 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
3-Monochloropropane-1,2-diol esters (3-MCPDE) are food contaminants commonly found in refined vegetable oils and fats, which have possible carcinogenic implications in humans. To investigate this clinically, we conducted an occurrence level analysis on eight categories of retail and cooked food commonly consumed in Malaysia. This was used to estimate the daily exposure level, through a questionnaire-based case-control study involving 77 subjects with renal cancer, with 80 matching controls. Adjusted Odds Ratio (AOR) was calculated using the multiple logistic regression model adjusted for confounding factors. A pooled estimate of total 3-MCPDE intake per day was compared between both groups, to assess exposure and disease outcome. Among the food categories analysed, vegetable fats and oils recorded the highest occurrence levels (mean: 1.91 ± 1.90 mg/kg), significantly more than all other food categories (p < .05). Risk estimation found the Chinese ethnic group to be five times more likely to develop renal cancer compared to Malays (AOR = 5.15, p = .001). However, an inverse association was observed as the 3-MCPDE exposure among the Malays (median: 0.162 ± 0.229 mg/day/person) were found to be significantly higher than the Chinese (p = .001). There was no significant difference (p = .405) in 3-MCPDE intake between the cases (median: 0.115 ± 0.137 mg/day/person) and controls (median: 0.105 ± 0.151 mg/day/person), with no association between high intake of 3-MCPDE and the development of renal cancer (OR = 1.41, 95% CI: 0.5091–2.5553). Thus, there was insufficient clinical evidence to suggest that this contaminant contributes to the development of renal malignancies in humans through dietary consumption. Further research is necessary to support these findings, which could have significant public health ramifications for the improvement of dietary practices and food safety measures.
Introduction
Renal cancer ranks eleventh most common cancers worldwide in men in 2020 (Sung et al. Citation2021), with renal cell carcinoma (RCC) being the most common malignant tumour of the kidney constituting 90% of all renal malignancies in adults (Hsieh et al. Citation2017). In Malaysia, renal cancer contributed around 2.5% of all cancers amongst men and only 0.9% amongst women between 2012 and 2016 (Azizah et al. Citation2019). Although the exact pathogenesis of RCC remains unclear, potential risk factors have been identified such as genetic factors, pre-existing chronic kidney disease, family history, smoking, obesity, hypertension, analgesic use, and occupational exposure to chemicals (e.g. cadmium, asbestos) (Chow et al. Citation2010; Choueiri et al. Citation2014; Scelo and Larose Citation2018). Some studies have suggested the role of dietary habits increasing the risk of RCC, highlighting the risks involved with the intake of meat-based protein, and alcohol consumption (Daniel et al. Citation2011; Lew et al. Citation2011). However, in contrast to other cancers such as gastric and colorectal cancer, the relationship between food intake and RCC does not appear to be as significant (Weikert et al. Citation2006)
Pre-clinical studies have linked the incidence of renal cancer development to the consumption of chloropropanols, specifically 3-monochloropropane-1,2-diol (3-MCPD). 3-MCPD is a contaminant formed during food production, especially in cases where foods containing fat and salt are processed via acid hydrolysis at high temperatures, such as in the manufacturing of vegetable protein hydrolysate and soy sauce (Crews et al. Citation2013). It is primarily found in its esterified form as a mixture of both, mono- and di-esters in processed edible oils and fats (MacMahon, Begley, et al. Citation2013), attributed to the presence of organochlorine compounds as the active precursors during high-temperature refining (Freudenstein et al. Citation2013; Tiong et al. Citation2018). From previous in vivo studies, the kidney, and testes were identified as critical organs for 3-MCPD, as shown by the significant cellular responses in these organs. Dietary 3-MCPD, given through drinking water in F344 rats, was reported to increase the incidence of renal tubule neoplasms in both sexes (Sunahara Citation1993). One study found the development of both renal cell carcinomas and Leydig cell tumours in male rats, which showed a dose-related positive trend on chronic consumption, indicating clear carcinogenic activity (Cho et al. Citation2008). However, conflicting findings were reported in more recent studies on different strains of mice, where oral 3-MCPD did not result in any neoplastic or non-neoplastic results upon histopathological testing (Jeong Citation2010; Lee et al. Citation2017).
3-MCPD has been classified as possibly carcinogenic to humans (Group 2B) by the International Agency for Research on Cancer (IARC) and the European Scientific Committee on Food (SCF) (International Agency for Research on Cancer (IARC) Citation2000; European Commission (EC) Citation2001). The Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA) established a group provisional maximum tolerable daily intake (PMTDI) of 4 µg/kg body weight/day for 3-MCPD using the benchmark dose modelling approach (Joint FAO/WHO Expert Committee on Food Additives (JECFA) Citation2018). A BMDL10 value of 0.20 mg/kg body weight per day in male rats was selected by the panel as the reference point for possible renal effects in humans (EFSA Panel on Contaminants in the Food Chain (CONTAM) Citation2018).
Many studies have demonstrated contamination by 3-MCPD in its esterified form in various food items. High concentrations of 3-MCPD esters (3-MCPDE) have been found in refined vegetable oils, particularly palm and palm-derived oils (MacMahon, Mazzola, et al. Citation2013; Li et al. Citation2015; Abd Razak et al. Citation2019). Other notable foodstuffs with this contaminant include heat-treated food (e.g. processed meat, fish products) (Merkle Citation2018; Inagaki Citation2019), confectionaries, frozen/canned foods (Shimamura et al. Citation2021), and infant food including human breast milk (Becalski et al. Citation2018) and infant formula milk (Becalski et al. Citation2015; Beekman et al. Citation2020; Nguyen and Fromberg Citation2020).
As 3-MCPDE are considered potentially toxic due to the release of 3-MCPD from its esters on digestion, its common presence in our diet has raised general concern. To investigate this clinically, we aimed to assess the extent of exposure to dietary 3-MCPDE via occurrence level analysis of common Malaysian foods. This data was then used to investigate any association between 3-MCPDE intake and renal cancer development through dietary exposure assessment in a retrospective case-control study.
Materials and methods
3-MCPDE occurrence level in food
Selection and sampling of food items
Food items selected to be analysed were based on the highest priority for data collection from the open call for data report published by European Food Safety Authority (EFSA) in October 2014. The list was aligned to the Malaysian diet, taking into account the consumption data available in the Malaysian Adult Nutrition Survey (MANS) (Institute for Public Health (IPH) Citation2014). All the food items selected were known to have high vegetable oil content, either as an ingredient or through their preparation techniques (e.g. frying, grilling). The most common vegetable oils found in the samples were palm oil (palm olein), palm kernel oil, sunflower oil, and canola oil. Food items were categorised based on composition and preparation method, which consisted of vegetable fats and oils (margarine, mayonnaise, shortening, creamer), milk and milk products (milk, butter), confectionaries (biscuits, cakes, buns, chocolate, tarts), snacks (crackers, potato chips), local kuih-muih (Malaysian sweet or savoury bite-sized desserts), cooked fried/grilled dishes, cooked stewed/boiled dishes, and fast food (burger, fries, nuggets, pizza, sausage, hot dogs) ().
Table 1. Food categories analysed for 3-MCPDE occurrence.
A stratified sampling plan based on the method used by the Malaysian Food Composition Database (Tee Citation1997) was used for sample collection, which followed a specific defined stratum (by retail sale point). A minimum of three cooked samples were purchased randomly from different outlets (restaurant or stall), selecting freshly cooked/prepared samples. For processed retail foods, the top national brands (by retail sale point) identified through a market survey, were selected for sampling from major grocery chains in the states of Selangor, Negeri Sembilan, Perlis, Kelantan, Sarawak and the Federal Territory of Putrajaya. For each brand, one kilogram of the sample was purchased. The collection was conducted between the months of November 2017 and December 2018.
Sample preparation
Liquid food items such as evaporated/concentrated creamers and margarine were sampled in their original glass/plastic/aluminium containers. Other solid or semi-solid retail and cooked food samples were freeze-dried in resampling plastic bottles.
Storage. Samples were then homogenised using a commercial blender and subsequently stored at −80 °C and freeze-dried for five days (Beta 2-8 LDplus, Christ, Germany). Upon freeze-drying, the samples were ground into fine powders, transferred into airtight containers and stored at −20 °C for transport.
Determination of 3-MCPDE level in food samples
Analysis was conducted following the validated method from the American Oil Chemists’ Society (AOCS) Official Method Cd 29a-13 on edible oils and fats (American Oil Chemists’ Society (AOCS) Citation2017), and Ermacora and Hrnčiřík (Citation2014). This is an indirect method for the determination of 3-MCPDE in edible oils and various oil-based food products by acid transesterification and derivatisation of the released free form with phenylboronic acid (PBA). Internal standard (IS) was applied for quantification of the analytes using respective deuterated fatty acid esters. Working standard solutions were prepared as shown in .
Table 2. Preparation of standard solutions.
The preparation of calibration standard solutions were set by adding the appropriate volume (µL) of the respective working standard solutions in a 0.1 g blank olive oil sample prior to the sample extraction procedure.
The content of bound 3-MCPDE is reported in milligrams per kilogram (mg/kg). Analytical results below the limits of detection (LOD) and limit of quantification (LOQ) were treated according to the Instructions for electronic submission of data on chemical contaminants in foods to GEMS/Food – Appendix 5: Evaluation of low-level contamination of foods. All quality assurance and quality control (QA/QC) parameters in the analysis were assessed in accordance with QA/QC guidelines, norms, and accredited procedures as followed by the Department of Chemistry, Malaysia.
Data analysis
Prior to analysis, data was cleaned and revised for discrepancies. Tests for normality were done using Kolmogorov–Smirnov and Shapiro–Wilk statistics, showing the data for the food samples was not normally distributed. Hence, non-parametric tests were applied for descriptive statistics. 3-MCPDE occurrence levels in medians, interquartile range, and percentile range for each food category were calculated using Statistical Package for Social Sciences, SPSS (version 23 for Windows, 2015, SPSS Inc.). The statistical significance of the difference between food groups was assessed using Mann–Whitney’s (MW) test for two groups and Kruskall–Wallis’s (KW) test for three groups or more. The level for significance was designated as p < .05.
Association of renal cancer and 3-MCPDE: case-control study
Study design and population
A retrospective case-control study design was adapted in view of the low prevalence rate of renal cancer in Malaysia, and the long latency period between exposure and disease manifestation. Subject recruitment was conducted between August 2017 and September 2018 in central, northern and southern regions of Peninsular Malaysia. Adult patients diagnosed with primary renal cancer, paired with adult controls matched based on age group, gender, and residence, were recruited. Potential cases from Kuala Lumpur Hospital (central region), Selayang Hospital (central region), Penang Hospital (northern region) and Sultan Ismail Hospital, Johor Bharu (southern region) were identified from diagnostic pathology records from the relevant departments. Selected hospitals were chosen on the basis of being referral centres for renal cancer with dedicated oncology departments.
Inclusion criteria for cases were patients with histologically confirmed primary adenocarcinoma of the kidney (ICD-10 C64) (World Health Organisation (WHO) Citation1992), aged above 18 years old, with good mental status. A simple short-term memory test developed by the University of Washington, USA was used as a screening tool for memory capability, available online: https://faculty.washington.edu/chudler/stm0.html (accessed on 24th August 2017). Eligible subjects were required to recall more than 70% of the letters in the sequence to participate. Patients who were too ill, having psychiatric disorders, psychotic features, or severe communication problems such as speech or hearing impairment were excluded from the study.
Inclusion criteria for controls were defined as those with (1) no known medical illness; (2) aged above 18 years old; (3) no history of symptoms that are associated with kidney cancer; (4) normal blood pressure; (5) normal blood sugar level, normal full blood count, renal function and urine dipstick test. History of signs and symptoms associated with renal cancer include the presence of blood in the urine (haematuria), unilateral low back pain (not caused by injury), a lump on the side or lower back (unilateral or bilateral), fatigue, significant loss of weight and appetite (not caused by dieting), prolonged fever (not caused by an infection and that doesn’t subside), and anaemia (Sungur and Azak Citation2019; Atkins Citation2021). Normal blood pressure was defined as systolic pressure ≤140 mmHg and diastolic pressure ≤90 mmHg) measured using Omron upper arm automatic blood pressure monitor HEM-7124. Blood parameters assessed included full blood count, kidney function and random blood sugar level, obtained from the latest laboratory reports from issued medical records. Normal venous plasma glucose was defined as a fasting blood sugar level of ≤7.0 mmol/L or a random blood sugar level of ≤11.1 mmol/L. Healthy controls were identified and recruited from the general population at the Klinik Kesihatan Kuala Lumpur and the participating government hospitals outpatient clinics.
The study was approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID: 17-904-35863) and was performed in accordance to the Declaration of Helsinki (World Medical Association). Written informed consent was obtained from all participants.
Sample size and sampling method
The study was conducted with independent cases and controls. Prior data indicated that the probability of exposure among controls is 0.69 (Azizah et al. Citation2016). If the true probability of exposure among cases was 0.89, 64 cases and 64 controls were required to reject the null hypothesis of the exposure rates for case and controls being equal (power = 0.8). The Type I error probability associated with this test of this null hypothesis is 0.05. We used an uncorrected chi-squared statistic to evaluate this null hypothesis. By using the power and sample size calculator (PS version 3.1.2) (Dupont and Plummer Citation1998) with the case to control the ratio of 1:1, the total sample needed was approximately 128 respondents. Considering the probable dropout rate of 20%, the total number of subjects required was 154, with 77 cases and 77 healthy controls.
The controls were selected from respective outpatient clinic visitors based on convenient sampling following the matching criteria.
Data collection
Sociodemographic questionnaire
A pre-tested socio-demographic questionnaire and food frequency questionnaire (FFQ) were used for data collection. The pre-test was conducted among visitors of Hospital Kuala Lumpur outpatient clinics in August 2017 before proceeding with the actual study. The interview was conducted on a one-to-one basis, with the duration of 20 min in the clinical setting or homes of the respondents by pre-trained interviewers in order to minimise inter-observer bias. The socio-demographic questionnaire contains data such as gender, date of birth, age range, race, anthropometric parameters, household income, residential area, and education level. The risk factors investigated were smoking habit (active smoking, passive smoking, ex-smoker, non-smoker), personal medical history, family medical history of cancer or genetic diseases (first, second degree), and chronic hazard exposure (toxic chemicals, radiation, pollutants). Each variable investigated is further defined under Appendix 1 (Variable definitions).
Food frequency questionnaire (FFQ)
Consumption data were collected via a semi-quantitative food frequency questionnaire, referencing the outline of the Diet History Questionnaire (DHQ) (Subar et al. Citation2001). The questionnaire included 34 selected food items commonly consumed by Malaysians, from each category in . Each food item was presented with accompanying food images depicting the type of food and serving sizes as a reference.
The consumption value for each listed food item is defined as the frequency of habitual intake within the 12 months prior to diagnosis for cases, and 12 months prior to the date of interview for control subjects. Data on intake frequency (per day, per week, per month, per year, or never), number of servings (per meal), and size of serving (piece, slice, packet, teaspoon, or tablespoon) were recorded. The conversion of food frequency to the estimated amount of food intake (in grams) was calculated using the formula developed by the Wessex Institute of Public Health Medicine, University of Southampton (Margetts and Nelson Citation1995) as the following;
This value was then used to calculate the estimated daily intake (EDI) value of 3-MCPDE in each food item in the questionnaire, by multiplying with the occurrence levels obtained prior, considering the frequency of intake for each respondent.
Data analysis
A descriptive analysis on normality and distribution of cases and control in terms of age, race, marital status, education, household income, residential area, and occupation were calculated using the Kruskal–Wallis test (non-normal distribution). For the risk factors, a measure of association using odds ratio (OR) was calculated using the binary/multiple logistic regression model. Initially, univariate analysis was conducted to analyse the association of individual variables to renal cancer (crude OR). The variables with p < .25 were selected for multivariate analysis for the association, by adjusting for potential confounders that may affect the overall risk (adjusted OR).
For each food item in the questionnaire, the measured 3-MCPDE occurrence level was multiplied with the frequency of consumption to give the EDI (µg/kg body weight/day) for each subject. The findings were then pooled according to their respective groups (case/control) to give a total EDI (mg/day) for each group. This was followed by an independent sample t-test (for normally distributed data), or Kruskal–Wallis and Mann–Whitney test (for non-normally distributed data), conducted to find any relation between intake level and disease outcome. Participants who consumed more than the PMTDI (4 µg/kg body weight/day) were considered to be of high exposure, and those who consumed less were considered of low exposure. All tests were two-sided, and a p value of <.05 was considered statistically significant. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist (Von Elm et al. Citation2007) was used to guide the reporting of this observational study.
Results
3-MCPDE occurrence level in Malaysian foods
In total, 251 food samples of 58 different food items were analysed for 3-MCPDE occurrence level, as summarised in . Results showed a non-normal distribution of pooled values per category, which is reported as the median, interquartile range (IQR), and range for each food item in mg/kg. Detected levels of 3-MCPDE were within the working range of 0.3 µg/g–9.3 µg/g. Final reporting was done on the amount of 3-MCPDE present in the sample, expressed on a food weight basis in milligrams per kilogram (mg/kg). The LOD and LOQ were calculated to be 0.11 mg/kg and 0.16 mg/kg, respectively. As the LOD and LOQ are not equal, a simple estimate of the median was made for the purpose of health risk assessment. All non-detectable (ND) results were set as equal to the LOD, as the non-quantified values were less than 60% of the overall data (World Health Organisation (WHO) Citation2002).
Table 3. 3-MCPDE occurrence levels in Malaysian food items (by categories).
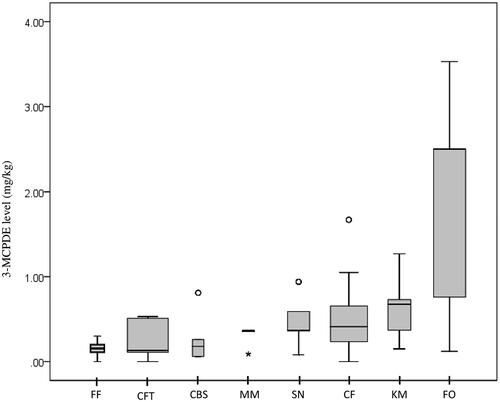
3-MCPDE was detected in 238 (94.8%) of the sampled food items. Levels exceeding the LOQ were detected in 212 samples (84.4%), of which 40 samples were from vegetable fats and oils (n = 42, 95.2%), 29 local kuih-muih (n = 30, 96.7%), 46 fried cooked food (n = 48, 95.8%), 12 stewed cooked food (n = 15, 80%), 25 snacks (n = 29, 86.2%), 13 fast food (n = 18, 72.2%), 11 milk and milk products (n = 16, 68.8%), and 36 confectionaries (n = 53, 67.9%). Overall, individual occurrence levels for all food samples analysed ranged from non-detectable (ND) to 7.77 mg/kg of wet weight, with a median of 0.44 ± 1.01 mg/kg. The highest levels were detected in the shortening samples (median: 3.53 ± 4.18 mg/kg), while all samples of chocolate bars (confectionary) and pizza (fast food) were below detectable levels. The difference in 3-MCPDE levels between the eight food groups was statistically significant (χ2 KW=74.59; p = .001). Vegetable fats and oils recorded the highest values (mean: 1.91 ± 1.90 mg/kg), significantly higher than all other food categories (p < .05).
Concerning cooked/prepared food, the highest levels overall were detected in the kuih-muih category. Kuih-muih (median: 0.68 ± 0.41 mg/kg) had significantly higher levels than confectionaries (p = .03), stewed/boiled foods (p = .011) and the fast-food group (p = .01), which recorded the lowest occurrence (median: 0.16 ± 0.17 mg/kg). Seventy percent of samples from the kuih-muih group gave a median level above 0.60 mg/kg, while all fast-food samples recorded levels below 0.30 mg/kg. Kuih denderam, a fried sweetmeat adapted from a South Indian desert adhirasam, contained the highest level overall (median: 1.27 mg/kg). This was followed by more popular varieties such as prawn fritters, banana fritters, vadai (Indian savoury lentil fritters), curry puffs, fried spring rolls, and cakoi (long deep-fried strip of dough made from wheat flour) which had similar readings between 0.65 and 0.75 mg/kg.
A wide level range was seen within the cooked-fried food category, with the highest levels detected in fried anchovies (median: 1.67 mg/kg) and butter prawn samples (median: 1.05 mg/kg). The remaining 80% consisting of common dishes such as fried rice (nasi goreng), fried rice noodle (bihun goreng), fried wheat noodle (mee goreng), Indian flatbread (roti canai) and stir-fried flat rice noodle (char-kuey-teow) were all below 0.80 mg/kg. Outlier values were detected for samples of beef curry (boiled/stewed), murukku (snacks), fried anchovies (cooked fried) and evaporated milk (milk and milk products) (). Despite higher levels of exposure to vegetable oil, the pairwise comparison showed 3-MCPDE levels in cooked fried food did not differ significantly when compared to stewed or boiled food samples (p = 1.0).
Figure 1. 3-MCPDE occurrence level per food category (mg/kg). Box plots represent the median, interquartile range, minimum and maximum levels in selected food categories. FF: Fast food; CFT: Confectionary; CBS: Cooked (boiled/stewed); MM: Milk and milk products; SN: Snacks; CF: Cooked (fried); KM: Kuih-muih; FO: Fats and oils Ο Outlier values: more than 1.5 × IQR (Boiled/stewed food: Beef curry; Snacks: Murukku; Cooked fried food: Fried anchovies) * Extreme outlier value for evaporated milk: 0.09 ± 0.091 mg/kg.
Sociodemographic distribution
depicts the demographic comparisons of recruited subjects in our case-control study. A total of 157 participants were enrolled, involving 77 cases and 80 controls. Overall, the subjects were largely residences from the central region (77.1%), followed by the northern region (12.7%), and the southern region (10.2%) of Peninsular Malaysia. The mean age for cases was 56.9 years (SD = 13.39), and for controls was 54.9 years (SD = 13.92). Eighty-eight percent of the cancer patients were above the age of 40. There was no significant difference in terms of age (p = .974) between the two groups. A majority of the renal cancer patients were elderly men, between the 50 and 65 age bracket, residing in the Klang Valley. Of the control subjects, 71.3% were living in urban areas, and none from rural areas, compared to 14% of the cases.
Table 4. Sociodemographic distribution of study subjects.
The case group was mainly composed of Chinese (42.9%) or Malays (40.5%), while the control group was largely made up of Malays (66.3%). In terms of social background, most of the subjects were educated until secondary school. However, more than half of the recorded cases were from the lower income group, earning < RM1000/month, compared to controls. This can be attributed to the fact that most of these patients are no longer working after being diagnosed with the disease.
Association between risk factors and renal cancer
Our study found no significant difference (p > .05) between the cases and controls in terms of the prevalence of obesity, history of smoking, first-degree family history of cancer, second-degree family history, or exposure to hazards e.g. toxic chemicals, pollutants, or radiation. Among the diagnosed cases, almost half of the patients (47%) had pre-existing hypertension, and 22% were diabetics on treatment. Less than 5% of them had any pre-existing kidney diseases or were diagnosed with other organ malignancies. The majority of cases (85%) had no history of prolonged use of diuretics or analgesics prior to diagnosis.
After adjusting for age, gender, race and other potential confounders, Chinese were found to be five times more likely to develop renal cancer compared to Malays (AOR = 5.15, p < .05), but with no significance when compared to other races as a whole (non-Chinese). Other main risk factors investigated (obesity, regular hazard exposure, first/second-degree family history, cigarette smoking) did not yield statistical significance. Regarding dietary pattern, even with higher content of 3-MCPDE present in refined vegetable oils, eating fried food at least once daily did not show any increased risk among subjects compared to those who consume only once weekly or once monthly ().
Table 5. Measure of association for risk factors of renal cancer.
Comparison of 3-MCPDE intake between case and control groups
The estimated individual EDI (mg/day/person) and total EDI of 3-MCPDE for both groups are tabulated in . As the distribution of data in both groups was highly skewed, the comparison was done using the Mann-Whitney test. Collectively, the renal cancer patients did consume higher amounts of 3-MCPDE per day (median: 0.115 ± 0.137 mg/day/person). However, the differences were not statistically significant (p = .405) when compared to the controls (median: 0.105 ± 0.151 mg/day/person) (). The total EDI for all participants was up to 31.9 mg/day, with 62.7% (20.6 mg/day) contributed by the consumption of vegetable oil-based food items.
Figure 2. Graphic representation comparing the mean estimated daily intake (mg/day) of 3-MCPDE according to (A) group (case/control) and (B) ethnicity with 95% CI (T-Bar).
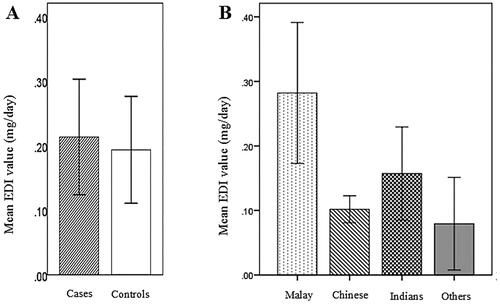
Table 6. Comparison of total EDI and individual EDI of 3-MCPDE (case and control group).
In general, the Malays had the highest 3-MCPDE exposure among all races (median: 0.162 ± 0.229 mg/day/person). EDI of Malay cases was higher than their controls in both gender, but the difference was not statistically significant (p = .13) (). The pairwise post-hoc comparison showed that 3-MCPDE EDI levels were significantly higher in the Malays compared to the Chinese (p < .001) (). This was evident for both genders, being exposed to significantly higher amounts daily, only when compared to their Chinese counterparts (p = .001, p = .008). The extent of exposure did not differ in consideration of gender (p = .170), the different age groups (p = .317), and between the overweight and non-overweight subjects (p = .367) for both case and control groups.
Table 7. Comparison of EDI (mg/day/person) of 3-MCPDE between groups considering ethnicity and gender.
Out of the total 157 subjects, 29 were considered high exposure to 3-MCPDE effects, as they exceeded the maximum recommended daily intake of 4 µg/kg body weight/day. This group comprised 15 cases and 14 controls, of which 80% were Malay. The total daily 3-MCPDE intake for each of these highly exposed subjects ranged between 4.01 and 76.49 µg/kg body weight/day. When compared between high exposure to low exposure subjects, no association could be made between high intake of 3-MCPDE and the development of renal cancer (OR = 1.41, 95% CI: 0.5091–2.5553).
Discussion
The relevant legislation currently in force such as Commission Regulation (EU) 2020/1322 and the Malaysian Food Act 1983 and Regulations 1985 (European Commission (EC) Citation2020; Laws of Malaysia Citation2021) defines the maximum levels for 3-MCPD and 3-MCPDE but only in certain foods specifically soy sauces, HVP, vegetable oils and fats, fish oils and infant formula. There is no regulation currently drafted for cooked or prepared foods outside these categories. Taking into account the 3-MCPDE contamination found in the 251 food items assessed in the current study, 223 samples (88.8%) of different food categories recorded occurrence levels above 0.1 mg/kg wet weight. 44.4% of these food items generally may contain flavour enhancers or soy sauce as ingredients such as cooked fried foods and processed foods e.g. hot dogs, potato chips, and instant noodles. Based on a recent study by Wong et al. in Malaysia (Wong Citation2020), the use of chicken-based seasoning could have contributed to the high amounts in savoury cooked fried dishes, as the seasoning sampled in their study all exceeded the maximum tolerable limit of 0.02 mg/kg. This was attributed to the addition of soy sauce or acid-hydrolysed soybeans as ingredients in chicken-flavoured seasoning cubes, which may be the source of the chloropropanol contamination.
Further discussions on the role of vegetable oils in 3-MCPDE contamination in a foodstuff have prompted amendments to Regulation (EC) No 1881/2006. This involves efforts to mitigate the problem, starting from the manufacturing of the raw ingredients during preparation. It is evident that the top categories with the highest occurrence level among the cooked foods (i.e. kuih-muih, cooked fried food), all involved preparation through deep-frying. These findings imply that the cooking method by frying, tends to show a higher presence of 3-MCPDE level in general, as all kuih-muih, snacks (keropok lekor, keropok ikan), and fried dishes tested were prepared in a similar manner. The main choice of frying oil in Malaysia being palm oil may also be a contributing factor, as palm oil in itself has been found to contain higher 3-MCPDE levels compared to other vegetable oils (Abd Razak et al. Citation2019; Liu Q et al. Citation2021). Efforts in reducing these contaminants have extensively been evaluated. Some reports suggest modifying steps in the processing of vegetable fats and oils, starting from the initial selection and washing of the raw material, prior to refining. Reduced usage of organic chlorine-containing compounds during pre-treatment and washing with water and ethanol could remove critical reactants and pre-cursors of 3-MCPD (Matthäus et al. Citation2011; Li Citation2016). Some publications also suggested the prospect of utilising radical scavengers, comprising polyphenols from other foods such as rosemary extract, to induce degradation of the compound, from the final processed product (Zhang et al. Citation2016; Yıldırım and Yorulmaz Citation2018).
From our case-control study, we found that ethnic Chinese were at a higher risk of developing renal cancer when compared to other populations in Malaysia. This correlates with the Malaysia National Cancer Registry findings 2007–2011 (Azizah et al. Citation2016) and the recently published report for 2012–2016 (Azizah et al. Citation2016), where renal cancer has the highest reported incidence among the Chinese. Male incidence was twice as high as for females and contributed 2.5% among all other cancers (Azizah et al. Citation2016). Similarly, in our study, the male case were double the number of female cases, but there was no evidence of increased cancer risk concerning different gender. With regard to age group, documentation of age at diagnosis should have been considered to properly map the age distribution of this disease, as renal cancer has a higher prevalence among the elderly within the 60–70 age bracket (Protzel Citation2012).
Cigarette smoking and obesity are established risk factors associated with renal cancer development (Scelo and Larose Citation2018). Though this dose-response relationship was not shown in this study, cigarette smoking increases the risk of renal cancer by around 20 to 40%, positively correlated to the smoking intensity and duration (Hunt Citation2005; Liu et al. Citation2019). Obesity being another major risk factor did not increase risk in our study, as the present weight of the chronic renal cancer patients recorded may be reduced due to cancer-related weight loss. In addition, our study did not find a significant association between a family history of cancer and hazard exposure (toxic chemicals, radiation, pollutants) which is inconsistent with other studies (Hu et al. Citation2002; Ljungberg and Choi Citation2011; Purdue Citation2011).
Another potential risk factor investigated concerns dietary pattern, where the frequency of fried food intake was compared between subjects. Despite higher occurrence levels, consuming fried food frequently at least once daily did not present a higher risk compared to infrequent consumption (weekly/monthly). In terms of ethnicity, we found that the Malays were exposed significantly more than the Chinese to 3-MCPDE through their dietary choices. This is mainly due to a higher preference for local kuih-muih and cooked-fried foods among the Malays from our survey. One population study, conducted in a northern state of Malaysia compared the different dietary preferences of Malay and Chinese adolescents, also showed that Malay adolescents had significantly higher scores for the unhealthy Western-based food pattern, characterised by high intake of processed foods, which were high in fat, salt and sugars (Abdullah et al. Citation2016). Even so, they were not reflected to have a higher risk of developing renal cancer in our study. This supports the notion that dietary pattern may not be as significant as other risk factors in the context of renal cancer development, as opposed to obesity, hypertension, smoking (Al-Bayati et al. Citation2019) and family history (Clague et al. Citation2009).
Thus, there is insufficient prevailing clinical evidence to suggest that this contaminant directly causes renal malignancies in humans, as cancers, in general, are known to be multifactorial (Wu et al. Citation2018). We can only infer these effects through evidence from previous animal studies, which have indicated that the kidney and testis were the main target organs for 3-MCPD-induced carcinogenicity (Buhrke Citation2017; Oberemm Citation2017; Mahmoud Citation2019). To our knowledge, there has not been any similar study conducted for comparison, involving 3-MCPDE exposure from cooked or prepared food from Asia investigating renal cancer per se. A recent umbrella review of meta-analyses investigated the role of specific food categories (e.g. fruits, vegetables, meat, beverages) and dietary nutrients (e.g. dietary fibres, vitamins, proteins), with up to class III level of evidence linking diet to RCC incidence (Liao et al. Citation2022).
Limitations of this study must be noted. Our study included cases of primary kidney cancer with histologically confirmed adenocarcinoma of the kidney (ICD-10 C64) (World Health Organisation (WHO) Citation1992), but excluded other histological types, such as renal sarcoma, transitional cell carcinoma, and lymphoma, which would give a better general representation of kidney cancer. The subjects’ recruitment in our study was largely hospital-based, with control subjects recruited via convenient sampling, which may be subject to selection bias. We did not perform matching on race or ethnicity, as it precludes assessment of these factors as potential risk factors. This resulted in the recruitment of control subjects with a Malay ethnic majority (more than 60%). Regardless, the proportion of control subjects recruited was found to be representative of the Malaysian population as a whole in terms of major ethnic group composition (Department of Statistics Malaysia Citation2022). Another notable limitation in subject recruitment was the identification of the healthy control subjects, where the medical history is largely self-reported. Therefore, we cannot exclude information bias.
Recall bias cannot be excluded, as in most case-control studies. We observed that the case subject’s recollection of dietary pattern prior to diagnosis were less definitive when compared to the control subjects. As some subjects were diagnosed more than five years ago, accurate recollection on food consumption frequency was a challenge. This was further contributed with the possible change of dietary habits as a result of illness, e.g. reduced appetite, orthorexia, and food cravings (Nucci et al. Citation2022). We also acknowledge the dietary recall periods may not reflect the actual timeframe of relevant significant exposure, but the recollection of habitual intake is considered to minimise the recall bias in these studies (Zhang et al. Citation2002). Inter-observer and interviewer bias are also commonly found in retrospective data collection. However, these were kept minimal as interviews were conducted by the same trained interviewers throughout the study.
There are several strengths of our study in terms of subject recruitment. The diagnosis of renal cell carcinoma was validated through a detailed histopathology review of medical records, as well as a high response rate among cases and controls. Furthermore, the repeatability of the food items consumption assessment was guaranteed by the use of a validated questionnaire structure, which was pre-tested on the general population prior to study commencement. Our food sample selection is also an advantage as it closely reflects the actual exposure estimate from the Malaysian diet as some samples involve cooked, prepared, ready-to-eat meals. Careful consideration of our findings is advisable, due to the limited precision of the risk estimates, and the uncertainties of exposure assessment commonly associated with studies involving dietary surveys (EFSA Citation2006).
Conclusions and recommendations
From our study, it is evident that 3-MCPDE and its equivalents are indeed present in common Malaysian foods, especially from the kuih-muih, snacks and fried dishes’ category. Despite this, it is still too early to distinguish any causal relationship of developing renal cancer being directly due to high 3-MCPDE consumption in humans, as the results available from our case-control study do not support this hypothesis. Other similar clinical studies are also limited on this issue, making any presumptions of the presence or absence of a direct or inverse correlation debatable. Further studies on larger cohorts of different populations may be of benefit.
From a public health perspective, we should not disregard the fact that we are exposed to this compound daily, and the toxic risks associated with it. Our occurrence level data can be used as a baseline for redefining the current recommendations for chloropropanol contaminants concerning 3-MCPDE in cooked or prepared foods. Risk management through mitigation strategies along the food supply chain should also be strengthened, to keep the risk of exposure at the lowest.
Author contribution
Conceptualisation: Tasnim K. Ami Fazlin SM., Fairulnizal MN. Methodology: Tasnim K., Ami Fazlin SM., Ruhaya S., Lalitha P., Mohamad Hasnan A. Formal analysis: Siti Hajar MR., Lau MS., Norhayati MK., Nurul Izzah A., Lalitha P., Laila Rabaah AS., Zawiyah S., Nurhazwani AR., Investigation: Siti Hajar MR., Lau MS., Tasnim K, Siti Khuzaimah M, Sughanti J, Syazwani SA, Norhayati MK, Nurul Huda R. Resources: Tasnim K, Siti Hajar MR., Lau MS., Ami Fazlin SM., Norhayati MK., Fairulnizal MN, Ruhaya S., Lalitha P., Laila Rabaah AS., Zawiyah S., Nurhazwani AR., Ros Suzanna AB., Rohan M., Teoh BW., Khoo SC., Lim CS. Data Curation: Siti Hajar MR., Lau MS. Writing – Original Draft: Siti Hajar MR. Writing – Review & Editing: Siti Hajar MR., Nurul Izzah A., Ami Fazlin SM., Lau MS. Visualization: Siti Hajar MR., Nurul Izzah A., Ami Fazlin SM. Supervision: Ami Fazlin SM., Ros Suzanna AB., Rohan M., Teoh BW, Khoo SC., Lim CS. Project administration: Ami Fazlin SM. Funding acquisition: Tasnim K, Ami Fazlin SM.
| Abbreviations | ||
| 3-MCPD | = | 3-Monochloropropane-1,2-diol |
| 3-MCPDE | = | 3-Monochloropropane-1,2-diol esters |
| AOCS | = | American Oil Chemists’ Society |
| AOR | = | Adjusted Odds Ratio |
| CI | = | Confidence Interval |
| COR | = | Crude Odds Ratio |
| EC | = | European Commission |
| EDI | = | Estimated Daily Intake |
| GC-MS | = | Gas Chromatography-Mass Spectrometry |
| HVP | = | Hydrolysed Vegetable Protein |
| IS | = | Internal Standard |
| LOD | = | Level of Detection |
| LOQ | = | Level of Quantification |
| MANS | = | Malaysian Adult Nutrition Survey |
| OR | = | Odds Ratio |
| PBA | = | Phenylboronic Acid |
| PMTDI | = | Provisional Maximum Tolerable Daily Intake |
| RCC | = | Renal Cell carcinoma |
| TDI | = | Tolerable daily intake |
Acknowledgement
The authors would like to thank the Director General of Health Malaysia for his permission to publish this article. We would like to acknowledge all the officers and staff involved from Herbal Medicine Research Centre, Nutrition, Metabolic & Cardiovascular Research Centre, Institute for Medical Research, Food Safety and Quality Division, Ministry of Health, and Department of Chemistry, Malaysia who have contributed directly or indirectly towards the successful conduct of this study. We also extend our warm gratitude and appreciation to the Director of Institute for Medical Research, Institute for Public Health, Senior Director of Food Safety and Quality Division, Ministry of Health, Director General of Department of Chemistry, Malaysia, and all the directors of the government hospitals and clinics involved in our survey (Hospital Kuala Lumpur, Hospital Pulau Pinang, Hospital Johor Bahru, Hospital Selayang and Klinik Kesihatan Kuala Lumpur).
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Abd Razak RA, Ahmad Tarmizi AH, Abdul Hammid AN, Kuntom A, Ismail IS, Sanny M. 2019. Verification and evaluation of monochloropropanediol (MCPD) esters and glycidyl esters in palm oil products of different regions in Malaysia. Food Addit Contam Part A. 36(11):1626–1636. doi:10.1080/19440049.2019.1654139
- Abdullah N-F, Teo PS, Foo LH. 2016. Ethnic differences in the food intake patterns and its associated factors of adolescents in Kelantan, Malaysia. Nutrients. 8(9):551. doi:10.3390/nu8090551
- Al-Bayati O, Hasan A, Pruthi D, Kaushik D, Liss MA. 2019. Systematic review of modifiable risk factors for kidney cancer. Urol Oncol. 37(6):359–371. doi:10.1016/j.urolonc.2018.12.008
- American Oil Chemists’ Society (AOCS). 2017. Official method Cd 29c-13. 2- and 3-MCPD Fatty acid esters and glycidol fatty acid esters in edible oils and fats by GC/MS (difference method). Revised 2017. Official methods and recommended practises of the AOCS. 7th ed. AOCS.
- Atkins MB. 2021. Clinical manifestations, evaluation, and staging of renal cell carcinoma. Waltham (MA): UpToDate; [accessed 2021 May 31].
- Azizah AM, Nor Saleha IT, Noor Hashimah A, Asmah Z, Mastulu W. 2016. Malaysian National cancer registry report 2007–2011: Malaysia cancer statistic, data and figure. Malaysia: National Cancer Institute.
- Azizah AMN, Saleha ITN, Hashimah A, Asmah Z, Mastulu W. 2019. Malaysian National cancer registry report 2012–2016: Malaysia cancer statistic, data and figure. Malaysia: National Cancer Institute.
- Becalski A, Zhao T, Granvogl M, Arbuckle T. 2015. A pilot survey of 2-and 3- monochloropropanediol and glycidol fatty acid esters in baby formula on the Canadian market 2012–2013. J Food Compos Anal. 44:111–114. doi:10.1016/j.jfca.2015.08.004
- Becalski A, Zhao T, Granvogl M, Arbuckle T. 2018. An investigation of presence of 2-and 3-monochloropropanediol fatty acid esters in Canadian human milk samples. Food Addit Contam Part A. 35(10):1881–1889. doi:10.1080/19440049.2018.1506163
- Beekman JK, Grassi K, MacMahon S. 2020. Updated occurrence of 3-monochloropropane-1,2-diol esters (3-MCPD) and glycidyl esters in infant formulas purchased in the United States between 2017 and 2019. Food Addit Contam Part A. 37(3):374–390. doi:10.1080/19440049.2019.1706002
- Buhrke T, Schultrich K, Braeuning A, Lampen A. 2017. Comparative analysis of transcriptomic responses to repeated-dose exposure to 2-MCPD and 3-MCPD in rat kidney, liver and testis. Food Chem Toxicol. 106(Pt A):36–46. doi:10.1016/j.fct.2017.05.028
- Cho WS, Han BS, Nam KT, Park K, Choi M, Kim SH, Jeong J, Jang DD. 2008. Carcinogenicity study of 3-monochloropropane-1, 2-diol in Sprague–Dawley rats. Food Chem Toxicol. 46(9):3172–3177. doi:10.1016/j.fct.2008.07.003
- Choueiri TK, Je Y, Cho E. 2014. Analgesic use and the risk of kidney cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 134(2):384–396.
- Chow WH, Dong LM, Devesa SS. 2010. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 7(5):245–257. doi:10.1038/nrurol.2010.46
- Clague J, Lin J, Cassidy A, Matin S, Tannir NM, Tamboli P, Wood CG, Wu X. 2009. Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 18(3):801–807. doi:10.1158/1055-9965.EPI-08-0601
- Crews C, Chiodini A, Granvogl M, Hamlet C, Hrnčiřík K, Kuhlmann J, Lampen A, Scholz G, Weisshaar R, Wenzl T, et al. 2013. Analytical approaches for MCPD esters and glycidyl esters in food and biological samples: a review and future perspectives. Food Addit Contam Part A. 30(1):11–45. doi:10.1080/19440049.2012.720385
- Daniel C, Schwartz K, Colt J, Dong L, Ruterbusch J, Purdue M, Cross A, Rothman N, Davis F, Wacholder S. 2011. Meat-cooking mutagens and risk of renal cell carcinoma. Br J Cancer. 105(7):1096–1104.
- Dayan A. 1993. Solar and ultraviolet radiation. IARC monographs on the evaluation of carcinogenic risks to humans. Vol 55. J Clin Pathol. 46(9):880. doi:10.1136/jcp.46.9.880-a
- Department of Statistics Malaysia. 2022. Current Population Estimates, Malaysia, 2022. Prime Ministers Department, Department of Statistics Malaysia. www.dosm.gov.my.
- Dupont WD, Plummer WD. 1998. Power and sample size calculations for studies involving linear regression. Control Clin Trials. 19(6):589–601. doi:10.1016/s0197-2456(98)00037-3
- EFSA Panel on Contaminants in the Food Chain (CONTAM). 2018. Update of the risk assessment on 3‐monochloropropane diol and its fatty acid esters. EFSA J. 16(1):e05083.
- EFSA. 2006. Guidance of the scientific committee on a request from EFSA related to uncertainties in dietary exposure assessment. EFSA J. 438:1–54.
- Ermacora A, Hrnčiřík K. 2014. Development of an analytical method for the simultaneous analysis of MCPD esters and glycidyl esters in oil-based foodstuffs. Food Addit Contam Part A. 31(6):985–994.
- European Commission (EC). 2001. Opinion of the scientific committee on food on 3-monochloro-propane-1, 2-diol (3-MCPD). Brussel, Belgium: European Commission.
- European Commission (EC). 2020. Commission Regulation (EU) as amending Regulation (EC) No 1881/2006 as regards maximum levels of 3-monochloropropane diol (3-MCPD), 3-MCPD fatty acid esters and glycidyl fatty acid esters in certain foods. European Commission, Brussels, 2019. Official Journal of European Union. L 310:1–5.
- Freudenstein A, Weking J, Matthäus B. 2013. Influence of precursors on the formation of 3‐MCPD and glycidyl esters in a model oil under simulated deodorization conditions. Eur J Lipid Sci Technol. 115(3):286–294.
- Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V. 2017. Renal cell carcinoma. Nat Rev Dis Primers. 3:17009. doi:10.1038/nrdp.2017.9
- Hu J, Mao Y, White K. 2002. Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med. 52(3):157–164. doi:10.1093/occmed/52.3.157
- Hunt JD, Van der Hel OL, McMillan GP, Boffetta P, Brennan P. 2005. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 114(1):101–108. doi:10.1002/ijc.20618
- Inagaki R, Ito F, Shimamura Y, Masuda S. 2019. Effect of chloride on the formation of 3-monochloro-1, 2-propanediol fatty acid diesters and glycidol fatty acid esters in fish, meats and acylglycerols during heating. Food Addit Contam Part A. 36(2):236–243. doi:10.1080/19440049.2018.1562231
- International Agency for Research on Cancer (IARC). 2016. Outdoor Air Pollution. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 109:9.
- Institute for Public Health (IPH). (2014). National health and morbidity survey 2014: Malaysian adult nutrition survey (MANS). Vol. 3, Food consumption statistics of Malaysia. Kuala Lumpur: Institute of Public Health, Ministry of Health Malaysia.
- Institute for Public Health (IPH) 2015. National health and morbidity survey 2015 (NHMS 2015). Vol. 2, Non-communicable diseases, risk factors & other health problems. Kuala Lumpur: Institute for Public Health, Ministry of Health, Malaysia.
- International Agency for Research on Cancer (IARC). 2000. Some industrial chemicals: IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. Vol. 77. Lyon, France: IARC; p. 469–486.
- International Agency for Research on Cancer (IARC). 2012. Arsenic, metals, fibres, and dusts: international agency for research on cancer (IARC) monographs on the evaluation of carcinogenic risks to humans. Vol. 100. Lyon, France: IARC; p. 11.
- Jeong J, Han BS, Cho WS, Choi M, Ha CS, Lee BS, Kim YB, Son WC, Kim CY. 2010. Carcinogenicity study of 3-monochloropropane-1, 2-diol (3-MCPD) administered by drinking water to B6C3F1 mice showed no carcinogenic potential. Arch Toxicol. 84(9):719–729. doi:10.1007/s00204-010-0552-6
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2018. Safety evaluation of certain contaminants in food: prepared by the eighty-third meeting of the joint FAO/WHO expert committee on food additives (JECFA). 3-monochloro-1,2-propanediol esters and 3-monochloro-1,2-propanediol. WHO food additives series FAO JECFA monographs 19 Bis. No. 74. Geneva: World Health Organization; p. 665–761.
- Laws of Malaysia. 2021. Malaysia Food Act 1983 and Regulations 1985. Fourteenth a schedule (Regulation 38A). Maximum permitted proportion of 3-monochloropropane-1,2-diol (3-MCPD) in specific food. Petaling Jaya (Malaysia): International Law Book Services.
- Lee BS, Park SJ, Kim YB, Han JS, Jeong EJ, Son HY, Moon KS. 2017. Twenty-six-week oral carcinogenicity study of 3-monochloropropane-1,2-diol in CB6F1-rasH2 transgenic mice. Arch Toxicol. 91(1):453–464. doi:10.1007/s00204-016-1696-9
- Lew JQ, Chow W, Hollenbeck AR, Schatzkin A, Park Y. 2011. Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study. Br J Cancer. 104(3):537–541.
- Li C, Nie SP, Zhou YQ, Xie MY. 2015. Exposure assessment of 3-monochloropropane-1, 2-diol esters from edible oils and fats in China. Food Chem Toxicol. 75:8–13.
- Li CZ, Zhu J, Wang S, Nie S, Xie M. 2016. Formation of 3-chloropropane-1, 2-diol esters in model systems simulating thermal processing of edible oil. LWT-Food Sci Technol. 69:586–592.
- Liao Z, Fang Z, Gou S, Luo Y, Liu Y, He Z, Li X, Peng Y, Fu Z, Li D. 2022. The role of diet in renal cell carcinoma incidence: an umbrella review of meta-analyses of observational studies. BMC Med. 20(1):1–26.
- Liu X, Peveri G, Bosetti C, Bagnardi V, Specchia C, Gallus S, Lugo A. 2019. Dose-response relationships between cigarette smoking and kidney cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 142:86–93.
- Liu Q, Zhou P, Yang D. 2021. Chloropropanol and glycidyl esters content in edible vegetable oils in China in 2015-2017. Wei Sheng Yan Jiu. 50(1):75–78.
- Ljungberg BC, Choi HY. 2011. The epidemiology of renal cell carcinoma. Eur Urol. 60(4):615–621.
- MacMahon S, Begley TH, Diachenko GW. 2013. Occurrence of 3-MCPD and glycidyl esters in edible oils in the United States. Food Addit Contam Part A. 30(12):2081–2092.
- MacMahon S, Mazzola E, Begley TH, Diachenko GW. 2013. Analysis of processing contaminants in edible oils. Part 1. Liquid chromatography-tandem mass spectrometry method for the direct detection of 3-monochloropropanediol monoesters and glycidyl esters. J Agric Food Chem. 61(20):4737–4747.
- Mahmoud YI, Abo-Zied FS, Salem ST. 2019. Effects of subacute 3-monochloropropane-1, 2-diol treatment on the kidney of male albino rats. Biotech Histochem. 94(3):199–203.
- Margetts BM, Nelson M. 1995. Measuring dietary exposure in nutritional epidemiological studies. Nutr Res Rev. 8(1):165–178.
- Matthäus B, Pudel F, Fehling P, Vosmann K, Freudenstein A. 2011. Strategies for the reduction of 3‐MCPD esters and related compounds in vegetable oils. Eur J Lipid Sci Technol. 113(3):380–386.
- Merkle S, Ostermeyer U, Rohn S, Karl H, Fritsche J. 2018. Mitigation strategies for ester bound 2-/3-MCPD and esterified glycidol in pre-fried breaded and frozen fish products. Food Chem. 245:196–204.
- Nguyen KH, Fromberg A. 2020. Monochloropropanediol and glycidyl esters in infant formula and baby food products on the Danish market: occurrence and preliminary risk assessment. Food Control. 110:106980.
- Nucci D, Santangelo OE, Provenzano S, Nardi M, Firenze A, Gianfredi V. 2022. Altered food behavior and cancer: a systematic review of the literature. Int J Environ Res Public Health. 19(16):10299.
- Oberemm AB, Sawada S, Pink M, Frenzel F, Rozycki C, Meckert C, Zabinsky E, Braeuning A, Lampen A. 2017. Lanthanum chloride precipitation-based toxicoproteomic analysis of 3-monochloropropane-1, 2-diol toxicity in rat kidney reveals involvement of extracellular signal-regulated kinase 2. Arch Toxicol. 91(10):3247–3260.
- Protzel C, Marucchke M, Hakenberg OW. 2012. Epidemiology, aetiology, and pathogenesis of renal cell carcinoma. Eur Urol Suppl. 11:52–59.
- Purdue MP, Johansson M, Zelenika D. 2011. Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat Genet. 43(1):60–65.
- Scelo G, Larose TL. 2018. Epidemiology and risk factors for kidney cancer. J Clin Oncol. 36(36):JCO2018791905.
- Shimamura Y, Inagaki R, Oike M, Dong B, Gong W, Masuda S. 2021. Glycidol fatty acid ester and 3-monochloropropane-1,2-diol fatty acid ester in commercially prepared foods. Foods. 10(12):2905.
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. 2001. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the eating at America’s table study. Am J Epidemiol. 154(12):1089–1099.
- Sunahara G, Perrin I, Marchesini M. 1993. Carcinogenicity study on 3-monochloropropane-1,2-diol (3-MCPD) administered in drinking water to Fischer 344 rats. Unpublished report No. RE-SR93003 submitted to IMR by Nestec Ltd, Research & Development, Switzerland.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. 2021. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249.
- Sungur Ş, Azak E. 2019. Determination of fatty acid profile and 3-monochloropropane-1, 2-diol (3-MCPD) levels in bakery products. Nat Eng Sci. 4(2):114–124.
- Tee ES, Mohd. Ismail N, Mohd Nasir A, Khatijah I. 1997. Nutrient composition of Malaysian foods. 4th ed. Kuala Lumpur: Malaysian Food Composition Database Programme, Institute for Medical Research.
- Tiong SH, Saparin N, Teh HF, Ng TLM, Md Zain MZB, Neoh BK, Md Noor A, Tan CP, Lai OM, Appleton DR. 2018. Natural organochlorines as precursors of 3-monochloropropanediol esters in vegetable oils. J Agric Food Chem. 66(4):999–1007.
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 85:867–872.
- Weikert S, Boeing H, Pischon T, Olsen A, Tjonneland A, Overvad K, Becker N, Linseisen J, Lahmann PH, Arvaniti A. 2006. Fruits and vegetables and renal cell carcinoma: findings from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer. 118(12):3133–3139.
- Wong SL, Low KH, Jenatabadi HS, Mohamed CW, Khor SM. 2020. Estimation of the dietary intake and risk assessment of food carcinogens (3-MCPD and 1, 3-DCP) in soy sauces by Monte Carlo simulation. Food Chem. 311:126033.
- World Health Organisation (WHO). 1992. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: World Health Organization.
- World Health Organisation (WHO). 2000. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. Geneva, Switzerland: World Health Organization, 1998. World Health Organization Technical Report Series. 894:1–253.
- World Health Organisation (WHO). 2002. Instructions for electronic submission of data on chemical contaminants in foods - Appendix 5: evaluation of low level contamination in foods. GEMS/Food Electronic Reporting Manual for Contaminants in Food. World Health Organisation: Food Contamination Monitoring and Assessment Program (GEMS/FOOD). WHO/SDE/PHE/FOS/00.3. [Revised 2002 January].
- Wu S, Zhu W, Thompson P, Hannun YA. 2018. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun. 9(1):1–12.
- Yıldırım A, Yorulmaz A. 2018. The effect of rosemary extract on 3-MCPD and glycidyl esters formed during frying. Grasas Aceites. 569(4):273.
- Zhang HJ, Zhang M, Cheong LZ, Hu P, Zhao Y, Yu L, Wang Y, Jiang Y, Xu X. 2016. Mitigation of 3-monochloro-1, 2-propanediol ester formation by radical scavengers. J Agric Food Chem. 64(29):5887–5892.
- Zhang M, Yang Z, Binns C, Lee A. 2002. Diet and ovarian cancer risk: a case–control study in China. Br J Cancer. 86(5):712–717.
Appendices
Appendix 1. Variable definitions.
