and/or AAII. The other samples were purchased from local markets in different countries, including Belgium (Vanhaelen et al. Citation1994; Bieler et al. Citation1997), China and/or Japan, Korea and Taiwan (Hashimoto et al. Citation1999; Huang et al. Citation2005; Wei et al. Citation2005; Hsieh et al. Citation2006; Koh et al. Citation2006; Zhou et al. Citation2006; Kuo et al. Citation2010; Lee et al. Citation2002; Xu et al. Citation2013), Australia (Cheung et al. Citation2006), Hong Kong (Zhao et al. Citation2008; Wang & Chan Citation2014), the Netherlands (Martena et al. Citation2007), and Switzerland (Ioset et al. Citation2003). presents an analysis of these data plotting the number (a) and percentage (b) of AA-containing samples against their time of collection. The results obtained show that there is no specific trend or a reduction in the percentage of positive samples over the years.
Chemical analysis of AAs in recently collected PFS
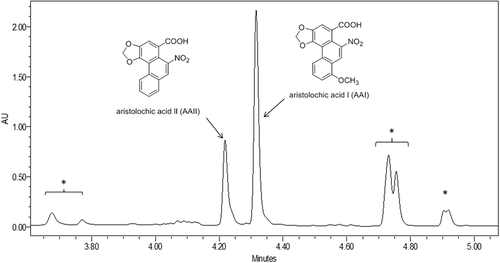
presents part of a UPLC chromatogram of a methanol extract from a PFS revealing the presence of both AAs, with AAI eluting at 4.3 min and AAII at 4.2 min. The accuracy of the method was evaluated by calculating the recovery of AAs from spiked samples. The average recovery was 92% for both AAI and AAII, and this was used to calculate the levels of AAI and AAII in the PFS samples using the calibration curves defined using commercially available reference compounds. Of the 18 PFS analysed, three samples (16.7%) tested positive and contained both AAI and AAII at levels that ranged from 2.1 to 594.8 and from 0.6 to 235.3 µg g–1 respectively (). For all these PFS, the level of AAI was 2.5–3.5-fold higher than AAII, supporting the fact that AAI is a major component in Aristolochia species (Schmeiser et al. Citation1996; Stiborová et al. Citation2003). Among the three positive samples, S2 (plant leaf) had the highest AA levels, followed by S9 (tincture) and S4 (globule). All samples that tested positive for AAs contain an ingredient called Aristolochia clematitis that is commonly known as Birthwort.
Table 2. Levels of aristolochic acid I (AAI) and aristolochic acid II (AAII) in positive samples of PFS (n = 3 independent analyses).
EDIs for combined exposure to AAs resulting from the consumption of PFS and herbal products
A combined exposure to AAI and AAII was calculated based on adding the levels of both AAI and AAII as such. The EDIs of AAs resulting from the consumption of PFS for a 70-kg person based on the recommended daily intake as indicated on the label of the respective supplement () are presented in . The EDIs amounted to 1.7 × 10–3–30 µg kg–1 bw day–1.
Table 3. Estimated daily intakes (EDIs) of AAs.
From the 206 positive AA-containing PFS and other herbal products reported in the literature, for only 159 samples were the actual levels of AAs available for further analysis and calculation of an EDI. For these 159 samples, EDIs were calculated and are presented in the supplemental data online.
Risk assessment of exposure to AAs from the consumption of PFS and herbal products using the MOE approach
In a next step, the MOEs were calculated based on the EDIs and the lowest BMDL10 of 10 µg AAs kg–1 bw day–1 () estimated from data reported for kidney tumour formation by a mixture of AAs (71% of AAI and 21% of AAII) upon oral exposure (Mengs et al. Citation1982) in rats. The MOE values thus obtained for S2, S4 and S9, presented in , were below 10,000, indicating a priority for risk management (EFSA Citation2005).
Table 4. Results from a BMD analysis of the data for kidney tumour formation in rats (Mengs et al. Citation1982) exposed to AAs using BMDS software version 2.5, a BMD of 10% and default settings based on assumption of equal potency of AAs.
Table 5. MOE values of AAs resulting from the consumption of PFS.
In addition, MOE values were also calculated for the collected literature data presented in the supplemental data online (Vanhaelen et al. Citation1994; Bieler et al. Citation1997; Hashimoto et al. Citation1999; Lee et al. Citation2002; Schaneberg & Khan Citation2004; Huang et al. Citation2005; Cheung et al. Citation2006; Hsieh et al. Citation2006; Zhou et al. Citation2006; Martena et al. Citation2007; Zhao et al. Citation2008; Kuo et al. Citation2010; Xu et al. Citation2013; Vaclavik et al. Citation2014; Wang & Chan Citation2014). shows the calculated MOEs based on the three samples analysed in the present study and the 159 samples reported in the literature for which actual levels of AAs were available enabling calculation of the respective EDIs. For 95.7% of these samples, the EDIs resulted in an MOE lower than 10,000, indicating a priority for risk management. It is interesting to note that of this 95.7%, about 64.2% of these PFS result in EDIs that indicate an MOE value lower than 10, indicating that the dose to which humans will be exposed when using these PFS will be in the range of the dose levels that caused kidney tumour formation in rats.
Figure 4. Number of samples with respective MOE values assuming a lifetime or 2 weeks of exposure to AAs based on samples analysed in the present study and reported in the literature. The MOE was calculated by dividing the lowest BMDL10 of AAs of 10 µg kg–1 bw day–1 for kidney tumour formation by the EDI of AAs from the PFS and other herbal products.
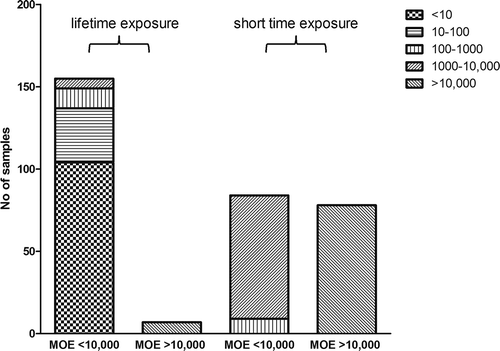
Considering that the exposure to AAs will not be lifelong but may be limited to shorter time intervals, and applying Haber’s rule (Felter et al. Citation2011) to calculate the risk for shorter-than-lifetime exposure, EDIs may be two to three orders of magnitude lower than assuming lifetime exposure. Since the labels of the respective products do not indicate a specific time period for use of the PFS, but rather indicate taking the preparation as recommended by a physician, i.e., until the disease is cured, it was assumed that 2 weeks would be a realistic minimum estimate for the period of use for the PFS. Assuming 2 weeks’ exposure, the MOE values would be 75 years × 52 weeks per year divided by 2 weeks = 1950 times lower. Taking this shorter exposure period into account, about 51.8% of the positive samples would still give rise to MOE values lower than 10,000 ().
Discussion
In the present study, a risk assessment for AAs resulting from the intake of PFS and herbal products was performed using the MOE approach. An overview of the level of AAs present in PFS and other herbal products from 1990 to 2016 showed that 206 out of 573 (36.0%) samples collected and analysed contained AAs. In order to determine the current exposure to AAs from the intake of PFS, 18 samples were purchased online and three of these 18 (16.7%) samples were shown to contain AAI at levels up to 594.8 µg g–1 and AAII at levels up to 235.3 µg g–1. It is interesting to note that although Aristolochia sp. have been banned from being present in food, including food supplements, AAs can still be found in some of the PFS. This conclusion is in line with what was found for other PFS samples and analysed after instalment of the ban in the Netherlands, the UK, the United States, Canada and Australia in 2001 (USFDA Citation2001; IARC Citation2002; Martena et al. Citation2007), and in Taiwan in 2003 (Lai et al. Citation2010).
The results clearly confirmed that PFS-containing AAs are still available on the market and easily accessible to the public despite the ban. The amount of AAs detected in the positive samples was comparable and within the range of what was reported based on an analysis of samples on the Dutch market 9 years ago (Martena et al. Citation2007) and also for samples collected in other countries including China, Australia, Japan, Korea, Taiwan and the United States (Hashimoto et al. Citation1999; Lee et al. Citation2002; Schaneberg & Khan Citation2004; Cheung et al. Citation2006; Chan et al. Citation2007; Vaclavik et al. Citation2014). The levels detected were also comparable with the levels of AAs found in the weight-loss regimen given to Belgian patients who developed AAN resulting in EDIs of 7–31 µg kg–1 bw day–1 (Vanhaelen et al. Citation1994; Bieler et al. Citation1997). When analysing the number of positive samples over the years, there was no specific trend showing a reduction in the percentage of positive samples found to contain AAs. In fact, for the current PFS that were obtained via the online market, the percentage of positive samples (16.7%) was in the same order of magnitude as the positive percentage found for the samples purchased online in 2004 (24.0%) (Schaneberg & Khan Citation2004) and 2013 (20.0%) (Vaclavik et al. Citation2014). It is of interest to note that AA-containing supplements could be accessible via the internet in countries that have regulations when ordered from countries that did not take any measures. The two positive products (S2, plant leaf and S9, tincture) were from Romania, while the globule (S4) was from Germany where measures have been taken. All these three positive samples were prepared from Aristolochia clematitis, a common plant in the wheat plantation in the Balkan region identified as the causative agent of Balkan endemic nephropathy (Grollman et al. Citation2009). EFSA has included this plant in the compendium of botanicals reported to contain toxic substances of concern (EFSA Citation2009) and all species of Aristolochia are prohibited to be present in food and PFS for sale in the Netherlands, the UK, the United States, Canada and Australia (USFDA Citation2001; IARC Citation2002; Martena et al. Citation2007).
In the current study, the calculation of MOEs for kidney tumour formation was based on the BMDL10 derived from rat data (Mengs et al. Citation1982). This rat study tested a mixture of AAs (71% of AAI and 21% of AAII) upon oral exposure. However, the composition of AAs in PFS and herbal products varies and can be different. The results of the present study showed levels of AAI that were 2.5–3.5-fold higher than AAII, in line with the mixture tested in the rodent bioassay. The composition could be different depending on the species (Xu et al. Citation2013) and part of the plant used (Zhao et al. Citation2008). In the present study combined exposure was applied to estimate the EDI of AAs by simple dose addition. In theory, one could also consider taking into account that the potency of AAI and AAII for DNA adduct formation and cancer induction may be different (Pfau et al. Citation1990; Dong et al. Citation2006; Mei et al. Citation2006; Chan et al. Citation2008) and use a so-called toxic equivalency (TEQ) approach for the risk assessment. This TEQ approach would require the definition of toxic equivalency factors (TEFs). shows an overview of the currently available data on relative potencies of AAI and AAII for relevant endpoints that could be of use in defining such TEFs. From this overview it can be concluded that data available for the definition of the TEFs are limited but seem to indicate that the difference in the relative potency of AAI and AAII would be small. This implies that when a combined AA exposure would be corrected using the TEQ approach, the overall MOE would be comparable when using the simple dose addition or a TEQ approach. Calculation of the MOEs for a lifetime exposure to AAs via PFS included in the present study resulted in MOEs that were below the default of 10,000 for 95.7% of all AA-containing samples. The default of 10,000 includes a factor 100 for interspecies differences and human variability in biokinetics and biodynamics, a factor of 10 for inter-individual uncertainties in cell cycle control and DNA repair, and another factor of 10 for the uncertainties arising from the fact of using the BMDL10 is not equivalent to no observed adverse effect level (NOAEL) and the effects can occur at a lower dose (EFSA Citation2005; Barlow et al. Citation2006). For 64.2% of the samples MOEs were even below 10, indicating the dose to which humans will be exposed when using these PFS and herbal products will be in the range of the dose levels inducing kidney tumour formation in rats. The results obtained confirm the priority for risk management of AA-containing PFS. When considering that the MOE was calculated based on lifetime exposure while the use of these PFS may be limited to shorter periods of time, the EDIs might be two to three orders of magnitude lower, e.g., 1950 times lower upon 2 weeks of exposure. However, even in this situation MOEs would still be lower than 10,000 for more than 50% of the AA-containing PFS and other herbal products.
Table 6. DNA adduct formation in the kidney of rats exposed to either AAI, AAII or a mixture of AAs obtained from the literature.
In conclusion, although the use of botanicals and botanical ingredients containing or suspected to contain plant species of the genus Aristolochia are no longer permitted in the market in many countries in the world, consumers are not yet fully protected from exposure to AA-containing PFS and other herbal products. Taken together, the present study indicates that exposure to AAs via PFS and other herbal products in the human populations even after several years of instalment of the legal restrictions is still of concern and a priority for further risk management, especially for people who frequently use the respective food supplements and herbal products.
Supplementary data
Download MS Word (92.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Abdullah R, Alhusainy W, Woutersen J, Rietjens IMCM, Punt A. 2016. Predicting points of departure for risk assessment based on in vitro cytotoxicity data and physiologically based kinetic (PBK) modeling: the case of kidney toxicity induced by aristolochic acid I. Food Chem Toxicol. 92:104–116.
- Arlt VM, Stiborova M, Schmeiser HH. 2002. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 17:265–277.
- Barlow S, Renwick A, Kleiner J, Bridges J, Busk L, Dybing E, Edler L, Eisenbrand G, Fink-Gremmels J, Knaap A, et al. 2006. Risk assessment of substances that are both genotoxic and carcinogenic: report of an international conference organized by EFSA and WHO with support of ILSI Europe. Food Chem Toxicol. 44:1636–1650.
- Bieler CA, Stiborova M, Wiessler M, Cosyns J-P, De Strihou CVY, Schmeiser HH. 1997. 32p-post-labelling analysis of DNA adducts formed by aristolochic acid in tissues from patients with Chinese herbs nephropathy. Carcinogenesis. 18:1063–1067.
- Chan W, Lee KC, Liu N, Cai Z. 2007. A sensitivity enhanced high-performance liquid chromatography fluorescence method for the detection of nephrotoxic and carcinogenic aristolochic acid in herbal medicines. J Chromatogr. 1164:113–119.
- Chan W, Yue H, Poon WT, Chan Y-W, Schmitz OJ, Kwong DW, Wong RN, Cai Z. 2008. Quantification of aristolochic acid-derived DNA adducts in rat kidney and liver by using liquid chromatography–electrospray ionization mass spectrometry. Mutat Res/Fund Mol Mech Muta. 646:17–24.
- Cheung TP, Xue C, Leung K, Chan K, Li CG. 2006. Aristolochic acids detected in some raw Chinese medicinal herbs and manufactured herbal products–a consequence of inappropriate nomenclature and imprecise labelling? Clin Toxicol. 44:371–378.
- Debelle FD, Vanherweghem J-L, Nortier JL. 2008. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 74:158–169.
- Dong H, Suzuki N, Torres MC, Bonala RR, Johnson F, Grollman AP, Shibutani S. 2006. Quantitative determination of aristolochic acid-derived DNA adducts in rats using 32p-postlabeling/polyacrylamide gel electrophoresis analysis. Drug Metab Dispos. 34:1122–1127.
- EFSA. 2005. European Food Safety Authority. opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 282:1–31.
- EFSA. 2009. EFSA compendium of botanicals that have been reported to contain toxic, addictive, psychotropic or other substances of concern. EFSA J. 7:281.
- EFSA. 2012. Guidance on selected default values to be used by the EFSA scientific committee, scientific panels and units in the absence of actual measured data. EFSA J. 10:2579.
- EMEA. 2000. The European Agency for the evaluation of medicinal products, position paper on the risks associated with the use of herbal products containing aristolochia species. Document EMEA/HMPWP/23/00. London; p. 1–10.
- Felter SP, Conolly RB, Bercu JP, Bolger PM, Boobis AR, Bos PM, Carthew P, Doerrer NG, Goodman JI, Harrouk WA. 2011. A proposed framework for assessing risk from less-than-lifetime exposures to carcinogens. Crit Rev Toxicol. 41:507–544.
- Grollman AP, Scarborough J, Jelakovic B. 2009. Aristolochic acid nephropathy: an environmental and iatrogenic disease. Adv Mol Toxicol. 3:211–227.
- Hashimoto K, Higuchi M, Makino B, Sakakibara I, Kubo M, Komatsu Y, Maruno M, Okada M. 1999. Quantitative analysis of aristolochic acids, toxic compounds, contained in some medicinal plants. J Ethnopharmacol. 64:185–189.
- Hsieh S-C, Huang M-F, Lin B-S, Chang H-T. 2006. Determination of aristolochic acid in Chinese herbal medicine by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr. 1105:127–134.
- Huang C-Y, Tseng M-C, Lin J-H. 2005. Analyzing aristolochic acids in Chinese herbal preparations using LC/MS/MS. J Food Drug Anal. 13:125–131.
- IARC. 2002. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: World Health Organization; p. 68–128.
- Ioset J-R, Raoelison G, Hostettmann K. 2003. Detection of aristolochic acid in Chinese phytomedicines and dietary supplements used as slimming regimens. Food Chem Toxicol. 41:29–36.
- Koh H, Wang H, Zhou S, Chan E, Woo S. 2006. Detection of aristolochic acid I, tetrandrine and fangchinoline in medicinal plants by high performance liquid chromatography and liquid chromatography/mass spectrometry. J Pharm Biomed Anal. 40:653–661.
- Kuo C-H, Lee C-W, Lin S-C, Tsai I-L, Lee -S-S, Tseng YJ, Kang -J-J, Peng F-C, Wei-Chu L. 2010. Rapid determination of aristolochic acids I and II in herbal products and biological samples by ultra-high-pressure liquid chromatography–tandem mass spectrometry. Talanta. 80:1672–1680.
- Lai M-N, Lai J-N, Chen P-C, Hsieh S-C, Hu F-C, Wang J-D. 2010. Risks of kidney failure associated with consumption of herbal products containing Mu Tong or Fangchi: a population-based case-control study. Am J Kidney Dis. 55:507–518.
- Lee T-Y, Wu M-L, Deng J-F, Hwang D-F. 2002. High-performance liquid chromatographic determination for aristolochic acid in medicinal plants and slimming products. J Chromatogr B. 766:169–174.
- Martena MJ, van der Wielen JC, van de Laak LF, Konings EJ, de Groot HN, Rietjens IMCM. 2007. Enforcement of the ban on aristolochic acids in Chinese traditional herbal preparations on the Dutch market. Anal Bioanal Chem. 389:263–275.
- Medsafe. 2003. Herbal, traditional and complementary medicines. Wellington: New Zealand Medicines and Medical Devices Safety Authority.
- Mei N, Arlt VM, Phillips DH, Heflich RH, Chen T. 2006. DNA adduct formation and mutation induction by Aristolochic acid in rat kidney and liver. Mutat Res/Fund Mol Mech Muta. 602:83–91.
- Mengs U, Lang W, Poch J-A. 1982. The carcinogenic action of aristolochic acid in rats. Arch Toxicol. 51:107–119.
- Paini A, Scholz G, Marin-Kuan M, Schilter B, O’Brien J, van Bladeren PJ, Rietjens IMCM. 2011. Quantitative comparison between in vivo DNA adduct formation from exposure to selected DNA-reactive carcinogens, natural background levels of DNA adduct formation and tumour incidence in rodent bioassays. Mutagenesis. 26:605–618.
- Pfau W, Schmeiser HH, Wiessler M. 1990. 32p-postlabelling analysis of the DNA adducts formed by aristolochic acid I and II. Carcinogenesis. 11:1627–1633.
- Schaneberg B, Khan I. 2004. Analysis of products suspected of containing Aristolochia or Asarum species. J Ethnopharmacol. 94:245–249.
- Schilter B, Andersson C, Anton R, Constable A, Kleiner J, O’Brien J, Renwick A, Korver O, Smit F, Walker R. 2003. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem Toxicol. 41:1625–1649.
- Schmeiser HH, Bieler CA, Wiessler M, De Strihou CVY, Cosyns J-P. 1996. Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res. 56:2025–2028.
- Speijers G, Bottex B, Dusemund B, Lugasi A, Tóth J, Amberg‐Müller J, Galli CL, Silano V, Rietjens IMCM. 2010. Safety assessment of botanicals and botanical preparations used as ingredients in food supplements: testing an European Food Safety authority‐tiered approach. Mol Nutr Food Res. 54:175–185.
- Stiborová M, Frei E, Arlt VM, Schmeiser HH. 2008. Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy. Mutat Res/Rev Mutat Res. 658:55–67.
- Stiborová M, Frei E, Sopko B, Sopková K, Marková V, Laňková M, Kumstýřová T, Wiessler M, Schmeiser HH. 2003. Human cytosolic enzymes involved in the metabolic activation of carcinogenic aristolochic acid: evidence for reductive activation by human NAD(P)H: quinone oxidoreductase. Carcinogenesis. 24:1695–1703.
- USFDA. 2001. Aristolochic acid: FDA warns consumers to discontinue use of botanical products that contain aristolochic acid. US Food and Drug Administration; 2001 April 11; Silver Spring (MD).
- Vaclavik L, Krynitsky AJ, Rader JI. 2014. Quantification of aristolochic acids I and II in herbal dietary supplements by ultra-high-performance liquid chromatography–multistage fragmentation mass spectrometry. Food Addit Contam. 31:784–791.
- Van den Berg SJPL, Restani P, Boersma MG, Delmulle L, Rietjens IMCM. 2011. Levels of genotoxic and carcinogenic compounds in plant food supplements and associated risk assessment. Food Nutr Sci. 2:989–1010.
- Vanhaelen M, Vanhaelen-Fastre R, But P, Vanherweghem J-L. 1994. Identification of aristolochic acid in Chinese herbs. Lancet. 343:174.
- Vanherweghem J-L, Tielemans C, Abramowicz D, Depierreux M, Vanhaelen-Fastre R, Vanhaelen M, Dratwa M, Richard C, Vandervelde D, Verbeelen D, Jadoul M. 1993. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet. 341:387–391.
- Wang Y, Chan W. 2014. Determination of aristolochic acids by high-performance liquid chromatography with fluorescence detection. J Agric Food Chem. 62:5859–5864.
- Wei F, Cheng XL, Ma LY, Jin WT, Schaneberg BT, Khan IA, Lin RC. 2005. Analysis of aristolochic acids and analogues in medicinal plants and their commercial products by Hplc‐Pad‐Esi/Ms. Phytochem Anal. 16:222–230.
- Xu Y-Q, Li X-W, Liu G-X, Wang X, Shang M-Y, Li X-M, Cai S-Q. 2013. Comparative study of the contents of analogues of aristolochic acid in two kinds of aristolochiae fructus by high-performance liquid chromatography. J Nat Med. 67:113–122.
- Zhang H, Cifone M, Murli H, Erexson G, Mecchi M, Lawlor T. 2004. Application of simplified in vitro screening tests to detect genotoxicity of aristolochic acid. Food Chem Toxicol. 42:2021–2028.
- Zhao Z-Z, Liang Z-T, Jiang Z-H, Leung KS-Y, Chan C-L, Chan H-Y, Sin J, Man T-O, Law K-W. 2008. Comparative study on the aristolochic acid I content of Herba Asari for safe use. Phytomedicine. 15:741–748.
- Zhou X, Zheng C, Sun J, You T. 2006. Analysis of nephroloxic and carcinogenic aristolochic acids in aristolochia plants by capillary electrophoresis with electrochemical detection at a carbon fiber microdisk electrode. J Chromatogr. 1109:152–159.