 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ethoxyquin (EQ) is an antioxidant supplemented to feed ingredients, mainly fish meal, which is currently under re-evaluation for use in the food production chain. EQ is partly metabolized into several metabolites of which the ethoxyquin dimer (EQDM) accumulates most in the farmed fish fillet. In this study, the feed-to-fillet transfer of dietary EQ and EQDM in Atlantic salmon fillet was investigated, and a physiologically based pharmacokinetic (PBPK-) two-compartmental model was developed, based on experimental determined EQ and EQDM uptake, metabolism, and elimination kinetics. The model was verified with an external data-set and used to simulate the long term (>1.5 years) EQ and EQDM feed-to fillet transfer in Atlantic salmon under realistic farming conditions such as the seasonal fluctuations in feed intake, growth, and fillet fat deposition. The model predictions showed that initial EQDM levels in juvenile fish are the driving factor in final levels found in food-producing animals, while for EQ the levels in feed, and seasonal variations were the driving factor for food EQ levels.
Introduction
Ethoxyquin (EQ; 1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline) is an aromatic amine with the ability to scavenge lipid peroxide radicals and thus terminate the spontaneous oxidation of unsaturated lipids in fish feeds and feed ingredients. In Atlantic salmon (Salmo salar), dietary EQ is rapidly metabolized into several metabolites of which the ethoxyquin dimer (EQDM; 1,8ʹ-di(1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline) is one of the dominant ones that accumulate in the fillet (Bohne et al. Citation2007). EQDM seems to have a higher potential to accumulate in Atlantic salmon fillet than its parent compound (Bohne et al. Citation2007, Citation2008). In commercial Norwegian farmed Atlantic salmon surveyed in 2007, average levels of muscle EQDM were around 730 µg kg−1, while EQ levels averaged EQ around 55 µg kg−1 (Lundebye et al. Citation2010). Results from an annual feed monitoring program commissioned by the Norwegian Food Safety Authorities showed that during the years 2007–2017 the average EQ concentration in Norwegian fish feeds was 17 ± 24 mg kg−1 with a minimum and maximum concentration of 0.5 and 224 mg kg−1 (including both EQ and its metabolite EQDM; n = 503) (NFSA Citation2018). After extensive (>1 year) storage, metabolites are formed in the salmon feed with EQDM as one of the major metabolites, i.e. 30–40% of the total EQ+metabolites, (He et al. Citation2000; Negreira et al. Citation2017).
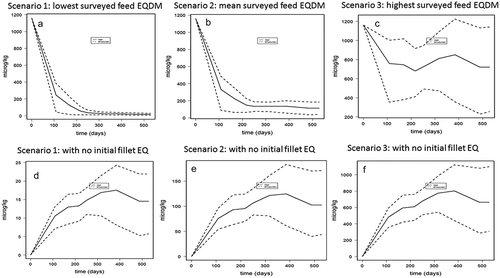
Figure 6. (a–f). Whole seawater production cycle model predictions (mean and 95% lower and upper bound) of fillet ethoxyquin dimer (EQDM) concentrations (µg kg−1 ww) in Atlantic salmon fed lowest (scenario 1, 0.64 mg kg−1), mean (scenario 2, 4.6 mg kg−1), and highest (scenario 3, 29.4 mg kg−1) EQ levels surveyed in Norwegian commercial salmon feeds in 2017 and assuming a maximum 40% metabolisation of dietary EQ into EQDM. Prediction with an initial fish fillet EQDM value of 1198 µg kg−1 ww are given in the upper panels (a–c for scenario 1, 2,and 3, respectively), and predictions without initial EQDM levels are given in the lower panels (d–f for scenario 1, 2, and 3, respectively).
EQ, and other antioxidant such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), have been authorized in feed for all animal species with a maximum concentration of 150 mg kg−1 feed, alone or in combination with other authorized synthetic antioxidants (Council Directive 70/524/EEC, replaced by Council Regulation (EC) No. 1831/2003) (EC Citation2003). EQ is not an approved food additive in the EU, nevertheless it may be transferred from feed to fillet, and this potential transfer has received increasing attention from a food safety perspective. In the EU, the use of EQ as feed ingredient additive is currently under re-evaluation for use, or prohibition, in the food production chain. In order to provide a pro-active approach to ensure consumer safety, feed legislation ought to assure compliance with food safety legislation. Like feed contaminants, information on the transfer of additives from feed to animal food products is essential for appropriate human risk assessment (Leeman et al. Citation2007) on the use of feed additives as well as for harmonization of legislation of feed additives throughout the food product chain (van Raamsdonk et al. Citation2009). Transfer models have been used to describe the fate of feed xenobiotics in farmed animals, including farmed Atlantic salmon, and are valuable tools in predicting food xenobiotic levels at different feed level scenarios (Hoogenboom et al. Citation2010; Berntssen et al. Citation2011b).
The feed-to-food transfer of xenobiotics is often assessed in physiological based toxico-kinetic (PBK) models, which are based on xenobiotic-specific uptake and elimination kinetics (Nichols et al. Citation2004; Berntssen et al. Citation2007, Citation2011a, Citation2011b, Citation2013; Brambilla et al. Citation2007). Despite the extensive use of EQ, only a few studies have reported on the EQ uptake and elimination kinetics in farmed fish. More importantly, the kinetic information (including metabolisation from the parent compound EQ) on EQDM is lacking or incomplete. Earlier trials with Atlantic salmon established concentration dependent uptake rates/absorption efficiencies for EQ with a rapid elimination and half-lives around 2.1–2.6 days (Bohne et al. Citation2008) or 7 days (Skaare et al. Citation1977). The EQDM was not eliminated, but rather increased, during a 14 days withdrawal period (Bohne et al. Citation2008). The present study provides long-term EQDM elimination kinetics, which is needed to assess the toxico-kinetic metabolisation of feed EQ and transfer of EQDM to the edible part of the fish. The use of physiological based toxico-kinetic models allows taking into account physiological changes such as feed intake, organ (fillet) growth, and fillet fat deposition (Berntssen et al. Citation2011b, Citation2013). These are important variables when predicting transfer and metabolisation under realistic long-term (> 1 year) seawater farming conditions with seasonal fluctuations in these physiological parameters. Earlier published physiological based toxico-kinetic (PBK) multicompartmental models for fat-soluble compounds described the physiological changes in Atlantic salmon, such as fillet growth and fat deposition (Berntssen et al. Citation2011b, Citation2013). The present study aims to provide for the first time the combined uptake, metabolisation, and elimination kinetics of EQ and EQDM from the muscle of Atlantic salmon. These kinetics are derived from the experimental data from an exposure study in which Atlantic salmon were fed EQ spiked feeds for 90 days followed by a 90-day depuration period. The kinetics parameters are implemented in an Atlantic salmon (PBK) multicompartmental model, which is used to predict the transfer and metabolisation of EQ and EQDM during an entire seawater food production cycle.
Material and methods
Ethical statement
The experiment was approved by the Norwegian National Animal Research Authority (Mattilsynet; FOTS ID: 9004) and performed in compliance with national and international ethical standards.
Diet preparation
EQ-low diets were produced in a single batch as 5-mm extruded pellets by EWOS Innovation (Bergen, Norway), according to an established commercial feed formulation (Harmony Debio, organic fish feed). The control feed was not free of EQ due to the presence of EQ in feed ingredients other than fishmeal, and the control background level was 0.47 mg EQ kg−1. The feed preparation is described in detail by Bernhard et al. (Citation2019). Briefly, EQ fortified diets were produced by vacuum coating 8 v/w% EQ (Capsoquin batch no. S-5162, 99% purity; courtesy of Industrial Técnica Pecuaria, S.A., Spain) enriched fish oil to low EQ salmon feeds. The fortification level (~150 mg kg−1) was chosen as the previous maximum content for EQ in the fish feed (150 mg kg−1) to ensure high enough dietary concentration to give detectable levels of EQ and EQDM in fish muscle to assess kinetic uptake and elimination rates. Measured levels were 119 ± 7 mg kg−1 (mean ± SD, n = 6). The fortified feeds were stored at −18°C before and during the dietary exposure period. Feed samples were taken during the trial, and the analysis did not show any degradation during the experimental period. Concentrations of EQDM were below the limit of quantification (LOQ <0.07 mg EQDM kg−1 feed) during the cold storage.
Feeding trial
Fish fed 119 mg EQ kg−1 were sampled over a 90-day exposure period, followed by a 90-day depuration period in which the fish were fed a low-EQ control diet. The feeding trial was carried out at NOFIMA (Sunndalsøra, Norway) between July and October 2016. The experimental conditions are described in Bernhard et al. (Citation2019). Briefly, post-smolt (Salmobreed, both genders) were randomly distributed among six fibreglass tanks (840 l; ~70 fish per tank). At the beginning of the experiment, the weight of fish was 213 ± 35 g, (mean ± standard deviation; n = 20). Prior to the trial start, fish were acclimatized in their tanks to the control feed for a period of 14 days. Fish were reared under a 24-h light regime and fed by automatic feeders in six meals a day to a feed intake level approximating 0.80% of body weight per day. The feeding rate was adjusted for growth biomass increase, which was assessed by measured average weight gain of the sampled fish per sampling time point, and biomass reduction due to sampling. Uneaten pellets were collected in a flow-over system and registered daily thus providing exact feed intake values daily. Water temperature, pH, oxygen availability and salinity were monitored daily during the experiment. During the exposure period, five fish from each tank were sampled at day 0, 2, 4, 10, 20, 45 and 90. During the depuration period, five fish per tank were sampled at day 0, 3, 6, 22, 40, 60 and 90. To avoid potential water contamination by EQ from the diet and faeces, a high water flow (~15 L min−1) was maintained through the tanks. Water samples were taken 1 h after feeding at the final exposure sampling, and no EQ residues (LOQ<0.1 mg L−1) were detected. At sampling, the fish were anaesthetised in a bath of tricaine methanesulfonate (FINQUEL MS-222; ~60 mg L−1) before body weight and fork-tail length were measured. Fish were stored at −28°C and at the end of the experiment all fish were thawed and filleted (whole fillet muscle without skin on the left side of the salmon), five fish per tank were pooled (n = 3 per sampling point), and analysed for EQ and EQDM.
EQ and EQDM analyses
Ethoxyquin levels were quantified by reversed-phase high-performance liquid chromatography with fluorescence detection, using an external standard curve. After extraction with hexane from muscle, samples were saponified in ethanol-NaOH. EQ and EQDM from feed samples were extracted directly with 0.1% (w/v) solid acetic acid in acetonitrile as previously described by Bohne et al. (Citation2007), with modifications described by Ørnsrud et al. (Citation2011).
Modelling approach
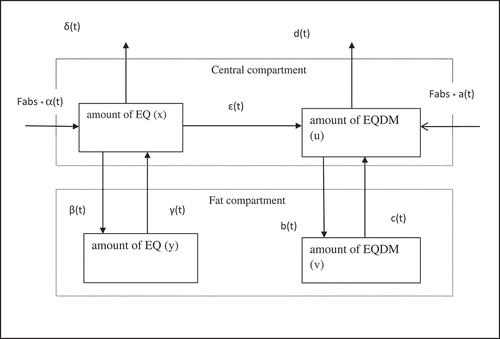
Based on the results from previous Atlantic salmon feed-to fillet transfer studies, a two-compartmental model (Berntssen et al. Citation2011b) was used in which a parental chemical compound is transformed into a main accumulating metabolite (Berntssen et al. Citation2013), that describes the flow, metabolism and excretion of EQ and EQDM. The first compartment can be interpreted as the combination of central and muscle compartment, and the second compartment as the fat compartment. The fillet compartment consists of part of the central compartment and part of the fat compartment. Metabolism of EQ into EQDM takes part in the central compartment
In the model description (), x and y are the amounts of EQ in the central and fat compartment, respectively. The parameters u and v indicates the amounts of EQDM in the central and fat compartments, respectively. The entities α(t) and a(t) are the intake rates of EQ and EQDM, respectively, Fabs is the fraction absorption parameter, β(t) and b(t) are the transport rates of EQ and EQDM from the central to the fat compartment, respectively, and γ(t) and c(t) are the reversed transport rates from the fat compartment to the central compartment. The entities δ(t) and d(t) are the excretion rates of EQ and EQDM out of the central compartment, respectively. Last, ε(t) is the metabolism rate of the formation of EQ into EQDM. Note that the structures of the models related to EQ and EQDM are similar. The two differences are that in the model related to EQ, the excretion out of the central compartment is described by three rates (δ(t) and ε(t) followed by d(t))instead of one rate value d(t) in the model related to EQDM, and as a result in the model related to EQDM the inflow is described by three rate values (Fabs * a(t) and Fabs * α(t) followed by ε(t)) instead of one rate value (Fabs * α(t)) in the model related to EQ.
Figure 1. A serial two-compartment modelling scheme to assess the transfer of dietary ethoxyquin (EQ) and transformation into the metabolite ethoxyquin dimer EQDM to the fillet accumulation in Atlantic salmon fed with EQ. The whole fish can be divided into a central- and fat compartment. The central compartment can be divided into the muscle fibre fraction of the fillet and other non-fat organs such as liver, kidney, gills etc. The fat compartment in which EQ and EQDM will accumulate can be divided into a fillet part and fat in other organs such abdominal fat. The parameters x and y are the amounts of EQ in the central and fat compartment, respectively, while u and v indicates the amounts of EQDM in these compartments, respectively. The entities α(t) and a(t) are the intake rates of EQ and EQDM, respectively, Fabs is the fraction absorbed parameter of EQ and EQDM, β (t) and b(t) are the transport rates of EQ and EQDM from the central to the fat compartment, respectively, and γ (t) and c (t) are the reversed transport rates from the fat compartment to the central compartment. ε (t) is the metabolism rate of the formation of EQ into EQDM. The entities δ (t) and d (t) are the excretion rates of EQ and EQDM out of the central compartment, respectively.
Model parameterisation and assumptions
The fish used in this trial was not free of EQDM at the start of the experimental period, due to EQDM from prior feeding with commercial feed. The two weeks of acclimatization with low EQ feed were not sufficient to deplete the fish of EQDM as opposed to EQ. So, the initial amount of EQDM was treated as a model parameter to be estimated. Consequently, the model parameters to be estimated were the compartment transition rates (δ, β, ε, γ, d, b and c, see ), the Fabs parameter, and the initial amount of EQDM in the body of the salmon. Since only total fillet concentration were analysed, and not the separate fat or central compartments, the statistical model had to be simplified while finding a good model fit. This means that not all model parameters (EQ and EQDM fat-to-central and vice versa transport rates;β (t), γ (t) b (t) and c (t)) could be estimated separately. Hence, assumptions on these parameters had to be made. As models assuming the initial EQDM amount in the fat or the central compartment gave similar model fits, given the relatively high fat solubility of EQDM, it was assumed that the initial amount of EQDM resides in the fat compartment. As a verification additional analyses of pure fat samples (belly fat from six fish at the end of the EQ exposure period) indeed showed high (5–8 µmol kg−1fat) levels of EQDM. After stopping the intake of EQ the EQ concentration showed a fast decrease, but the EQDM concentration showed a slow decrease. Therefore, it was assumed that the EQDM fat-to-compartment transition rate was smaller than the EQ fat-to-compartment transition rate. Furthermore, as the data did not allow identification of EQ and EQDM central-to-fat-compartment and fat-to-central-compartment transition rates, all these compartment transition rates were assumed to be equal, except for the EQDM fat-to-compartment transition rate. So, referring to , the following restrictions were put on the model parametrisation: β(t) = γ(t) = b(t) ≠ c(t), δ(t) = d(t), and γ(t) > c(t). The R-routine NLS (non-linear least squares model fitting) was used to fit the model to the data. Since it was not guaranteed that the estimated parameter values would be positive, we estimated the log-transformed instead of nominal parameter values.
Model equations
The mathematical model equations describe the changes of the amounts of EQ and EQDM in the central and fat compartments.
The symbols used for the compartment transition rates above are in fact short-hand notations for more complex formulas (Berntssen et al. Citation2011c), e.g.
The parameter f denotes the fraction of cardiac output Q(t) flowing to and from the fat compartment, pc is a partition coefficient, Wc(t) is the weight of the central compartment. Likewise,
The parameter CL denotes the clearance (excretion of EQ or EQDM from the liver) per unit of liver weight. Plotting the and organ and body weight values against time showed that the ratios of all time-dependent weight values did not vary over time (less than 5% variation), so we assumed them constant. As a result, we assumed all compartment transition rates δ, β, ε, γ, d, b and c to be time-constant. However, note that the concentrations of EQ and EQDM were calculated as the ratio of the calculated amounts and the time-dependent compartment weights.
Model validation
The kinetic model that was based on experimental data from the present trial, was validated by fitting the model to another external data-set from an earlier published dietary EQ trial in Atlantic salmon (Bohne et al. Citation2008). In the Bohne et al. (Citation2008) experiment, as in the present study, there was an initial amount of EQDM. Therefore, for the validation of the present model with the external data set of Bohne et al. (Citation2008), it was assumed the amount at time t = 0 to be identical to the first measured value, and it was assumed that only the central compartment contained EQDM at time t = 0. Validation was performed by comparing model predicted values with the analysed levels from the external data set.
Model extrapolation to estimate fillet EQ and EQDM levels during a production cycle
The fitted model, that was based on 90 days exposure followed by 90 days depuration, was used to predict the fillet concentrations of EQ and EQDM in salmon during a whole seawater production cycle fed on different EQ and EQDM feed levels. The range of the input data used in the extrapolation model included three feed EQ/EQDM level scenarios, based on the lowest (1.6 mg EQ and 0.64 mg EQDM kg−1, scenario 1), highest (73.4 mg EQ and 29.4 mg EQDM kg−1, scenario 2) and mean (11.6 mg EQ and 6.6 mg EQDM kg−1, scenario 3) level of dietary EQ as surveyed in Norwegian produced commercial salmon feed in 2017 (Sanden et al. Citation2017), and assumed maximum 40% metabolisation of feed EQ into EQDM (He and Ackman Citation2000; Negreira et al. Citation2017). Further input data for the whole cycle model predicted fillet levels included average seasonal variations in daily feed intake, growth rate and fat deposition in Atlantic salmon farmed from about 80 g to about 4 kg during an 18-month period, as during a commercial production cycle (Berntssen et al. Citation2010b, Citation2016; Lock et al. Citation2011). The daily dietary EQ/EQDM intake per salmon, weight gain and relative fillet fat deposition during a seawater production cycle varied based on season and water temperature as well as fish size. We assumed the initial amount of EQDM in a 80 g pre-smolt being 1198 µg kg−1 in the extrapolation, based on the highest analysed initial values in the fish used in the present study. When making a prediction for all time points during an entire production cycle, we assumed all estimated parameter values to be unchanged over time. This assumption was valid because the data did not point at the model parameters (compartment transition rates, metabolic rate, and excretion rates) being dependent on time.
Statistics
All EQDM and EQ exposure concentrations were corrected for residue levels in fish fed the control diet. For the modelling medium bound values (LOQ) were used for values lower than the detection level (0.021 µg kg−1 for EQ and 0.063 µg kg−1 for EQDM). All data are converted from mg kg−1 ww to µmol kg−1 ww as to compensate for the difference in molecular mass between EQ and EQDM (217 and 432 g mol−1, respectively). All significant differences in EQ and EQDM fillet levels between the different time points were assessed as following: A Kolmogorov–Smirnov test was used to assess normality of distribution of each treatment (Zar Citation1984). All data were found to be normally distributed. Statistical differences for parameters between time points were assessed by ANOVA, followed by Tukey’s HSD post hoc test. Significant differences among the group were set at p < .05 (Zar Citation1984). All statistics were performed using the program Statistica (Statsoft Inc., Tulsa, USA).
Results and discussion
Fillet levels during uptake and elimination
gives the fillet concentrations (in µmol kg−1) of EQ and EQDM in Atlantic salmon fed with EQ spiked feed (119 mg kg−1, or 548 µmol kg−1) for 90 days, followed by a 90-day depuration period during which the fish were fed 0.47 mg EQ kg−1 (2 µmol kg−1). EQ had a rapid mono-phasic, nearly linear, uptake until day 20 after which the fillet EQ levels stabilized to a presumed steady state. Between day 20 and 90 of the exposure period, fillet EQ levels decreased which coincided with a reduced feed intake during this period (feed intake 0.85% BW day−1 for feeding period 0–20 days and 0.70% BW day−1 for feeding period 20–90 days). However, no significant (p < .05) differences were observed in fillet levels between day 20 and 90. After three days of depuration, EQ levels fell significantly from the start of the depuration (day 90). EQ showed a rapid elimination with near-zero levels after 22 days in the depuration period. The estimated overall half-life of EQ was 7.8 ± 0.9 days, which is longer than the half-lives of 2.1–2.6 days previously reported for Atlantic salmon (Bohne et al. Citation2008), but similar to the half-life of 7 days for total EQ products described by (Skaare et al. Citation1977). The difference in half-life in this study compared to Bohne et al. (Citation2008) could be explained by the differences in the duration of the elimination period (14 days in (Bohne et al. Citation2008) versus 90 days in the present study). When only the first 22 days of depuration in the present study were included in the half-life assessment, the estimated half-life for EQ was 3.1 ± 1.3 days.
Figure 2. Upper panel: fillet concentration (µmol kg−1 ww) of ethoxyquin (EQ) and its main metabolite ethoxyquin dimer (EQDM) in Atlantic salmon fed EQ spiked feed (119 mg kg−1, EQ, EQDM< LOQ 0.07 mg kg−1) for 90 days, followed by a 90 day depuration period with low EQ feed (0.47 mg kg−1). The dashed vertical line indicates the beginning of the depuration period. Lower panel: EQ and EQDM fillet concentration during the depuration period (n = 5 per sample point given as mean value and error bars represent standard deviation).
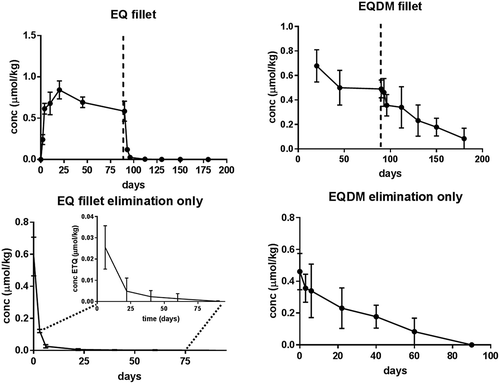
The fish used in this trial was not free of EQDM at the start of the experimental period, due to EQDM from prior feeding with commercial feed. The two weeks of acclimatization with control EQ feed were not sufficient to deplete the fish of EQDM as opposed to EQ. Hence, accumulation could not be assessed. The fillet EQDM levels were not significantly different during the dietary exposure period (from day 20–90). In the depuration period, fillet EQDM decreased significantly after 22 days from the start of the depuration (day 90), indicating a far lower elimination than the parent compound EQ. At the end of the elimination period, EQDM fillet levels were 30% of those at the start of the elimination period (day 90). As no EQDM was present in the feed during the exposure period, all EQDM was assumed to be formed from metabolisation of the parent compound EQ. The estimated overall fillet half-life of EQDM was 71 days, thus a period exceeding 3–6 months would be required to obtain EQDM free fish. This long half-life for EQDM also explains the high EQDM levels in the fish at the start of the trial and warrants a different design of future trials, requiring long term rearing (likely from first-feeding onwards) of fish on EQ free diets.
Model performance and validation
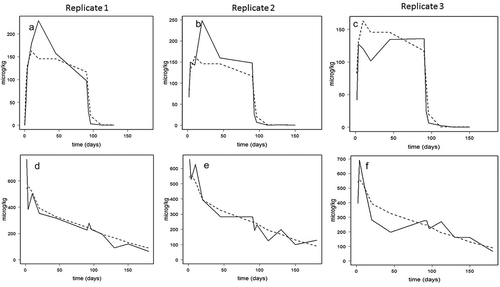
Different model parameterisations (see section Model parameterisation) were fitted to the experimental data from the replicate tanks. No significant differences in model parameters (e.g. Fabs) were observed among the replicates, thus model-parameters were set to be equal for all tanks. The model parameters used in the predictive model are given in the supplementary data (Table 1). shows the fit of the model to the experimental data per individual tank (3 tanks in total). As shown, for two of the three replicates the model underestimates the peak EQ concentration at the end of the exposure period, while for the third replicate the model peak and experimental data overlapped. For EQDM the model gives a slight underestimation of the elimination for two of the replicate tanks. The deviation in model prediction among the different replicates can be attributed to biological and experimental variation within the trial.
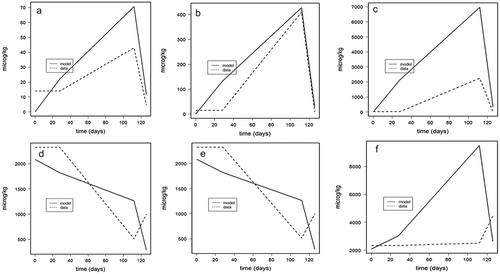
Figure 3. (a–f). Model predicted fillet concentrations (µg kg−1 ww) (broken line) and experimental data (even line) for ethoxyquin (EQ) (a–c; upper panels) and its main metabolite ethoxyquin dimer (EQDM) (d–f; lower panels) in three replicate tanks (replicate 1, 2, 3) in Atlantic salmon fed EQ spiked feed (119 mg kg−1, EQ, EDQ < LOQ 0.07 µg kg−1) for 90 days, followed by a 90-day depuration period with control feed (0.47 mg EQ kg−1).
The model was validated by applying the model fitting to another experimental data-set and comparing the predicted to the data concentration values. The external data-set used, was based on an earlier published trial in which Atlantic salmon (initial weight ~200 g) was fed EQ enriched feed with a concentration of 18, 107, or 1800 mg kg−1 EQ for 12 weeks followed by a two weeks depuration period (Bohne et al. Citation2008). The results of the model validation showed a good fit between the predicted and the actual fillet concentrations in case of low (18 mg kg−1) or intermediate (107 mg kg−1) dietary EQ levels ( for fillet EQ and EQDM, respectively). However, at the highest dietary EQ levels (1800 mg kg−1), the model over-estimated the fillet concentration ( for fillet EQ and EQDM, respectively). The highest level used in the trial conducted by Bohne et al. (Citation2008), by far exceeded the average EQ levels surveyed in commercial available salmon feeds (11.6 mg EQ kg−1) in 2017 (Sanden et al. Citation2017) and the former EU maximum limit for EQ in animal feeds (150 mg kg−1). Comparing the ratios of the data concentration values to the data intake values, it seems that some non-linear processes may take place that are not covered by our model at the highest exposure level (1800 mg kg−1). This might reflect an oversaturation EQ metabolism, transport from central to the fat compartment, and/or excretion capacity at these high exposure levels compared to the model transition rates that were assessed at more moderate exposure levels (119 mg kg−1). Hence, the current model would give an over-estimation of fillet EQ concentrations fish are fed with feed with high EQ levels. For the feed concentration from the external data set (Bohne et al. Citation2008) that are in the same range as the present trial (107 versus 119 mg kg−1) and feed concentrations in the range typically found in commercial salmon feeds (18 versus 11.6 mg kg−1), a good fit was found verifying the model ().
Figure 4. (a–f). Model verification for fillet ethoxyquin (EQ) (upper panels a–c) and fillet ethoxyquin dimer (EQDM) (lower panels d–f) for Atlantic salmon fed EQ enriched feed at concentrations of 18 (a,d), 107 (b,e), or 1800 (c,f) mg EQ kg−1 for 12 weeks followed by a two-week depuration period (Bohne et al. Citation2008).
Model life cycle fillet prediction levels at different feeding scenarios
The model was used to predict fillet EQ and EQDM for farmed Atlantic salmon are fed different levels of EQ and EQDM for an average full seawater production cycle. In addition to the different feed concentrations, the long-term model input parameters included feed intake, growth, and development of the fat compartment which reflect the seasonal variations related to seawater temperature and maturation of a market sized Atlantic salmon (Berntssen et al. Citation2010a, Citation2010b, Citation2016). The surveyed mean (scenario 2) as well as minimum (scenario 1) and maximum (scenario 3) feed EQ concentration found in 2017 (Sanden et al. Citation2017) was used in the model as feed input data and an assumed maximum 40% presence of the EQDM metabolite (Negreira et al. Citation2017). The long-term predictions for a mean (scenario 1) EQ fillet concentrations of a market size Atlantic salmon (4–5 kg) were ~7 µg kg−1 ww with minimum (scenario 1) and maximum (scenario 3) predicted EQ fillet levels of 4 and 45 µg kg−1 ww, respectively (). For EQDM, the predicted mean fillet levels after a whole life cycle were ~95 µg kg−1 ww, with minimum and maximum fillet values of 80–550 µg kg−1 ww, respectively (). The final predicted EQ fillet levels were lower than the predicted EQDM levels, as expected due to the bio-transformation of EQ into EQDM (ε(t), ) and the lower overall fillet elimination rate of EQDM compared EQ (half-life of 71 versus 8 days, respectively). The model predicted values for EQ were in the same range as found in commercial Norwegian farmed Atlantic salmon in 2016, of 6.6 (1–32) µg kg−1 ww (mean, minimum-maximum) (TeLalab (Citation2016), unpublished data). Model-predicted EQDM values were in the lower range of the levels analysed in commercial Norwegian farmed Atlantic salmon in 2016, with values of 191 (13–875) µg EQDM kg−1 ww (mean, minimum-maximum) (Telalab-Greenpeace 2016, unpublished data). However, considering the variation in EQDM found in farmed salmon the long-term model EQDM prediction were within the range of what could be expected in commercially produced Atlantic salmon fillet.
For EQ, in addition to the levels in the feed, long-term fillet predictions were driven by seasonal variations in feed intake (), which related to the difference in water temperature (e.g. (Berntssen et al. Citation2011c). In contrast, EQDM levels in market size Atlantic salmon (4–5 kg) did not vary with seasonal variations in feed intake (), but were dominated by the initial EQDM levels in the juvenile fish (weighing ~0.02 kg) at the start of the seawater production cycle. When EQDM model predictions were made without the initial presence of EQDM (), the long term predicted EQDM values followed more the seasonal variations with final EQDM levels that are much lower compared to when juvenile fish contained EQDM as initial input values (mean EQDM of 18 versus 95 µg kg−1 ww, for Figure 6d and a, respectively). For EQ, the lower initial fillet values in juvenile Atlantic salmon (~4–6 µg kg−1 ww) had no significant influence on the seasonal variations or the predicted fillet EQ levels in the final market-sized salmon ().
Figure 5. (a–f). Whole seawater production cycle model predictions (mean and 95% lower and upper bound) of fillet ethoxyquin (EQ) concentrations (µg kg−1 ww) in Atlantic salmon fed lowest (scenario 1, 1.6 mg kg−1), mean (scenario 2, 11.6 mg kg−1), and highest (scenario 3, 73. 4 mg kg−1) EQ levels surveyed in Norwegian commercial salmon feeds in 2017. Prediction with an initial fish fillet EQ value of 4–6 µg kg−1 ww are given in the upper panels (a–c for scenario 1, 2,and 3, respectively), and predictions without initial EQ levels are given in the lower panels (d–f for scenario 1, 2, and 3, respectively).
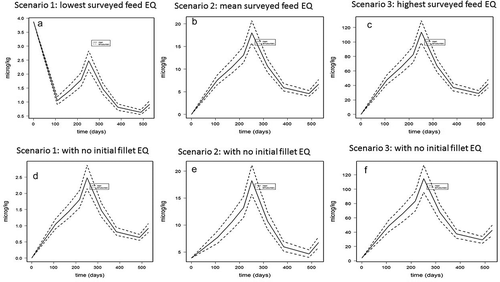
In the present trial, initial EQDM fillet levels in fish of ~200 g were between 841 and 1198 µg kg−1 ww, while in an earlier study the mean initial EQDM concentrations in a 200 g fish was 2326 ± 718 µg kg−1 ww (Bohne et al. Citation2008). These values are higher than those found in market size (4–5 kg) Norwegian commercially farmed Atlantic salmon (mean 191 µg kg−1 ww). Similarly, for the EQDM long-term model predictions, EQDM decreased rather than increased during a long-term seawater food production cycle (). The higher EQDM levels in juvenile fish compared to the market size fish can be attributed to the fact that juvenile Atlantic salmon receive feed that has a higher inclusion level of fish meal (the main source for EQ and EQDM in commercial salmon feed) compared to adult fish. Adult Atlantic salmon are fed with increasingly higher energy feeds in which the fat content is increased and the protein content decreased e.g. (Berntssen et al. Citation2005). In order to reduce EQDM levels in farmed market size Atlantic salmon, the main focus has to be on the feed for juvenile fish, which is one of the main drivers for the final levels found in farmed Atlantic salmon at the end of the production cycle. In addition to the initial EQDM level in the juvenile Atlantic salmon, the presence of the EQDM metabolite in the feed itself contributes to the fillet accumulation of EQDM. The transformation of EQ into metabolites in commercial salmon feeds depends on storage conditions and time, and would hence varies considerably (Berntssen et al. Citation2005). A more detailed screening of the contribution of EQ metabolites in commercial feeds has been performed (Negreira et al. Citation2017). Future feed surveillance programmes should quantify levels of EQ metabolites in commercial salmon feeds as used on farming sites. These feed metabolite levels, in addition to the initial metabolite levels in juvenile fish fillet, are needed as input data to improve long-term model prediction of the feed-to-fillet transfer of both EQ and especially EQDM. The model application includes the level of initial EQDM values in the juvenile Atlantic salmon, which is one of the main factors for predicting future final levels in market sized Atlantic salmon.
Health-based guidance values, such as acceptable daily intake (ADI) provide guidance on safe consumption of substances taking into account safety data, uncertainties in these data, and the likely exposure. The Joint FAO/WHO Meetings on Pesticide Residues established an ADI for EQ, and several metabolites in plants (methylethoxyquin (MEQ), dehydromethylethoxyquin (DHMEQ) and dihydroethoxyquin (DHEQ)) as pesticides in 2005 (FAO Citation2005), however, the EFSA was unable to validate this value (EFSA Citation2013, Citation2015). The EFSA lacked suitable data to conclude on safe levels of EQ as a feed additive in terms of a target animal (fish) safety and consumer safety. The model described and validated in the present study enables a prediction of the level of EQ and EQDM in fish fillets based on feed levels. This combined with toxicological data (Bernhard et al. Citation2018b) is essential information for assessing the safety of EQ as a feed additive for fish.
Supplemental Material
Download MS Word (23.1 KB)Acknowledgments
This study was financed by the Norwegian Seafood Research Fund (FHF), The Marine Ingredients Organisation (IFFO) Marine Harvest ASA, EWOS AS/Cargill Aqua Nutrition, Biomar AS, Skretting AS and Europharma (FHF project no. 901327). The authors would like to thank the personnel at NOFIMA Sunndalsøra for carrying out the fish feeding trial, and the laboratory technicians at NIFES for performing the analyses.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Bernhard A, Rasinger JD, Betancor MB, Caballero MJ, Berntssen MHG, Lundebye AK, Ornsrud R. 2019. Tolerance and dose-response assessment of subchronic dietary ethoxyquin exposure in Atlantic salmon (Salmo salar L.). PLoS One. 14:1–36.
- Bernhard A, Rasinger JD, Wisløff H, Kolbjørnsen Ø, Myrmel LS, Berntssen MHG, Lundebye A-K, Ørnsrud R, Madsen L. 2018b. Subchronic dietary exposure to ethoxyquin dimer induces microvesicular steatosis in male BALB/c mice. Food Chem Toxicol. 118:608–625.
- Berntssen MHG, Giskegjerde TA, Rosenlund G, Torstensen BE, Lundebye AK. 2007. Predicting world health organization toxic equivalency factor dioxin and dioxin-like polychlorinated biphenyl levels in farmed Atlantic salmon (Salmo salar) based on known levels in feed. Environ Toxicol Chem. 26:13–23.
- Berntssen MHG, Julshamn K, Lundebye AK. 2010a. Chemical contaminants in aquafeeds and Atlantic salmon (Salmo salar) following the use of traditional- versus alternative feed ingredients. Chemosphere. 78:637–646.
- Berntssen MHG, Lock EJ, Zeilmaker MJ, Van Eijkeren JCH. 2013. Toxicokinetic model assessment on the dechlorination of dietary toxaphene CHB-62 into CHB-44 in Atlantic salmon (Salmo salar L.). Food Addit Contam Part A-Chem. 30:1581–1589.
- Berntssen MHG, Lundebye AK, Torstensen BE. 2005. Reducing the levels of dioxins and dioxin-like PCBs in farmed Atlantic salmon by substitution of fish oil with vegetable oil in the feed. Aquacult Nutr. 11:219–231.
- Berntssen MHG, Maage A, Julshamn K, Oeye BE, Lundebye AK. 2011a. Carry-over of dietary organochlorine pesticides, PCDD/ Fs,PCBs, and brominated flame retardants to Atlantic salmon (Salmo salar L.) Fillets. Chemosphere. 83:95–103.
- Berntssen MHG, Olsvik PA, Torstensen BE, Julshamn K, Midtun T, Goksoyr A, Johansen J, Sigholt T, Joerum N, Jakobsen JV, et al. 2010b. Reducing persistent organic pollutants while maintaining long chain omega-3 fatty acid in farmed Atlantic salmon using decontaminated fish oils for an entire production cycle. Chemosphere. 81:242–252.
- Berntssen MHG, Sanden M, Helge H, Ø L. 2016. Modelling scenarios on feed-to-fillet transfer of dioxins and dioxinlike PCBs in future feeds to farmed Atlantic salmon (Salmo salar). Chemosphere. 163:413.
- Berntssen MHG, Valdersnes S, Rosenlund G, Torstensen BE, Zeilmaker MJ, van Eijkeren JCH. 2011b. Toxicokinetics and carry-over model of alpha-hexabromocyclododecane (HBCD) from feed to consumption-sized Atlantic salmon (Salmo salar). Food Addit Contam Part A-Chem. 28:1274–1286.
- Berntssen MHG, Valdersnes S, Rosenlund G, Torstensen BE, Zeilmaker MJ, van Eijkeren JCH. 2011c. Toxicokinetics and carry-over model of alpha-hexabromocyclododecane (HBCD) from feed to consumption-sized Atlantic salmon (Salmo salar). Food Addit Contam. 28:1274–1286.
- Bohne VJB, Hamre K, Arukwe A. 2007. Hepatic metabolism, phase I and II biotransformation enzymes in Atlantic salmon (Salmo Salar, L) during a 12 week feeding period with graded levels of the synthetic antioxidant, ethoxyquin. Food Chem Toxicol. 45:733–746.
- Bohne VJB, Lundebye AK, Harare K. 2008. Accumulation and depuration of the synthetic antioxidant ethoxyquin in the muscle of Atlantic salmon (Salmo salar L.). Food Chem Toxicol. 46:1834–1843.
- Brambilla G, Dellatte E, Fochi I, Iacovella N, Miniero R, Di Domenico A. 2007. Depletion of selected polychlorinated biphenyl, dibenzodioxin, and dibenzofuran congeners in farmed rainbow trout (Oncorhynchus mykiss): A hint for safer fish farming. Chemosphere. 66:1019–1030.
- [EC] Election Commission. 2003. Regulation (EC) no 1831/2003 of the European parliament and of the council of 22 September 2003 on additives for use in animal nutrition.
- [EFSA] European Food Safety Authority. 2013. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for ethoxyquin according to article 12 of regulation (EC) no 396/2005. EFSA J. 11(5):3231, 3225.
- [EFSA] European Food Safety Authority. 2015. Safety and efficacy of ethoxyquin (6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline) for all animal species EFSA panel on additives and products or substances used in animal feed (FEEDAP). EFSA J. 13:4272.
- [FAO] Food and Agriculture Organization. 2005. FAO (Food and Agriculture Organisation of the United Nations). Ethoxyquin. In: PESTICIDE RESIDUES IN food – 2005. Report of the joint meeting of the FAO panel of experts on pesticide residues in food and the environment and the WHO expert group on pesticide residues. Plant Production and Protection Paper 183. Rome (Italy): FAO.
- He P, Ackman RG. 2000. HPLC determination of ethoxyquin and its major oxidation products in fresh and stored fish meals and fish feeds. J Sci Food Agric. 80:10–16.
- Hoogenboom R, Zeilmaker M, van Eijkeren J, Kan K, Mengelers M, Luykx D, Traag W. 2010. Kaolinic clay derived PCDD/Fs in the feed chain from a sorting process for potatoes. Chemosphere. 78:99–105.
- Leeman WR, Van Den Berg KJ, Houben GF. 2007. Transfer of chemicals from feed to animal products: the use of transfer factors in risk assessment. Food Addit Contam. 24:1–13.
- Lock EJ, Fjelldal PG, Torstensen BE, Bjornevik M, Breck O, Johansen J, Reynolds P, Sigholt T, Joerum N, Jakobsen JV, et al. 2011. Dietary decontaminated fish oil has no negative impact on fish performance, flesh quality or production-related diseases in Atlantic Salmon (Salmo Salar).. Aquacult Nutr. 17(E760–):E772.
- Lundebye AK, Hove H, Mage A, Bohne VJB, Hamre K. 2010. Levels of synthetic antioxidants (ethoxyquin, butylated hydroxytoluene and butylated hydroxyanisole) in fish feed and commercially farmed fish. Food Addit Contam Part A-Chem. 27:1652–1657.
- Negreira N, Regueiro J, Valdersnes S, Berntssen MHG, Ornsrud R. 2017. Comprehensive characterization of ethoxyquin transformation products in fish feed by traveling-wave ion mobility spectrometry coupled to quadrupole time-of-flight mass spectrometry. Anal Chim Acta. 965:72–82.
- [NFSA] National Food Security Act. 2018. Overvåkings- og kartleggingsprogrammer (In Norwegian). [accessed 2018 May 28]. https://www.mattilsynet.no/fisk_og_akvakultur/akvakultur/for/#overvakings_og_kartleggingsprogrammer.
- Nichols JW, Fitzsimmons PN, Whiteman FW. 2004. A physiologically based toxicokinetic model for dietary uptake of hydrophobic organic compounds by fish – II. Simulation of chronic exposure scenarios. Toxicol Sci. 77:219–229.
- Ornsrud R, Arukwe A, Bohne V, Pavlikova N, Lundebye AK. 2011. Investigations on the metabolism and potentially adverse effects of ethoxyquin dimer, a major metabolite of the synthetic antioxidant ethoxyquin in salmon muscle. J Food Prot. 74:1574–1580.
- Sanden M, Hemre GI, Maage A, Lunestad BT, Espe M, Lie KK, Lundebye AK, Amlund H, Waagbø R, Ørnsrud R. 2017. Programme for surveillance of fish feeds yearly report for samples collected in 2016. NIFES report in Norwegian. https://nifes.hi.no/report/overvakning-fiskefor-2017/.
- Skaare JU, Roald SO. 1977. Ethoxyquin (EQM) residues in Atlantic salmon measured by fluorimetry and gas chromatography (GLC). Nord Vet Med. 29,:232–236.
- Telelab. 2016. Ethoxuquin in farmed fish. [accessed 2018 Jun 19]. https://www.greenpeace.de/sites/www.greenpeace.de/files/publications/1612_laborergebnisse_ethoxyquininspeisefisch_tela_0.pdf.
- van Raamsdonk LWD, van Eiikeren JCH, Meijer GAL, Rennen M, Zeilmaker MJ, Hoogenboom LAP, Mengelers M. 2009. Compliance of feed limits, does not mean compliance of food limits. Biotechnol Agron Soc. 13:51–57.
- Zar JH. 1984. Biostatistical analysis. New York: Prentice-Hall, Englewood Cliffs.
