Abstract
The use of herbal supplements for improved sexual performance is a common practice amongst the youth and some senior citizens in Ghana. These products are considered ‘natural’ and greatly preferred over synthetic alternatives due to the assurance of little to no adverse effects by producers. However, the high rate of adulteration often compromises their safety. Forty herbal supplements, of which 25 were previously shown to result in medium to high intake of phosphodiesterase type-5 (PDE-5) inhibitors using a PDE-Glo bioassay, were further investigated using liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis to examine the reliability of the bioassay and whether the observed higher responses could be ascribed to inherent plant constituents or adulterants. Results showed significant amounts of vardenafil, tadalafil and especially sildenafil, in 2, 1 and 10 samples, respectively, with total concentration levels resulting in estimated daily intakes (EDIs) above 25 mg sildenafil equivalents with six supplements even having EDIs above 100 mg sildenafil equivalents. Only one sample contained a natural ingredient (icariin), but its concentration (0.013 mg g−1) was too low to explain the observed potency in the bioassay. The estimated concentrations of PDE-5 inhibitors in 35 supplements, according to the bioassay, were in line with those of the LC–MS/MS analysis. However, discrepancies were observed for five supplements. Further examination of one of the latter supplements using the PDE-Glo bioassay to select the positive fraction and further examination with LC–MS/MS and 1H-NMR revealed the presence of hydroxythiohomosildenafil, a sildenafil analogue not yet included in the liquid chromatography–mass spectrometry reference library. This study demonstrates the significance of applying a tiered approach, where the use of a bioassay is followed by chemical analysis of bioactive samples in order to identify unknown bioactive compounds.
Introduction
Erectile dysfunction (ED) is a global issue, which has gained much attention in recent years (Ayta et al. Citation1999; Patel et al. Citation2014). Various interventions, including the use of drugs are recommended for the treatment and/or management of ED (Levine Citation2000; Rew and Heidelbaugh Citation2016). Most of these drugs are known as phosphodiesterase type-5 inhibitors (PDE-5i). These drugs inhibit PDE-5 enzyme activity in the corpus cavernosum, resulting in the accumulation of cGMP, which subsequently promotes downstream relaxation of smooth muscle cells, allowing adequate blood flow to the penis thereby enhancing erection. Examples of approved PDE-5i include sildenafil citrate (Viagra), tadalafil (Cialis), vardenafil hydrochloride (Levitra) and avanafil (Stendra) (Patel et al. Citation2014). However, there are many unlicensed analogues of these compounds, which manufacturers often use to avoid the detection of potential adulteration of natural products.
In most African settings, herbal-based sexual enhancers/aphrodisiacs are most common and preferred over the synthetic alternatives mainly due to their natural origin. However, many studies have shown that most of these herbal aphrodisiacs are adulterated with either approved synthetic PDE-5i or their unapproved analogues (Low et al. Citation2009; Savaliya et al. Citation2010; Mans et al. Citation2013; Reeuwijk et al. Citation2014; Gilard et al. Citation2015; Bujang et al. Citation2017; Tucker et al. Citation2018, Kee et al. Citation2018; Lee et al. Citation2021). shows the prevalence of adulteration of herbal aphrodisiacs in different studies.
Table 1. Prevalence of adulterated herbal products with active pharmacological ingredients.
Approved PDE-5i are prescription-only drugs that are used strictly upon medical recommendation with appropriate monitoring. Their use as adulterants in herbal products raises legal issues as well as public health concerns. This is especially the case when unapproved drug analogues are used (European Medicines Agency Citation2008), as studies have shown that although these analogues may function similarly as the parent drug, differences in their chemical structures may alter their pharmacokinetic and pharmacodynamic profiles, including their toxicity (Venhuis and De Kaste Citation2012; Bujang et al. Citation2017). Furthermore, PDE-5i are strongly contraindicated in patients using organic nitrate drugs, α-adrenergic blockers and CYP3A4 inhibitors (Langtry and Markham Citation1999; Kostis et al. Citation2005). The combined intake of these drugs may synergistically promote increased relaxation of smooth muscle cells, resulting in a drastic reduction of the systemic blood pressure (Kostis et al. Citation2005; Kloner Citation2007). The sudden drop in blood pressure (hypotension) may possibly lead to other cardiovascular effects and in extreme cases, sudden shock or even death (Langtry and Markham Citation1999; Gur et al. Citation2013). Patients advised against PDE-5 inhibition drugs may resort to herbal alternatives due to the belief that they can safely induce the same pharmacological effect while avoiding expected adverse effects (Blok-Tip et al. Citation2004). Nevertheless, due to their high prevalence of adulteration, consumers may unknowingly expose themselves to unanticipated risks (Poon et al. Citation2007; Bakota et al. Citation2017).
Several liquid chromatography–mass spectroscopy (LC–MS)-based methods are employed for the identification and quantification of adulterants commonly used in natural products (Gratz et al. Citation2004; Patel et al. Citation2014). However, these methods may not be able to identify new analogues, especially when they are unavailable in the LC–MS reference library (Jiru et al. Citation2019). To overcome this challenge, a tiered approach, where products are first screened using a bioassay to determine their bioactive properties, followed by analysis of ‘suspect samples’ with chemical analytical methods, to either confirm the presence of known compounds, or conduct further investigations to confirm the presence of novel analogues is much suited.
A previous study conducted on a series of forty herbal supplements from the Ghanaian market revealed that 36 (90%) were able to inhibit PDE-5 enzyme activity according to a PDE-Glo bioassay (Akuamoa et al. Citation2021). Based on the estimated daily intakes (EDIs), 11 out of the 36 positive supplements were categorised as resulting in a low intake (L; <25 mg day−1), 16 in a medium intake (M; 25–100 mg day−1) and 9 in a high intake (H; >100 mg day−1). A daily dose of 25 mg is regarded as the minimal dose required for a pharmacological effect and a dose above 100 mg as potentially leading to serious adverse health effects. In the present study, all 40 herbal supplements were again analysed using LC-MS-based techniques, first of all to identify and quantify the compounds responsible for the observed high inhibition in majority of the supplements, and to determine if the identified compounds were inherent plant constituents or pharmaceutical ingredients intentionally added by manufacturers. Analysing supplements with a low response enabled assessment of the accuracy of the bioassay in selecting supplements of interest. One of the five samples with an unexplained high response was further examined to demonstrate as a proof-of-concept the advantage of employing a tiered approach involving the application of a bioanalytical method.
Material and methods
Supplements
The same samples tested in a previous study by Akuamoa et al. (Citation2021) were further analysed in this study. Supplementary Table S1 in the previous study presents the list of the 40 supplements and their allocated sample ID, origin and instructions for use. Based on their responses and corresponding EDIs in the bioassay, they were further categorised either as negative (N), low (L), medium (M) or high (H) intake (, Akuamoa et al. Citation2021).
Chemicals and reagents
Compounds used in this study were purchased from ChemCruz (UK and USA) and Carbosynth (UK) with purity between 95% and 99%, unless indicated otherwise (Supplementary Table S1). Hydroxythiohomosildenafil (HTHS) (purity >98%) was purchased from Toronto Research Chemicals (Toronto, Canada). Acetonitrile (Lot No. 1332591) and methanol (Lot. 1328761) (HPLC Supra-Gradient) were purchased from Biosolve, (Valkenswaard, The Netherlands), acetic acid (Lot. 1.00063) (100%) and formic acid (Lot. 1.00264) were from Merck (Darmstadt, Germany) and ammonium formate (Lot. 17843) was from Fluka, (Munich, Germany). Sterile syringe filters (0.45 µm cellulose acetate membrane) were purchased from VWR International (North America) and Whatman 0.2 µm pore size Mini-PrepTM PTFE filter media with polypropylene housing (CAT. US203NPUORG) and syringeless filter devices were from G.E Healthcare (Buckingham, UK). Ultra-pure water was prepared using a Milli-Q water purification system (Ref. A+). The PDE-Glo phosphodiesterase assay kit (Promega, CAT No. V1361) was acquired from Fisher Scientific (Madison, WI, USA), Phosphodiesterase 5A1 human recombinant (CAT No. E9034) and 3-isobutyl-1-methylxanthine (IBMX) (CAT No. I5879) were purchased from Sigma-Aldrich (St. Louis, USA) and Coaster 96-well, flat bottom, non-treated, non-sterile white polystyrene assay plates from Corning (New York, USA).
Sample extraction
A hundred milligram or microlitre sample was aliquoted into polypropylene vails and 1 mL extraction solvent (ACN/H2O (80/20, v/v)) was added. Next, mixtures were vortexed (Vortex-2 Gene) at speed 5 for 1 min then placed in a multi-tube vortex mixer (Heidoph Reax 2) for 30 min and subsequently centrifuged (Eppendorf Centrifuge 5415 R) (985g) for 5 min at 22 °C. Supernatants were collected and transferred into new vials.
Sample screening with LC-full-scan high-resolution MS
Prior to confirmation and quantification of compounds, extracts were initially screened using LC-full-scan high resolution MS (QExactive Orbitrap MS, Thermo Fisher Scientific, San Jose, CA, USA) to identify a broad range of natural and synthetic compounds. For this, 250 µL of each sample extract was aliquoted into dilution vials and 250 µL extraction solvent (1% acetic acid in ACN:H2O (80/20 (v/v)) was added. Mixtures were homogenised and filtered through a 0.45 µm cellulose acetate membrane syringe (VWR International (North America)). Next, 50 µL of each filtrate was pipetted into a 500 µL capacity integrated filter vial, 450 µL extraction solution was added and the resulting sample extract was analysed. Measurements were carried out in positive and negative mode with and without fragmentation using LC-full-scan high-resolution MS. The source was operated in positive and negative mode using the following parameters: electrospray voltage 3.5/2.5 kV (pos/neg), sheath gas 47 arbitrary units, auxiliary gas 11 arbitrary units. The heater in the source was set to 300 °C and the heated capillary in the mass spectrometer operated at 412 °C. Data were acquired by continuously alternating scan events: one without and one with fragmentation (both m/z 70–1000). For fragmentation, collision energies (NCE) of 30 and 80 eV were used. In full-scan mode a resolution setting of 70,000 was applied and in tandem mass spectroscopy (MS/MS) mode of 50,000. This resulted in the identification of 13 compounds of interest. Calibration curves of these compounds were then prepared for confirmation and quantification.
Targeted compound analysis by LC–MS/MS
Of each extract, 250 µL was aliquoted into new dilution vials while 250 µL extraction solvent (1% acetic acid in MeOH:H2O:ACN (70:20:10 (v/v)) was added. Solutions were homogenised and filtered through a 0.45 µm cellulose acetate membrane syringe. Extracts were further diluted 10-, 100- and 1000-fold with MeOH:H2O (50/50 v/v). Peak areas were quantified by means of external calibration curves (5, 10, 25, 50, 100, 250, 500 and 1000 ng mL−1) using available commercial standards of the compounds identified during the screening process.
Confirmation analysis was performed based on an in-house protocol with slight modifications. The liquid chromatography–tandem mass spectrometry (LC-MS/MS) system consisted of an injection and pump system from Shimadzu (Hertogenbosch, The Netherlands) coupled to an Applied Biosystems (AB) Sciex QTRAP 5500 mass spectrometer (W.M., USA), operated in the ESI + mode. The analytes were eluted through an Atlantis T3 (Waters, 3.0 × 100 mm, 3 µm) LC column, which was connected to a SecurityGuard C18 precolumn (Waters, 20 × 4.0 mm ID). The elution program used consisted of two mobile phases: 5.0 mM ammonium formate prepared in ultrapure deionised water (A) and methanol (B), both of which contained 0.1% formic acid. The LC gradient started with 90% solvent A for 1 min, followed by a change to 100% solvent B over 8 min. It was maintained at 100% solvent B for 5.5 min and reverted to 90% solvent A for 6.5 min, making a total runtime of 20 min. The column was maintained at a constant temperature of 30 °C and equilibrated with the initial mobile phase composition for at least 270 s before running the next injection. Elution flow rate was 0.40 mL min−1. Detection was performed in MRM-mode with a probe temperature of 500 °C, an entrance potential of 10 V, a decluttering potential of 96 V, and a dwell time of 10 ms. The retention times of icariin, sildenafil, tadalafil and vardenafil were 8.19, 7.59, 8.34 and 7.64 min, respectively. The precursor ions of icariin, sildenafil, tadalafil and vardenafil were selected as (m/z) 677.2, 475.1, 390.2 and 489.0, while the product ions were selected as (m/z) 369.0, 100.2, 135.1 and 169.0 with the collision energies (CE) of 43, 32, 22 and 40 (V) respectively. Data analysis was performed with Microsoft Excel version 2016.
The calibration curve of the identified compounds was used to determine their concentration and subsequently expressed in mg sildenafil equivalents using the so-called relative potency (REP) values established by Bovee et al. (unpublished) as provided in Supplementary Table S2. The REP values (Table S2) were derived from an in vitro experiment by dividing the IC50 of sildenafil by the IC50 of each identified compound (PDE-5i). The established IC50 was based on how much of the compound (PDE-5i) is needed to induce the same effect (inhibiting PDE-5 enzyme activity) as sildenafil in vitro by 50%. Estimated compound concentrations were multiplied by their respective REP value (relative to sildenafil) and summed up to obtain the total concentration, and expressed in mg sildenafil equivalents per gram or mL of sample.
Fractionation of sample extract
Isolation of unknown PDE-5i in one of the supplements (S13) was carried out following an in-house method. The same sample extract used for the confirmation analysis was used without dilutions. Compound fractionation was performed using a Kinetex 2.6 µm Polar-C18 100 A (50 × 2.1 mm) LC column (Phenomenex, NL) fitted to a Nexera X2 U(H)PLC from Shimadzu (Tokyo, Japan). Two mobile phases were used, i.e. 100% ultrapure deionised water (A) and 100% ACN (B). The runtime was set at 15 min and fractions were repeatedly collected at a specified time frame based on peaks expressed as a function of time. The ACN phase of the collected fractions was evaporated under a continuous stream of nitrogen at 40 °C. The residual solutions were initially stored at −20 °C for 2 h, then at −80 °C for 30 min. Next, fractions were freeze dried overnight, and then evaporated at −54 °C under 0.047 mbar pressure in a vacuum, and stored at −20 °C for further analysis. Portion of the fractions were reconstituted in DMSO and re-tested in the PDE-Glo bioassay to estimate their inhibition potential.
Sample analysis using the PDE-Glo bioassay
The PDE-Glo bioassay was performed following the protocol described in detail in a previous study (Akuamoa et al. Citation2021). Briefly, a 5 µL aliquot of sample extract, 7.5 µL PDE-5 enzyme and 12 µL cGMP (20 µM) were pipetted into the wells of a Coaster 96-well plate. This was mixed and incubated in the dark for 90 min. A termination solution (12 µL) containing termination buffer + 100 mM 3-isobutyl-1-methylxanthine was used to terminate the reaction after the incubation period. Next, detection solution (12 µL) consisting of detection buffer + protein kinase was added, mixed for 5 min and incubated for another 20 min. Finally, 50 µL kinase glo reagent containing kinase glo substrate + kinase glo buffer was added and incubated for another 10 min. The Biotek Synergy HT (Vermont, USA) was used to measure luminescence signals in relative light units (RLUs). Data analysis and graphs were plotted using Microsoft Excel version 2016. Active fractions were subsequently analysed using time-of-flight mass spectrometry (TOF-MS).
LC-TOF-MS analysis for identification of unknown PDE-5i
Active fractions identified by the PDE-Glo bioassay were divided into two portions of 45 µL and analysed using an Agilent 1200 Series LC system coupled to a Bruker micro-TOF mass spectrometer, and operated in positive and negative mode. For analysis, 5 µL of dissolved active fractions in ACN:H2O (80:20 v/v %) was injected onto an Alltima HP C18 column (155 × 2.2 mm, 3 µm). Two mobile phases consisting of 100% ultrapure deionised water (A) and 100% ACN (B) were used. The LC analysis started at 80/20 A/B (v/v), then to 60/40 in 10 min and changed to 40/60 in 15 min. Subsequently, the solvents went back to starting conditions (80/20) in 20 min and equilibrated for another 15 min. The total runtime was 60 min. Elution flow rate was 1 mL min−1. During the measurement the detection was performed in the mass range of 99–1501 m/z with mass resolution of 15,000 FWHM. Peaks with the highest intensity were collected and their masses were recorded. Next, collected fractions were dried and the structure of their constituents was elucidated using 1H-NMR.
Compound identification by 1H-NMR analysis
The structure of the identified mass was elucidated by 1H-NMR analysis following an in-house protocol. The dried fractions were evaluated using a Bruker Avance III 600 MHz NMR spectrometer, 3 mm NMR tubes and a cryogenic NMR probe. Quantitative 1H-NMR spectra were recorded after dissolving the dried fraction in 200 µL DMSO-D6 and 2400 scans were collected using an 1 D NOESYGPPR pulse sequence. In addition to the 1D 1H NMR data, 2D NMR data sets, 2D 1H-1H COSY and 2D 1H-1H TOCSY were assessed as well.
Verification and confirmation of newly identified constituent
The identified compound was verified by spiking the active fraction with the pure compound. Analysis of pure compound, unspiked and spiked active fraction was carried out concurrently using the TOF-MS. Peaks from the unspiked active fraction were compared to that of the spiked active fractions. Confirmation of compound identity was based on co-elution of the suspect compound in the active fraction and the pure compound in a single peak having the same mass spectrum and increased intensity (Supplementary Figure S1).
Results and discussion
Forty herbal supplements collected from various markets and pharmacies in Accra (Ghana) were previously analysed using the PDE-Glo phosphodiesterase bioassay to estimate their PDE-5 inhibition potentials (Akuamoa et al. Citation2021). Based on the results obtained, it was evident that 36 (90%) of the selected supplements had the ability to inhibit PDE-5 enzyme activity to varying degrees. According to the EDIs of each supplement, eleven out of the 36 positive supplements were categorised as low intake (L; <25 mg), 16 as medium intake (M; 25–100 mg) and 9 as high intake (H; >100 mg). In the current study, all 40 supplements were subjected to further investigations using combined chemical analytical procedures, first of all to establish if the observed inhibition potentials were caused by inherent plant constituents or the effect of synthetic PDE-5i used as adulterants, and secondly to confirm the accuracy of the bioassay in aiding the selection of supplements of major concern.
LC/MS analysis of supplements
Screening with LC-full-scan high-resolution MS resulted in the identification of 13 PDE-5i (data not shown). LC-MS/MS was subsequently used for confirmation and quantification of these compounds. The concentration of individual PDE-5i was expressed in mg sildenafil equivalents using the so-called relative potency (REP) values established by Bovee et al. (unpublished) (Supplementary Table S2). To this end, the concentration of each compound was multiplied by its REP value relative to sildenafil (REP = 1) and summed up. A cut-off of 1 mg g−1 was selected for sildenafil as reporting level, as lower concentrations could be the result of cross-contamination during the analysis from supplements with very high levels. Moreover, only higher levels clearly pointed at purposeful adulteration to obtain the desired outcome, therefore, low levels were considered less relevant. For other analogues, a cut-off of 0.1 mg g−1 was applied since cross-contamination during analysis was less likely and with regard to the higher REPs for vardenafil. It should be considered that using these cut-offs may result in the elimination of supplements where a relatively low level might still result in an intake larger than 25 mg per day due to a high daily dose (especially in the case of liquids samples).
shows only the results for supplements with concentrations above the cut-off limits and their corresponding EDIs. The concentration of sildenafil in 11 supplements (28%) was above 1 mg g−1. Considering other PDE-5i, three supplements contained low amounts of tadalafil, vardenafil and traces of other compounds but below 0.1 mg g−1. Icariin, a known natural PDE-5i and an active constituent of the genus Epimedium, was detected by LC–MS/MS in only one supplement (S13) based on the retention time and mass spectrum. However, the calculated LC-MS/MS concentration (0.013 mg g−1) was too low to explain the high response in the PDE-Glo bioassay. The level of icariin detected by the LC–MS/MS was higher than the amounts detected in a study by Polat and Coskun (Citation2016) in genus Epimedium using HPLC coupled with DAD detection. This was contrary to the levels (3.0–172.1 mg g−1) of icariin detected in Herba Epimedii in a study by Liu et al. (Citation2006) using the capillary zone electrophoresis (CZE). The current study implied that none of the supplements contained natural PDE-5i. When considering the recommended daily doses of supplements, 11 out of the 40 would result in intakes (EDI) at pharmacologically relevant levels (i.e. >25 mg day−1). For S20 this is mainly the result of the relatively high recommended daily dose. Due to high sildenafil concentrations, the EDIs for six supplements were above 100 mg, which may potentially cause adverse effects especially in vulnerable individuals. Although traces of other compounds were detected, sildenafil was the most identified adulterant. This may partly be attributed to the readily available manufacturing process of sildenafil (Terrett et al. Citation1996; Bujang et al. Citation2017; Kee et al. Citation2018).
Table 2. LC–MS/MS-based levels of individual compound(s) in supplements, their total concentrations, recommended daily doses and EDIs.
Comparison of bioassay and LC–MS analysis
Compound concentrations in each supplement as determined by LC–MS/MS were subsequently compared to the estimated concentrations based on their inhibition potentials in the previous PDE-Glo bioassay. Supplementary Table S1 shows the complete list, while shows only the comparison between supplements categorised as resulting in ‘medium’ or ‘high’ intake based on the PDE-Glo assay. Estimated levels were corrected for the fold-dilution. It should be noted that in the previous study, a 100- to 1000-fold dilution was required to classify a supplement as resulting in a low or high intake of PDE-5i. Still, a few of the samples were tested at a 10,000-fold dilution (not used for estimating daily intakes). However, for comparison with the LC-MS/MS results, the 10,000-fold dilution was included when available. The LC–MS determined concentrations for all supplements (S1, S2, S8, S9, S11, S12, S18, S26, S29, S31, S32, S33, S34, S36, S40) classified as ‘negative’ or ‘low’ based on the PDE-Glo assay were below the established cut-offs. This proved that the bioassay reported no false-negative results.
Table 3. Comparison between estimated concentrations in the PDE-Glo assay and the LC–MS/MS analysis. Only supplements classified as resulting in medium or high intake based on the bioassay are shown.
Comparison between the two methods showed that for 19 out of the 24 samples classified as medium or high, the response by the bioassay could be explained by the LC–MS/MS results. This included three samples (S5, S17 and S27) where in the bioassay a 100-fold diluted extract indicated a level above 8 mg g−1 and a 1000-fold diluted extract, a level below 1 mg g−1. This was based on a full inhibition at the 100-fold and no inhibition at the 1000-fold dilution. It is unclear what caused this steep dose–response, but in any case, no known PDE-5i could be identified. For seven other supplements, the estimated level based on the 100-fold dilution was quite low and LC–MS/MS analysis could also not detect the presence of a PDE-5i above the cut-off. For nine other samples the high bioassay response could well be explained by the level of sildenafil, considering the variation in the estimated level at different dilutions.
However, the comparison also revealed discrepancies for five supplements (i.e. S3, S13, S20, S35 and S38), for which the response by the bioassay thus indicated the presence of unknown active PDE-5i constituents. For instance, the concentration in S13 estimated by the bioassay based on the 1000- and 10,000-fold dilutions was 40 and 873 mg g−1 respectively, whereas levels of known PDE5i determined by LC-MS/MS were below the cut-off values. This disparity between the bioassay and the LC-MS/MS results pointed at the presence of unknown active compound(s) in the supplement, but not yet present in the reference library of the HR-FS-LC-(Orbitrap)-MS method.
Investigation of unknown PDE-5i in one supplement
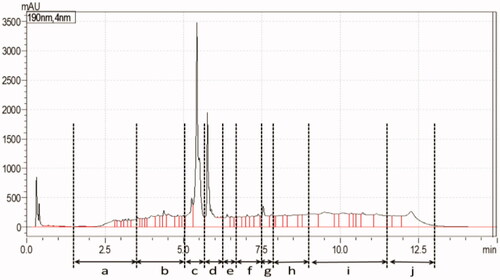
Supplement S13, which showed discrepancies between the two analyses, was selected as a model sample for further investigations. This was to demonstrate, as a proof-of-concept, the advantages of using a tiered approach. To this end an activity-based fractionation was performed using UPLC. Fractions showing peaks in the chromatogram at 190 nm were collected, as schematically presented in . Overall, 10 different fractions (peaks a–j) were repeatedly collected and pooled. Peak c was the highest and most obvious peak.
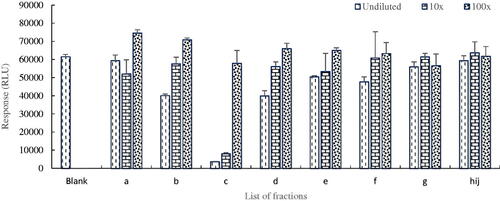
The collected fractions were subsequently analysed in the PDE-Glo bioassay to access their PDE-5 inhibition potentials. The principle of the assay results in a lower response (measured in RLUs) when inhibition of PDE-5 activity by the active component in the fraction is high, and vice versa (Akuamoa et al. Citation2021). In the absence of peaks, fractions h, i and j () were pooled together and tested as a single fraction. shows the response of each fraction in the various dilutions. The results obtained indicated that fraction c displayed the strongest inhibition potential and was therefore selected for further analysis of its constituent(s).
Figure 2. Inhibition potentials of collected fractions of S13 tested in the PDE-Glo bioassay, fraction c showing the presence of a PDE-5i.
Upon analysis of fraction c using the TOF-MS, the presence of a compound with a mass in the positive or negative mode, [M + 1]+ and [M-1]−, at m/z 521.2 and 519.2 respectively was elucidated, which pointed at a neutral mass of 520.2 m/z and a corresponding molecular formula C23H32N6O4S2. To further identify the unknown constituent, fraction c was analysed by 1H-NMR, for unequivocal identification of compound(s) present. Comparison of the data with the NMR spectrum of sildenafil revealed the unknown compound as an analogue of sildenafil having a hydroxyethyl group instead of a methyl group attached to the piperazinyl nitrogen and the oxygen atom replaced with a sulfur in the pyrazolopyrimidine moiety. Thus, the compound was identified as hydroxythiohomosildenafil, which was in line with the mass and structural composition derived from the TOF-MS data. However, hydroxythiohomosildenafil was first isolated in a study by Li et al. (Citation2009) using the ESI–MS/MS, NMR, UV and IR.
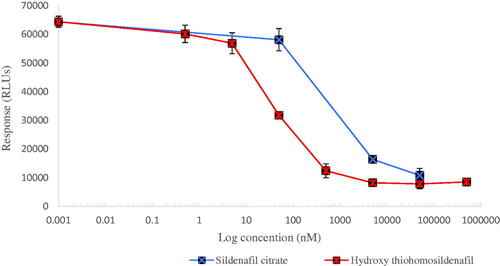
To confirm this, the commercially available standard of hydroxythiohomosildenafil was purchased and analysed by TOF-MS and 1H-NMR. This resulted in a similar retention time,mass spectrum and structure as the newly identified constituent. Next, a dose response curve for the activity of hydroxythiohomosildenafil in the PDE-Glo bioassay was constructed to establish its relative potency to sildenafil (). The results obtained revealed hydroxythiohomosildenafil to be substantially more potent than sildenafil with an IC50 of 80 nM as compared to the IC50 of 900 nM for sildenafil. This implied that hydroxythiohomosildenafil was about 12-fold more potent than sildenafil.
Figure 3. Concentration-dependent reduction in PDE-5 activity. IC50 values of 900 nM and 80 nM were calculated from the fitted dose–response curves for sildenafil and hydroxythiohomosildenafil, respectively, resulting in a REP value for hydroxythiohomosildenafil of about 12.
The reference library of the HR-FS-LC-(Orbitrap) MS was then updated to include hydroxythiohomosildenafil and the LC-MS/MS method optimised to also quantitatively detect this additional PDE-5i. Next, the concentration of hydroxythiohomosildenafil in S13 was estimated in sildenafil equivalents considering its relative potency factor (RPF) of 12. The estimated concentration of hydroxythiohomosildenafil in the sample according to the LC–MS/MS was 1 mg or, when multiplied with the RPF, 12 mg sildenafil equivalents per gram. This concentration was in line with 5 and 40 mg sildenafil equivalents per gram based on the effect of the 100- and 1000-fold dilutions in the bioassay. Based on the daily dose of 5.2 g the LC–MS-based level would result in an EDI of 63 mg sildenafil equivalents, clearly exceeding the 25 mg considered as effective dose.
Potential risks of adulterated herbal supplements
Adulteration of herbal supplements with synthetic PDE-5 inhibitors clearly deceives the user. It is even worse when unapproved drug analogues like hydroxythiohomosildenafil are used for adulteration purposes, with substantially higher intrinsic PDE-5 inhibition activity than sildenafil. This raises health concerns, especially when used in higher dose levels, since already a daily dose higher than 8 mg would give rise to exceedance of the highest therapeutic dose of 100 mg sildenafil equivalents. An overdose of sildenafil is likely to cause hepatotoxicity in vulnerable individuals (Wolfhagen et al. Citation2008; Enomoto et al. Citation2009; Nissan et al. Citation2016). Instances of sildenafil-related hepatic toxicity have been reported in a study by Graziano et al. (Citation2017).
Although high concentrations of PDE-5i in supplements may not result in fatal conditions on their own, pre-existing conditions such as hypertension and diabetes may likely potentiate the effects of these compounds and may even result in death (Matheeussen et al. Citation2015; Kee et al. Citation2018). Although in principle supplements with intakes above 25 mg are expected to elicit a pharmacological response, those above 100 mg may potentially cause adverse effects, however, it is equally important to address samples with EDIs below 25 mg sildenafil equivalents, especially for vulnerable consumers.
Conclusions
In the current study, 13 out of the 25 (54%) supplements, categorised as medium (M) and high (H) intake in the previous PDE-Glo bioassay (Akuamoa et al. Citation2021), were found to be adulterated with synthetic PDE-5i based on the results from LC–MS/MS. The EDIs of six of these supplements were higher than 100 mg sildenafil equivalents, thus exceeding the highest safe dose of Viagra (containing sildenafil as the active ingredient). The study also addresses the drawbacks regarding the use of chemical analysis only (i.e. chromatographic techniques) for screening, identification and quantification of adulterants in herbal products. The LC–MS/MS only identified compounds that were already in the reference library; however, a follow-up on one of the five samples with an unexplained response in the bioassay resulted in the identification of an undetected analogue, hydroxythiohomosildenafil. This confirms that a tiered approach, using a bioassay for selection of relevant samples and aiding in the identification of compounds present, has a clear advantage.
Collaboration
This project is a collaboration between the Department of Toxicology and Wageningen Food Safety Research at Wageningen University and Research.
Supplemental Material
Download Rich Text Format File (3.5 MB)Supplemental Material
Download MS Word (99.1 KB)Acknowledgements
The authors acknowledge the technical support of Mirjam Klijnstra during the repeat analysis of the sample series.
Disclosure statement
The authors declare that they have no competing financial interest or personal relationship that could influence the work reported in this paper.
Additional information
Funding
References
- Akuamoa F, Hoogenboom RL, Hamers A, Rietjens IM, Bovee TF. 2021. PDE-5 inhibitors in selected herbal supplements from the Ghanaian market for better erectile function as tested by a bioassay. Toxicol In Vitro. 73:105130. doi:https://doi.org/10.1016/j.tiv.2021.105130
- Ayta IA, McKinlay JB, Krane RJ. 1999. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 84(1):50–56. doi:https://doi.org/10.1046/j.1464-410x.1999.00142.x
- Bakota EL, Kelly AT, Walterscheid JP, Phatak DR. 2017. A case report of fatal desmethyl carbodenafil toxicity. J Anal Toxicol. 41(3):250–255. doi:https://doi.org/10.1093/jat/bkw128
- Blok-Tip L, Zomer B, Bakker F, Hartog KD, Hamzink M, Ten Hove J, Vredenbregt M, de Kaste D. 2004. Structure elucidation of sildenafil analogues in herbal products. Food Addit Contam. 21(8):737–748. doi:https://doi.org/10.1080/02652030412331272467
- Bujang NB, Chee CF, Heh CH, Rahman NA, Buckle MJ. 2017. Phosphodiesterase-5 inhibitors and their analogues as adulterants of herbal and food products: analysis of the Malaysian market, 2014-16. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 34(7):1101–1109. doi:https://doi.org/10.1080/19440049.2017.1336674
- Enomoto M, Sakaguchi H, Ominami M, Iwai S, Morikawa H, Tamori A, Kawada N. 2009. Sildenafil-induced severe cholestatic hepatotoxicity. Am J Gastroenterol. 104(1):254–255. doi:https://doi.org/10.1038/ajg.2008.18
- European Medicines Agency. 2008. Summary of product characteristics for sildenafil 2008. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-ProductInformation/human/000202/WC500049830.pdf
- Gilard V, Balayssac S, Tinaugus A, Martins N, Martino R, Malet-Martino M. 2015. Detection, identification and quantification by 1H NMR of adulterants in 150 herbal dietary supplements marketed for improving sexual performance. J Pharm Biomed Anal. 102:476–493. doi:https://doi.org/10.1016/j.jpba.2014.10.011
- Gratz SR, Flurer CL, Wolnik KA. 2004. Analysis of undeclared synthetic phosphodiesterase-5 inhibitors in dietary supplements and herbal matrices by LC-ESI-MS and LC-UV. J Pharm Biomed Anal. 36(3):525–533. doi:https://doi.org/10.1016/j.jpba.2004.07.004
- Graziano S, Montana A, Zaami S, Rotolo MC, Minutillo A, Busardò FP, Marinelli E. 2017. Sildenafil-associated hepatoxicity: a review of the literature. Eur Rev Med Pharmacol Sci. 21(suppl 1):17–22.
- Gur S, Kadowitz PJ, Gokce A, Sikka SC, Lokman U, Hellstrom WJG. 2013. Update on drug interactions with phosphodiesterase-5 inhibitors prescribed as first-line therapy for patients with erectile dysfunction or pulmonary hypertension. Curr Drug Metab. 14(2):265–269. doi:https://doi.org/10.2174/138920013804870600
- Jiru M, Stranska-Zachariasova M, Dzuman Z, Hurkova K, Tomaniova M, Stepan R, Cuhra P, Hajslova J. 2019. Analysis of phosphodiesterase type 5 inhibitors as possible adulterants of botanical-based dietary supplements: extensive survey of preparations available at the Czech market. J Pharm Biomed Anal. 164:713–724. doi:https://doi.org/10.1016/j.jpba.2018.11.007
- Kee CL, Ge X, Gilard V, Malet-Martino M, Low MY. 2018. A review of synthetic phosphodiesterase type 5 inhibitors (PDE-5i) found as adulterants in dietary supplements. J Pharm Biomed Anal. 147:250–277. doi:https://doi.org/10.1016/j.jpba.2017.07.031
- Kloner R. 2007. Erectile dysfunction and hypertension. Int J Impot Res. 19(3):296–302. doi:https://doi.org/10.1038/sj.ijir.3901527
- Kostis JB, Jackson G, Rosen R, Barrett-Connor E, Billups K, Burnett AL, Carson C, Cheitlin M, Debusk R, Fonseca V, et al. 2005. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference). Am J Cardiol. 96(12):85–93. doi:https://doi.org/10.1016/j.amjcard.2005.12.018
- Langtry HD, Markham A. 1999. Sildenafil. Drugs. 57(6):967–989. doi:https://doi.org/10.2165/00003495-199957060-00015
- Lee JH, Min AY, Park OR, Han JH, Yang YJ, Kim H, Baek SY. 2021. Detection of 94 compounds related to sexual enhancement including sildenafil, tadalafil, vardenafil and their analogues in various formulations of dietary supplements and food samples using HPLC and LC-MS/MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 38(5):769–781. doi:https://doi.org/10.1080/19440049.2021.1881623
- Levine LA. 2000. Diagnosis and treatment of erectile dysfunction. Am J Med. 109(9):3–12. doi:https://doi.org/10.1016/S0002-9343(00)00655-0
- Li L, Low MY, Aliwarga F, Teo J, Ge XW, Zeng Y, Bloodworth BC, Koh HL. 2009. Isolation and identification of hydroxythiohomosildenafil in herbal dietary supplements sold as sexual performance enhancement products. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 26(2):145–151. doi:https://doi.org/10.1080/02652030802368757
- Liu JJ, Li SP, Wang YT. 2006. Optimization for quantitative determination of four flavonoids in Epimedium by capillary zone electrophoresis coupled with diode array detection using central composite design. J Chromatogr A. 1103(2):344–349. doi:https://doi.org/10.1016/j.chroma.2005.11.036
- Low MY, Zeng Y, Li L, Ge XW, Lee R, Bloodworth BC, Koh HL. 2009. Safety and quality assessment of 175 illegal sexual enhancement products seized in red-light districts in Singapore. Drug Saf. 32(12):1141–1146. doi:https://doi.org/10.2165/11316690-000000000-00000
- Mans DJ, Callahan RJ, Dunn JD, Gryniewicz-Ruzicka CM. 2013. Rapid-screening detection of acetildenafils, sildenafils and avanafil by ion mobility spectrometry. J Pharm Biomed Anal. 75:153–157. doi:https://doi.org/10.1016/j.jpba.2012.11.031
- Mateescu C, Popescu AM, Radu GL, Onisei T, Raducanu AE. 2017. Spectroscopic and spectrometric methods used for the screening of certain herbal food supplements suspected of adulteration. Adv Pharm Bull. 7(2):251–259. doi:https://doi.org/10.15171/apb.2017.030
- Matheeussen V, Maudens KE, Anseeuw K, Neels H. 2015. A non-fatal self-poisoning attempt with sildenafil. J Anal Toxicol. 39(7):572–576. doi:https://doi.org/10.1093/jat/bkv071
- Nissan R, Poperno AY, Stein G, Shapira B, Fuchs S, Berkovitz R, Hess Z, Arieli M. 2016. A case of hepatotoxicity induced by adulterated “Tiger King”, a Chinese herbal medicine containing sildenafil. Curr Drug Saf. 11(2):184–188. doi:https://doi.org/10.2174/1574886311207040257.
- Patel DN, Li L, Kee CL, Ge X, Low MY, Koh HL. 2014. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges. J Pharm Biomed Anal. 87:176–190. doi:https://doi.org/10.1016/j.jpba.2013.04.037
- Polat DC, Coskun M. 2016. Quantitative determination by HPLC-DAD of icariin, epimedin A, epimedin B, and epimedin C in Epimedium (Berberidaceae) species growing in Turkey. Nat Prod Commun. 11(11):1934578X1601101. doi:https://doi.org/10.1177/1934578X1601101110
- Poon WT, Lam YH, Lai CK, Chan AY, Mak TW. 2007. Analogues of erectile dysfunction drugs: an under-recognised threat. Hong Kong Med J. 13(5):359–363.
- Reeuwijk NM, Venhuis BJ, de Kaste D, Hoogenboom RL, Rietjens IM, Martena MJ. 2014. Active pharmaceutical ingredients detected in herbal food supplements for weight loss sampled on the Dutch market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 31(11):1783–1793. doi:https://doi.org/10.1080/19440049.2014.958574
- Rew KT, Heidelbaugh JJ. 2016. Erectile dysfunction. Am Fam Physician. 94(10):820–827.
- Savaliya AA, Shah RP, Prasad B, Singh S. 2010. Screening of Indian aphrodisiac ayurvedic/herbal healthcare products for adulteration with sildenafil, tadalafil and/or vardenafil using LC/PDA and extracted ion LC-MS/TOF. J Pharm Biomed Anal. 52(3):406–409. doi:https://doi.org/10.1016/j.jpba.2009.05.021
- Terrett NK, Bell AS, Brown D, Ellis P. 1996. Sildenafil (VIAGRATM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg Med Chem Lett. 6(15):1819–1824. doi:https://doi.org/10.1016/0960-894X(96)00323-X
- Tucker J, Fischer T, Upjohn L, Mazzera D, Kumar M. 2018. Unapproved pharmaceutical ingredients included in dietary supplements associated with US Food and Drug Administration warnings. JAMA Netw Open. 1(6): e183337. doi:https://doi.org/10.1001/jamanetworkopen.2018.3337
- Venhuis BJ, De Kaste D. 2012. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: a history, analytical aspects and health risks. J Pharm Biomed Anal. 69:196–208. doi:https://doi.org/10.1016/j.jpba.2012.02.014
- Wolfhagen FH, Vermeulen HG, Rob A, Lesterhuis W. 2008. Initially obscure hepatotoxicity attributed to sildenafil. Eur J Gastroenterol Hepatol. 20(7):710–712. doi:https://doi.org/10.1097/MEG.0b013e3282f2bbb5.