Abstract
Sustainable food systems involve the recycling of biowaste and water. This study characterizes thirty-one top soil improvers of anthropogenic, animal, and green waste origin, along with eleven irrigation waters from rivers, channels, and civil wastewater treatment plants (cWWTPs) for the presence of antimicrobials. Liquid chromatography coupled with hybrid High-Resolution Mass Spectrometry (LC-HRMS/MS) was employed to identify forty-eight drugs belonging to the classes of sulfonamides (11), tetracyclines (7), fluoroquinolones (10), macrolides (12), amphenicols (3), pleuromutilins (2), diaminopyrimidines (1), rifamycins (1) and licosamides (1). Sludge from cWWTPs, animal manure, slurry, and poultry litter exhibited the highest loads for sulfonamides, tetracyclines, fluoroquinolones and macrolides (80, 470, 885, and 4,487 ng g−1 wet weight, respectively) with nor- and ciprofloxacin serving as markers for anthropogenic sources. In compost and digestate, antimicrobials were found to be almost always below the limits of quantification. Reused water from cWWTPs for irrigation in open-field lettuce production were contaminated in the range of 12-221 ng L−1 with sulfonamides, tetracyclines, and fluoroquinolones, compared to very few detected in channels and surface waters. The Antimicrobials Hazard Index (HI), based on the Predicted No Effect Concentration for Antimicrobial Resistance (PNECAMR), was significantly >100 in contaminated topsoil improvers from urban and animal sources. Accounting for worst-case inputs from topsoil improvers and irrigation water, as well as dilution factors in amended soil, fluoroquinolones only exhibited an HI around 1 in open fields for lettuce production. The origin of topsoil improvers plays a pivotal role in ensuring safe and sustainable leafy vegetable production, thereby mitigating the risk of Antimicrobial Resistance (AMR) onset in food-borne diseases and the transfer of AMR elements to the human gut flora.
Introduction
Climate change and the generally improvable environmental sustainability of food production systems (Holden et al. Citation2018) are pivotal factors necessitating the regular use of bio-waste from anthropogenic sources and reclaimed water in agriculture (EEA Citation2020a, Citation2020b). Historically, ensuring sufficient organic matter in agricultural soil for food security relied on the application of animal manure, slurry, and litter onto the soil. Today, organic carbon supplementation comes from inputs not only from traditional sources but also from compost and digestate derived from urban waste, including sludge from civil wastewater treatment plants (cWWTPs), green waste, and food-related household waste (Anderson et al. Citation2021). Furthermore, recent regulations (EU Regulation 2020/741) permit the direct use of effluents from cWWTPs in agriculture to reduce the water footprint. Authorized plants are required to provide a third-phase treatment for run-off water, aimed at reducing microbial loads and pathogens. Currently, there is no mention of end-of-waste criteria for pharmacological residues. However, the European Commission is consolidating a strategic approach to address the environmental impact of human and veterinary pharmaceuticals, including the presence of antimicrobial-resistant bacteria and related genetic elements (EU Commission Notice 2022). Within a One Health approach, the environmental introduction of pharmaceuticals, particularly antimicrobials, from the aforementioned sources in agriculture may result in: a) alterations of soil microbial communities, crucial within the context of 'safe soil’ assessment (Cycoń et al. Citation2019; Patyra et al. Citation2023); b) uptake of antimicrobials by plants, with green leafy vegetables identified as particularly sensitive, leading to residues in ready-to-eat vegetables (Matamoros et al. Citation2022); c) the creation of an environmental pool of antimicrobial genetic determinants that could be transferred to animals and humans, even through non-pathogenic bacteria (FAO and WHO Citation2023). As a result of the considerations above, this paper focuses on modelling a lettuce production cycle in an open field to calculate the associated inputs of selected antimicrobials in agricultural soils from topsoil improvers (TSI) and irrigation waters of different origin.
Materials and methods
Sampling
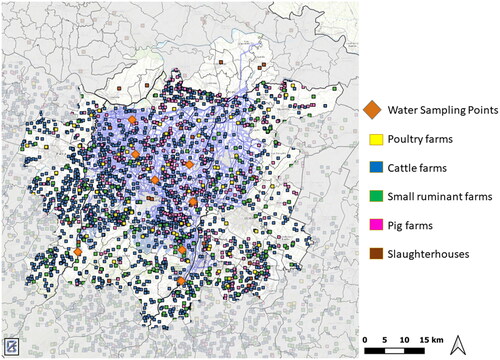
A convenient sampling of 31 topsoil improvers intended for agricultural use was conducted during the period of October 2020 to March 2021. The following main categories were included: sludge from cWWTPs and dairy processing plant (n = 5), mixed compost (n = 6), sludge treated with lime (n = 3), mixed compost and minerals (n = 2), digestates (n = 6) and topsoil improvers from animal sources (bovine manure, pig slurry, poultry litter; n = 9). Samples were obtained from the market, production plants and farms in accordance with national guidelines (Mecella Citation2001; ISPRA Citation2011) and were stored at +4 °C in the dark, until analysed. Analysed biosolids, how they appear, are shown in Figure S1 (Supplementary Materials). Independent samples of irrigation waters (n = 11) were collected from a primary Italian regional district dedicated to open-field green leafy vegetable production in Northern Italy, during the summer of 2022. The samples represented the following categories: surface waters from Apennine mountains rivers, as baseline samples (n = 2); irrigation channel waters from the Po River plain, which received contributions from both civil and animal farm effluents (n = 6); re-used waters from the third phase process of a civil wastewater treatment plant, receiving both civil and animal farming wastewater inputs (n = 3). Sampling sites were selected without the presence of an animal farm within a 1000 m radius ().
Agricultural parameters for antimicrobial input estimates
Producers of lettuce salads were interviewed to gather information about the open-field agricultural cycle. The soft topsoil used for lettuce production (10% clay, 70% sand; density 0.771 tons/cubic meter) is routinely amended with Top Soil Improvers (TSI) to a depth of 20 cm, just before the transplantation of small lettuce plants. Based on these parameters, the total weight of 1 hectare of soil intended for lettuce production, with a 0.20 m depth, is 1,542 tons. The amount of topsoil improver applied per hectare can vary based on the moisture content and nitrogen content of the product. Nitrogen inputs are restricted to prevent nitrate pollution in groundwater (EU Council Directive 91/676) and the presence of non-compliant nitrates in edible leaves (EU Regulation 1258/2011). These constraints limit the use of TSI to 0.03 tons of pelletized compost (16-18% moisture), poultry litter, or pig slurry per hectare on a wet weight (ww) basis. Composts and digestates (40–60% moisture) can be used up to 0.15 tons ww, and animal manure up to 0.30 tons ww. Biosolids from cWWTPs (95% moisture) cannot be directly applied to fields designated for horticulture but can be used up to 40% when incorporated into so-called ‘compost from sludge’, suitable for conventional horticulture. This information was considered in modelling antimicrobial inputs from cWWTP biosolids to lettuce production. The demand for irrigation water ranges from 5 to 10 L kg−1 of lettuce product harvested. Considering an average open-field lettuce production of 35 tons per hectare and adopting a conservative approach tailored to the summer season water needs, we calculated a water usage of 350,000 L/ha for a 2-month-long production cycle. Total antimicrobial inputs in cultivated soils are expressed as ppb (ng g−1 topsoil ww) under the lower-bound approach, (not quantified results posed equal to 0). Estimated environmental concentrations are affected by uncertainties related to the different half-lives of antimicrobials, soil texture, environmental conditions, daily inputs from irrigation water, and the fraction taken up by lettuce.
Antimicrobial analysis of topsoil improvers and irrigation waters
Chemicals: Acetonitrile LC-MS grade (ACN) and ammonia (25-30%) were purchased from Carlo Erba Reagents (Milan, Italy). Methanol LC-MS grade (MeOH) was purchased from Honeywell (Charlotte, NC, USA). Formic acid and ammonium acetate LC-MS grade were purchased from VWR (Radnor, PA, USA). Acetic acid LC-MS grade and EDTA disodium salt were purchased from Merck (Darmstadt, Germany). OASIS HLB cartridges (200 mg/6 mL) were purchased from Waters (Milford, MA, USA) and Strata X-C 200 (mg/6 mL) from Phenomenex (Torrance, CA, USA).
Sample preparation: Forty-eight antimicrobials belonging to the classes of sulfonamides (11), tetracyclines (7), fluoroquinolones (10), macrolides (12), amphenicols (3), pleuromutilins (2), diaminopyrimidines (1), rifamycines (1) and lincosamides (1) were analysed in the selected top soil improvers and irrigation waters. Because of intrinsic complexity of biosolids, it was necessary to apply two different sample processing protocols as summarized in : ‘protocol A’ which coincided with the procedure previously developed for river sediments by Sargenti et al. (Citation2020) and ‘protocol B’ here developed for a limited group of biosolid categories including digestate, pig slurry and bovine manure.
Table 1. Applied extraction and clean-up protocols to irrigation water and top soil improvers samples.
LC-HRMS analysis: LC-HRMS conditions were the same described by Moretti et al. (Citation2016). Briefly, a Thermo Ultimate 3000 High Performance Liquid Chromatography system (Thermo Scientific, San Jose, CA, USA) coupled to Q Exactive Plus high-resolution mass spectrometry (Thermo Scientific) operating in positive heated electrospray ionization (HESI) mode was used. Sheath and auxiliary gas were 35 and 15 arbitrary units, respectively. Spray voltage was set at 3.00 kV, capillary temperature at 300 °C, HESI temperature at 320 °C and S-Lens RF at 50.0 V. For acquisition a Full MS/dd-MS2 experiment was performing setting the following parameters: full scan: resolution 70000 FWHM (@200 m/z), AGC target 3e6, maximum injection time 300 ms and scan range 150-1200 m/z; dd-MS2: resolution 17500 FWHM (@200 m/z), AGC target 5e5, maximum injection time 80 ms, loop count 20, isolation window 2.4 m/z, minimum AGC targeted 1.00e3 and dynamic exclusion 2.0 s. The selected precursors and fragment ions as well as the retention times (RT) are listed in Table S1. Chromatographic separation was performed on a Poroshell 120 EC-C18 column (100 × 3.0 mm; 2.7 µm, Agilent Technologies, Santa Clara, CA, USA) using as mobile phases 0.1% formic acid (A) and MeOH (B) at flow rate of 0.250 mL min−1. The gradient was initiated with 5% eluent B for 1 min, continued with linear increase to 95% B in 19 min. This condition was maintained for 5 min. The system returned to 5% B in 1 min and was re-equilibrated for 4 min (run time: 30 min). The column temperature was + 30 °C and the sample temperature was kept at + 16 °C. Injection volume was 5 µL.
Method validation: Analyte identification (method selectivity) has been verified following the criteria stated in Commission Regulation 2021/808, considering both relative retention time (± 0.1 min) and the presence of at least two diagnostic ions with mass accuracy < 5 ppm as reported in Table S1. Precision and recovery factors have been evaluated using spiking experiments: six replicates in two different days at three different concentrations (main validation study). Antibiotics were quantified using matrix-matched calibration curves obtained adding analyte standard solutions to blank sample extracts. Internal standards (Table S1) were added to check analytical performances reached for each sample and to monitor relative retention times. For biosolids, the tested levels have been: 10 µg kg−1, 25 µg kg−1 and 100 µg kg−1, whereas for irrigation waters: 10 ng L−1, 50 ng L−1 and 100 ng L−1. The selected matrices for the main validation study on biosolids have been ‘sludge from cWWTP’ for Protocol A and ‘digestate’ for protocol B. Limits of Quantification (LOQs) were fixed at the first validation level, if appropriate. However, since the biosolids were very different from each other, LOQs and absolute recoveries had to be checked case-by-case during each analytical session by inserting both a blank sample and two spiked control samples. Estimated LOQs for each top soil improver category and for irrigation waters are reported in the Supplementary Materials (Tables S2 and S3).
Table 2. Determined antimicrobials (ng g-1 wet weight) in top soil improvers, according to the different origin.
Table 3. Determined antimicrobials (ng L−1) in the 11 samples of irrigation water, according to the different origin.
Risk assessment for the onset of antimicrobial resistance
The Hazard Index for the onset of antimicrobial resistance was computed as the ratio between the environmental concentrations in topsoil, irrigation water, and amended and watered soil, respectively, and the Predicted No Effect Concentration for antimicrobial resistance (PNECAMR) derived from Bengtsson-Palme and Larsson (Citation2016).
Results
In and we report the occurrence of the determined antimicrobials in the different top soil improvers and irrigation waters considered. Samples with no quantified antibiotics were omitted. Data are reported after recovery correction applying the relevant factor evaluated in the specific analytical batch. illustrates the computed inputs on agriculture fields according to the inventoried lettuce cultivation practices, under the worst-case scenarios. The Hazard Index for antimicrobial resistance (HIAMR), accounting for worst case concentration found in top soil improvers and irrigation water and for modelled inputs in soil are shown in . The extracted ion chromatograms of a standard solution of ciprofloxacin (A), a spiked sample (B), and a blank sample (C) are shown in .
Figure 2. ‘Extracted ion chromatograms referred to the analysis of the fluoroquinolone ciprofloxacin in top soil improvers and irrigation waters’. From top to bottom: analytical standard, cWWTP biosolid sample (, sample No. 20), and irrigation water sample from river (, ID #1298).
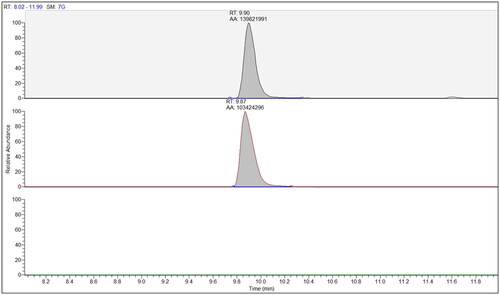
Table 4. Computed inputs (mg) and expected concentration (ng g−1) of antimicrobials from selected biosolids and irrigation waters in soils intended for lettuce production, according to the considered stancard agriculture practices.
Table 5. Hazard Index for antimicrobial resistance (HIAMR), accounting for worst case (wc) concentration (ppb) found in top soil improvers (TSI), irrigation water (IW) and for modelled inputs in soil.
Discussion
In this paper we tried to characterize the environmental pressure from antimicrobials in the production of a ready-to-eat leafy vegetables, as lettuce. This would represent the basis to support an holistic genomic and metagenomic based approach to the potential presence of foodborne pathogens and related virulence factors such as AMR, in the considered ready-to-eat food production environment (Gigliucci et al. Citation2017; Barbieri et al. Citation2023). The presence of antimicrobials in irrigation water (Slobodiuk et al. Citation2021; Seyoum et al. Citation2022; Solaiman et al. Citation2022) and topsoil improvers (Congilosi and Aga Citation2021; Wang et al. Citation2023; Ferreira et al. Citation2023) has been extensively described in previous studies, addressing: a) ecotoxicity effects, such as the alteration of soil functions due to the disruption of microbial communities; b) food safety aspects related to the transfer of pharmaceuticals to the edible portion of green leafy vegetables and human exposure through food intake (Bhalsod et al. Citation2018; Albero et al. Citation2019); and last but not least, c) the induction of Antimicrobial Resistance Genes in the soil microbiome, and possibly their transfer to foodborne pathogens (Mathews et al. Citation2022). Among these adverse effects, the induction of AMR genetic elements today represents the most sensitive health-based endpoint within a One Health Approach (ECDC, EMA, EFSA and OECD Citation2022).
Analysis
Results of the main validation studies are reported in Table S4 of Supplementary Materials. Absolute recoveries (recovery factors) of some antibiotic classes (in particular quinolones and macrolides in biosolids analysed with protocol A) were poor. Precision was considered acceptable since the coefficients of variation (CVwR) assessed in intra-laboratory reproducibility conditions were < 30%. As observed by other researchers (Jacobsen et al. Citation2004; Berendsen et al. Citation2015; Jansen et al. Citation2019; Mejías et al. Citation2023), manure, digestates, soil and sludges are very challenging matrices to which the same performance criteria cannot be applied as are generally adopted for food and feed (Regulation 2021/808). This is especially important when applying multi-residue methods, in which drugs from different families must be extracted and purified using a single sample treatment protocol. Indeed, antibiotics can undergo a wide variety of interactions based on their specific physico-chemical properties, such as complex formation or binding to solid materials. Moreover, due to the high difference among categories and the complexity of some of them (Figure S1), the validation parameters are only indicative as they were obtained on a single type of biosolid, namely ‘sludge from cWWTP’ (Protocol A) and ‘digestate’ (Protocol B). For this reason, during the analysis of real samples, analytical batches were organized by grouping the same category of biosolid (‘poultry litter’, ‘sludge with lime’, ‘pig slurry’ etc) and verifying method performance through two spiked samples prepared on the same type.
The method LOQs applied to irrigation waters were 10 ng L−1 for all the analytes (Table S3 of Supplementary Material), satisfying the requirements outlined in the EU Commission Implementing Decision 2022/1307, which established the following maximum acceptable LOQs for the monitoring of three antimicrobials in freshwaters: sulfamethoxazole (100 ng L−1), trimethoprim (100 ng L−1) and ofloxacin (26 ng L−1). Compliance with these limits ensures the applicability of our findings in irrigation water within legislative frameworks focused on priority substances relevant to ecotoxicity. Currently, the EU lacks legislative requirements for antimicrobial monitoring in top soil improvers (European Commission Regulation 2019/1009). Nevertheless, several national regulations in organic food production prohibit the use of sludge, sludge-derived compost and digestate (Lasaridi et al. Citation2018).
A positive list of active substances licensed in animal feed (EU Regulation 2019/4) includes the following antimicrobials, with final concentrations ranging from premixture integration up to 1 g kg−1 feed: amoxicillin, amprolium, apramycin, chlortetracycline, colistin, doxycycline, florfenicol, flumequine, lincomycin, neomycin, spectinomycin, sulfonamides (excluding sulfamethoxazole), tetracycline, oxytetracycline, oxolinic acid, paromomycin, penicillin V, tiamulin, thiamphenicol, tilmicosin, trimethoprim, tylosin, and valnemulin. Considering the toxicokinetics of these antimicrobials, LOQs in the range from 10 ng g−1 to 100 ng g−1 wet weight in topsoil improvers appears suitable for their analysis. It is essential to note that, during the development of analytical methods, colistins, high-priority polypeptide antibiotics for antimicrobial resistance, were also included (Saluti et al. Citation2018). Unfortunately, for these highly polar compounds, it was not possible to develop a suitable analytical procedure for biosolids (Binsker et al. Citation2022).
Antimicrobial profiling in topsoil improvers and irrigation waters
Among fluoroquinolones, ciprofloxacin appears to be a consistent marker of human sources, given its constant presence in biowastes from cWWTP (7/7) () and reused waters (1/3) (). These findings align well with evidence from other studies (Wang et al. Citation2023). In topsoil improvers of animal origin, animal manure consistently exhibits the presence of sulfonamides, often coupled with the synergic trimethoprim. Notably, the absence of sulfamethoxazole, an active principle mainly prescribed in human medicines coupled with trimethoprim (e.g. Bactrim™), is worth highlighting, except for its presence in reused water. Similar to ciprofloxacin, sulfamethoxazole could be likely considered a tracer of anthropogenic inputs/pressures.
Lincomycin seems to be distinctive of pig slurry, while tylosin A is associated with poultry litter. These antimicrobials are linked to medicated feed administration against respiratory diseases caused by mycoplasma, common in intensive pig and poultry farming systems, respectively.
Mixed composts and digestates, primarily composed of green and food household wastes, show minimal impact from antimicrobials, except for two samples revealing the unexpected presence of sulfanilamide at mg/kg levels. Sulfanilamide, an older antimicrobial, could reasonably be attributed to the breakdown product of methyl 4-aminophenylsulfonylcarbamate (Asulam™), a herbicide (EFSA 2021). This suggests a potential carry-over of phytosanitary contamination associated with green wastes used for composting (EFSA 2021).
Notably, the biosolid waste recovered from a dairy milk processing plant (sample No. 17, data not shown) indicates that this category of food plants waste may not align with biosolids from urban cWWTPs, as currently classified by legislation (Anderson et al. Citation2021). This is due to its different origin from raw milk, already compliant regarding antimicrobial residues in dairy products.
In a review conducted on three heavily impacted Italian rivers under anthropogenic pressures (Grenni et al. Citation2018), freshwater was found to be contaminated in the ranges of 1.30 - 124 ng L−1 for ciprofloxacin, 0.66 – 74.2 ng L−1 for spiramycin, 1.20 - 14.4 ng L−1 for oxytetracycline, 1.83 - 236 ng L−1 for sulfonamides, and 1.20 - 249 ng L−1 for lincomycin. In our study (), such contamination levels were not observed, primarily because most of the sampling sites were situated in rural and rural-urban areas, which do not face the substantial impact of high-density urban settlements. This observation suggests that a thoughtful urbanization design in areas dedicated to food production could be a crucial factor for ensuring safe and sustainable food systems.
Regarding the presence of ciprofloxacin in waters, it could be attributed to the metabolization of enrofloxacin, a fluoroquinolone commonly prescribed in intensive animal farms, as well as to the active principle present in human antimicrobials. The distinct presence of ciprofloxacin in biosolids derived from water treatment plants, together with its absence (<LOQ) in animal-derived soil improvers, suggests that its origin is mainly anthropogenic.
Our results in topsoil improvers are consistent with recent evidence provided by Matamoros et al. (Citation2022), where almost the same antimicrobial classes were found in biosolids from cWWTPs (sulfonamides, fluoroquinolones, tetracyclines), albeit at concentrations 10 times higher in a high-density urban settlement in Spain. In swine slurry, the presence of lincomycin confirms its role as a marker of pressure from intensive pig farming systems. Additionally, the absence of antimicrobials in mixed compost and digestate from green and household wastes supports their selection as a safer option for soil fertilization intended for vegetable food production.
Analyzing the irrigation water dataset (), a clear difference in antimicrobial loads is observed between reused waters (directly from cWWTPs after a third phase treatment) and other sources from rivers and agriculture channels. Reused waters could directly irrigate green-leafy vegetables, following their total E. Coli load (EU Regulation 2020/741). While literature on antimicrobial presence in reused waters is sparse, recent data from eight different Italian WWTPs reported a mean concentration of 27 ng L−1 for sulfamethoxazole with an RSD of 105% (Palumbo et al. Citation2022). This result aligns with our evidences, with concentrations ranging from 77 to 221 ng L−1 (N = 3). Freshwater from low-impact rivers (samples taken upstream of urban settlements) and canals experienced severe drought during sampling in summer 2022, thus reducing the dilution factor of urban and animal discharges. Regarding reused water, the third-phase oxidation process can break down several antimicrobials (Sagaseta de Ilurdoz et al. Citation2022). Therefore, the presence or absence and amounts of the determined substances in reused water may not be assumed as a proxy for the occurrence of AMR bacteria and related genetic factors.
Of particular interest is the presence of tiamulin, a diterpene antimicrobial with a pleuromutilin chemical structure similar to that of valnemulin. Tiamulin is administered widely in pig farms to combat respiratory diseases caused by gram-positive microorganisms and mycoplasma. Its presence in reused water aligns with the urban and animal farming wastewater inputs in the considered cWWTP where sampling activities were carried out.
Antimicrobial inputs in the lettuce cultivation cycle
This paper examines a two-month cycle of lettuce production during the summer season, considering the combined presence of antimicrobials from various sources, including topsoil improvers and irrigation waters. Employing a targeted multi-residue method, although not exhaustive of all potential antimicrobials beyond those typically considered in animal farming, our study yielded the following findings.
and results allow for a proposed risk ranking of antimicrobial inputs in agricultural soils from topsoil improvers and irrigation waters, respectively, based on their origin. It is evident that, except for two mixed compost samples with sulfanilamide, the regular use of mixed composts derived from green and household wastes, as well as sludges from dairy milk processing, can be deemed safe and sustainable. Similarly, irrigation from rivers and channels in low population density areas is considered safe.
On the contrary, the combination of animal manure and cWWTPs sludge-based composts with inputs from reused waters can lead to the presence of antimicrobials from different classes (sulfonamides + trimethoprim, tetracyclines, macrolides, quinolones). These combinations are not always present in specific human and veterinary medicines and, consequently, are not framed in the pre-marketing Environmental Risk Assessment of medicinal products for human or veterinary use. Addressing this aspect could be crucial in the near future, within the context of a comprehensive soil health strategy based on soil functions (Cycoń et al. Citation2019).
The computed final concentrations in soils after the 2-month lettuce cultivation cycle () acknowledge the following sources of uncertainties: the half-lives of different antimicrobials in soil, considering their binding to organic matter (Cycoń et al. Citation2019), and the reduction of antimicrobials through plant uptake (Albero et al. Citation2019). The variability in degradation rates reported in the literature reflects differences in soil composition, weather conditions, and the potential residual antimicrobial load in the soil after various production cycles involving contaminated topsoil improvers and irrigation waters. The overall balance is somewhat complex due to daily inputs from irrigation waters, leading to a phenomenon known as drug pseudo-persistence (Kumirska Citation2020): regular inputs compensate for overall losses, resulting in environmental persistence of the considered contaminant.
The assessment of Antimicrobial Resistance (AMR) has recently been incorporated into European wastewater legislation (EU Commission Notice 2022). , under the worst-case scenario, clearly indicates that the risk of AMR transfer in food production primarily stems from the use of topsoil improvers originating from cWWTPs sludges, animal manure, and slurry for almost all classes of determined antimicrobials (HI >1). In amended and irrigated soil, taking into account the associated dilution factors, the only concern is related to the presence of fluoroquinolones.
In terms of risk ranking, special attention should be given to the presence of fluoroquinolones, as AMR resistance to this class of antimicrobials is prioritized by the World Health Organization (WHO Citation2017). This prioritization is based on evidence of resistance in foodborne pathogens such as Campylobacter and Salmonella (high risk) and Shigella (medium risk).
In conclusion, the health-related risk assessment of safe and sustainable food systems demands a holistic approach based on the origin and source of environmental resources such as topsoil improvers and irrigation waters. The role of antimicrobials as facilitators of Antimicrobial Resistance (AMR) transfer from the environment to humans through food can be minimized by utilizing mixed composts and irrigation water sourced from non-anthropogenically impacted freshwater. This suggests a safe-by-design approach for agricultural settings.
Considering the ongoing trend of progressively limiting antimicrobial administration to farmed animals through medicated feeds and drinking water, it can be presumed that reused waters and topsoil improvers based on the recycling of civil wastewater treatment plant (cWWTP) sludges would constitute the primary pharmaceutical inputs in soils designated for agricultural practices. Ongoing research aims to correlate antimicrobials in selected matrices with the presence of antimicrobial-resistant foodborne pathogens and antimicrobial resistance genetic elements through microbiological, genomic, and metagenomic approaches.
Supplemental Material
Download MS Word (2.4 MB)Acknowledgments
Italian Ministry of Health grant no. RF-2019-12369714 ‘Emerging food safety risks from microbial hazards deriving from anthropogenic pressures in agricultural settings’. Principal Investigator dr Stefano Morabito.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anderson N, Snaith R, Madzharova G, Bonfait J, Doyle L, Godley A, Lam M, Day G. 2021. From: ricardo. Energy and Environment Sewage sludge and the circular economy. Version: 7 Date: 17/05/2021 EEA activity: 1.5.2. [accessed 2023 July 28]. https://forum.eionet.europa.eu/nrc-eionet-freshwater/library/urban-waste-water-treatment/sewage-sludge-and-circular-economy/download/en/1/Sewage%20Sludge%20and%20the%20Circular%20Economy%20-%20Final%20Report.pdf.
- Albero B, Tadeo J, del Mar Delgado M, Miguel E, Pérez RA. 2019. Analysis of multiclass antibiotics in lettuce by liquid chromatography–tandem mass spectrometry to monitor their plant uptake. Molecules. 24(22):4066. doi:10.3390/molecules24224066.
- Barbieri G, Gigliucci F, Brambilla G, Morabito S. 2023. A cross-cutting approach for the characterization of microbial emerging hazards in agriculture settings from circular economy-driven wastewater streams. Environ Health. Epub ahead of print. doi:10.1021/envhealth.3c00070.
- Bengtsson-Palme J, Larsson DJ. 2016. Concentrations of antibiotics predicted to select for resistant bacteria: proposed limits for environmental regulation. Environ Int. 86:140–149. doi:10.1016/j.envint.2015.10.015.
- Berendsen BJA, Wegh RS, Memelink J, Zuidema T, Stolker AAM. 2015. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta. 132:258–268. doi:10.1016/j.talanta.2014.09.022.
- Bhalsod GD, Chuang YH, Jeon S, Gui W, Li H, Ryser ET, Guber AK, Zhang W. 2018. Uptake and accumulation of pharmaceuticals in overhead- and surface-irrigated greenhouse lettuce. J Agric Food Chem. 66(4):822–830. doi:10.1021/acs.jafc.7b04355.
- Binsker U, Käsbohrer A, Hammerl JA. 2022. Global colistin use: a review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol Rev. 46(1):fuab049. doi:10.1093/femsre/fuab049.
- Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council 26.7.2022 EN. Off J Eur Union Law. 197:117.
- Commission Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. D. Of. Unión Eur. p. 1–114.
- Commission Regulation (EU) 1258/2011 of 2 December 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for nitrates in foodstuffs OJ L 320, 3.12.2011. p. 15–17.
- Commission Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/179/EC OJ L 180, 21.5.2021. p. 84–109.
- Congilosi JL, Aga DS. 2021. Review on the fate of antimicrobials, antimicrobial resistance genes, and other micropollutants in manure during enhanced anaerobic digestion and composting. J Hazard Mater. 405:123634. doi:10.1016/j.jhazmat.2020.123634.
- Cycoń M, Mrozik A, Piotrowska-Seget Z. 2019. Antibiotics in the soil environment—degradation and their impact on microbial activity and diversity. Front Microbiol. 10:338. doi:10.3389/fmicb.2019.00338.
- ECDC, EMA, EFSA and OECD. 2022. Antimicrobial resistance in the EU/EEA - A One Health response. [accessed 2023 Aug 11]. https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-one-health-response.
- [EEA] European Environmental Agency. 2020a. Report 04/2020 Bio-waste in Europe—turning challenges into opportunities. doi:10.2800/630938.
- [EEA] European Environmental Agency. 2020b. Report 17/2020 Water and agriculture: towards sustainable solutions. doi:10.2800/73735.
- [EFSA] European Food Safety Authority, Alvarez F, Arena M, Auteri D, Borroto J, Brancato A, Carrasco Cabrera L, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, et al. 2021. Updated peer review of the pesticide risk assessment of the active substance asulam (variant evaluated asulam-sodium). EFSA J. 19(11):e06921. doi:10.2903/j.efsa.2021.6921.
- European Commission Notice. 2022. Guidelines to support the application of Regulation 2020/741 on minimum requirements for water reuse (2022/C 298/01). [accessed 2023 Jul 28]. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52022XC0805(01)&from=EN.
- European Union Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources OJ L 375, 31.12.1991, p. 1.
- FAO and WHO. 2023. Foodborne antimicrobial resistance – compendium of codex standards. First revision. Rome: Codex Alimentarius Commission. doi:10.4060/cb8554en.
- Ferreira PFA, Xavier JF, Nunes JF, Fonseca IP, de Mattos de Oliveira Coelho S, Soares de Souza MM, da Silva Coelho I. 2023. Bacteria and antimicrobial resistance profile during the composting process of wastes from animal production. Braz J Microbiol. 54(2):1157–1167. doi:10.1007/s42770-023-00912-8.
- Gigliucci F, Brambilla G, Tozzoli R, Michelacci V, Morabito S. 2017. Comparative analysis of metagenomes of Italian top soil improvers. Environ Res. 155:108–115. doi:10.1016/j.envres.2017.02.004.
- Grenni P, Ancona V, Barra Caracciolo A. 2018. Ecological effects of antibiotics on natural ecosystems: a review. Microchem J. 136 (2018):25–39. doi:10.1016/j.microc.2017.02.006.
- Holden NM, White EP, Lange MC, Oldfield TL. 2018. Review of the sustainability of food systems and transition using the Internet of Food. NPJ Sci Food. 2(1):18. doi:10.1038/s41538-018-0027-3.
- ISPRA. 2011. Guidelines for composts sampling (in Italian). [accessed 2023 Jul 28]. https://www.isprambiente.gov.it/files/pubblicazioni/manuali-lineeguida/man-3-2001-compost/1-10-manuale_3_2011_compost-2.pdf-1.
- Jacobsen AM, Halling-Sørensen B, Ingerslev F, Hansen SH. 2004. Simultaneous extraction of tetracycline, macrolide and sulfonamide antibiotics from agricultural soils using pressurised liquid extraction, followed by solid-phase extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A. 1038(1–2):157–170. doi:10.1016/j.chroma.2004.03.034.
- Jansen LJM, van de Schans MGM, de Boer D, Bongers IEA, Schmitt H, Hoeksma P, Berendsen BJA. 2019. A new extraction procedure to abate the burden of non-extractable antibiotic residues in manure. Chemosphere. 224:544–553. doi:10.1016/j.chemosphere.2019.02.166.
- Kumirska J. 2020. Pharmaceutical residues in the environment. Molecules. 25(12):2941. doi:10.3390/molecules25122941.
- Lasaridi KE, Manios T, Stamatiadis S, Chroni C, Kyriacou A. 2018. The evaluation of hazards to man and the environment during the composting of sewage sludge. Sustainability. 10(8):2618. doi:10.3390/su10082618.
- Matamoros V, Escolà Casas M, Mansilla S, Tadić Đ, Cañameras N, Carazo N, Portugal J, Piña B, Díez S, Bayona JM. 2022. Occurrence of antibiotics in Lettuce (Lactuca sativa L.) and Radish (Raphanus sativus L.) following organic soil fertilisation under plot-scale conditions: crop and human health implications. J Hazard Mater. 436:129044. doi:10.1016/j.jhazmat.2022.129044.
- Mathews A, Naushad S, Duceppe MO, Kang M, Wang LR, Huang H. 2022. Complete genome sequence of a Listeria monocytogenes strain with antimicrobial resistance genes isolated from lettuce in Canada. Microbiol Resour Announce. 11(7):e0029822. doi:10.1128/mra.00298-22.
- Mecella G. 2001. Analytical methods of waters intended for irrigation and animal farming (Metodi di analisi delle acque per uso agricolo e zootecnico). Milano (Italy): Franco Angeli.
- Mejías C, Santos JL, Martín J, Aparicio I, Alonso E. 2023. Multiresidue method for the determination of critically and highly important classes of antibiotics and their metabolites in agricultural soils and sewage sludge. Anal Bioanal Chem. 415(29–30):7161–7173. doi:10.1007/s00216-023-04982-3.
- Moretti S, Cruciani G, Romanelli S, Rossi R, Saluti G, Galarini R. 2016. Multiclass method for the determination of 62 antibiotics in milk. J Mass Spectrom. 51(9):792–804. doi:10.1002/jms.3834.
- Palumbo MT, Russo S, Polesello S, Guzzella L, Roscioli C, Marziali L, Valsecchi L, Cappelli F, Pascariello S, Tasselli S, et al. 2022. Integrated exposure and algal ecotoxicological assessments of effluents from secondary and advanced-tertiary wastewater-treatment plants. Environ Toxicol Chem. 41(10):2404–2419., doi:10.1002/etc.5424.
- Patyra E, Nebot C, Gavilán RE, Kwiatek K, Cepeda A. 2023. Prevalence of veterinary antibiotics in natural and organic fertilizers from animal food production and assessment of their potential ecological risk. J Sci Food Agric. 103(7):3638–3644. doi:10.1002/jsfa/12435.
- Regulation (EU) 2019/4 of the European Parliament and of the Council of 11 December 2018 on the manufacture, placing on the market and use of medicated feed, amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and repealing Council Directive 90/167/EEC. Official Journal of the European Union L 4/7.1.2019, p. 1.
- Regulation 2020/741 of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse. Official Journal of the European Union L 177, 5.6.2020, p. 32.
- Sagaseta de Ilurdoz M, Sadhwani JJ, Vaswani Reboso J. 2022. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment - a review. J. Water Process Eng. 45:102474. doi:10.1016/j.jwpe.2021.102474.
- Saluti G, Diamanti I, Giusepponi D, Pucciarini L, Rossi R, Moretti S, Sardella R, Galarini R. 2018. Simultaneous determination of aminoglycosides and colistins in food. Food Chem. 266:9–16. doi:10.1016/j.foodchem.2018.05.113.
- Sargenti M, Bartolacci S, Luciani A, Di Biagio K, Baldini M, Galarini R, Giusepponi D, Capuccella M. 2020. Investigation of the correlation between the use of antibiotics in aquaculture systems and their detection in aquatic environments: a case study of the nera river aquafarms in Italy. Sustainability. 12(12):5176. doi:10.3390/su12125176.
- Seyoum MM, Lichtenberg R, Orlofsky E, Bernstein N, Gillor O. 2022. Antibiotic resistance in soil and tomato crop irrigated with freshwater and two types of treated wastewater. Environ Res. 211:113021. doi:10.1016/j.envres.2022.
- Slobodiuk S, Niven C, Arthur G, Thakur S, Ercumen A. 2021. Does irrigation with treated and untreated wastewater increase antimicrobial resistance in soil and water: a systematic review. Int J Environ Res Public Health. 18(21):11046. doi:10.3390/ijerph182111046.
- Solaiman S, Handy E, Brinks T, Goon K, Bollinger C, Sapkota AR, Sharma M, Micallef SA. 2022. Extended spectrum β-lactamase activity and cephalosporin resistance in Escherichia coli from U.S. Mid-Atlantic surface and reclaimed water. Appl Environ Microbiol. 88(15):e0083722. doi:10.1128/aem.00837-22.
- Wang J, Xu S, Zhao K, Song G, Zhao S, Liu R. 2023. Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic resistant bacteria (ARB) during sewage sludge treatment and disposal: a review. Sci Total Environ. 877:162772. doi:10.1016/j.scitotenv.2023.162772.
- WHO. 2017. News release: WHO publishes list of bacteria for which new antibiotics are urgently-needed. [accessed 2023 Aug 11]. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.