Abstract
Although various shape memory polymers (SMPs) or diverse applications have been widely reported, the SMPs based on rubbers have been rarely realized due to the low triggering temperature of rubbers. In another aspect, the SMPs based on sustainable substances are highly desired for the growing shortage in fossil resources. In the present study, we accordingly developed the sustainable SMPs with tunable triggering temperature, based on natural rubber (NR) and ferulic acid (FA) as the raw materials. Specifically, the SMPs are based on a crosslinked network of epoxidized natural rubber (ENR) crosslinked by in situ formed zinc ferulate (ZDF) via oxa-Michael reaction. The excellent shape memory effect (SME) is found in these SMPs, as evidenced by the high fixity/recovery ratio and the tunable triggering temperature. With the incorporation of natural halloysite nanotubes (HNTs), the stress and recovery rate of the SMPs are found to be tunable, which widens the application of this kind of SMPs. The combination of adoption of sustainable raw materials, and the excellent and tunable SME makes these SMPs potentially useful in many applications, such as various actuators and heat-shrinkable package materials.
1. Introduction
SMPs are smart materials that can change shape and memorize their original shape when exposed to an external stimulus, such as temperature, light, electricity and solvent [Citation1 –Citation5]. Heating is the most common way to trigger the shape memory effect (SME) [Citation6,Citation7]. Generally, SMPs possess a fixing phase provided by a crosslinked network as well as a transition phase supported by the glass transition and/or crystalline melting transition [Citation8]. SMPs are deformed at a temperature above the transition temperature (Ttrans) and the deformed sample is cooled to a temperature below Ttrans under stress to fix the temporary shape. When reheated above Ttrans without stress, SMPs recover their permanent shape due to entropy elasticity of polymer chains [Citation9]. SMPs have attracted significant attention due to their diverse applications such as medical devices, sensors, switches and self-deployable structures [Citation10 –Citation13].
Although vitrification is utilized as the transition mechanism in many SMPs, it is seldom used in elastomer-based SMPs because Tg of rubber is too low for practical applications [Citation14]. Crystalline compounds with relatively high melting point, such as fatty acid, are introduced into elastomers to act as transition phase. Weiss et al. [Citation15] mixed zinc stearate with a sulfonated ethylene-propylene-diene monomer (EPDM) (EPDM with pendent sulfonate groups) to prepare SMPs with a transition phase supported by zinc stearate. The resultant blends exhibited good SME and the length recovery was ~92%. It is conceivable that if Tg of rubber is able to approach to room temperature or above, rubber should also be a suitable choice for SMP fabrication. By this way, we may also tune the transition temperature by simply changing the amount of the curing agent. Chang et al. [Citation16] revealed that epoxidized natural rubber (ENR) crosslinked by 3-amino-1,2,4-triazole showed high Tg and exhibited excellent SME based on the vitrification mechanism.
Recently, extensive interest has focused on ‘sustainable’ polymers with biobased raw materials instead of petroleum-based resources due to the increasing consumption of limited fossil feedstock [Citation17 –Citation19]. Sustainable materials are those whose production is supported inexhaustibly by nature. A variety of sustainable sources have been explored for polymers, including vegetable oils, plant sugars, rosins and lignin [Citation20 –Citation23]. However, the main challenge of ‘sustainable’ polymer fabrication, namely physicochemical properties of products is hardly comparable or superior to their counterparts prepared from fossil resources, is urgent to be solved.
In our previous studies, the efficient crosslinking between ENR and zinc acrylate (ZDA) via oxa-Michael reaction has been revealed and SME based on glass transition has been demonstrated [Citation24,Citation25]. Very recently, the oxa-Michael reaction between zinc ferulate (ZDF) and ENR has also been revealed [Citation26]. The ZDF-cured ENR should be potentially ideal SMPs in virtue of tunable Tg and the utilization of sustainable raw materials including NR and FA. In ENR crosslinked by ZDF, the crosslinked networks serve as the permanent shape and the glass transition serves as the shape triggering mechanism. It should be emphasized that FA exhibits potential antioxidant capability due to a stabilized phenoxy radical arising from the phenolic nucleus and an extended side chain conjugation [Citation27,Citation28]. Besides, FA shows unique features of nontoxicity and pharmacological functions [Citation29,Citation30]. More importantly, FA is a sustainable material as a ubiquitous plant constituent of metabolism [Citation31]. Combining the sustainability of NR, the precursor of ENR, raw materials for the SMPs fabrication in the present work are sustainable and essentially nontoxic.
In the present study, the SMPs based on ENR/ZDF networks were fabricated. The transition behaviors and the SME were investigated. To better conform the practical applications, halloysite nanotubes (HNTs), a kind of naturally occurring nanotubular clay, were incorporated into the ENR/ZDF network to regulate the mechanical properties and SME of the SMPs. The effects of HNT incorporation on the mechanical properties and SME were evaluated and correlated with the structures of the composites.
2. Experimental methods
2.1. Raw materials
ENR with an epoxidization degree of 50% was produced by the Agricultural Products Processing Research Institute, Chinese Academy of Tropical Agricultural Science, Zhanjiang, P. R. China. Chemically pure FA was purchased from Aladdin Industrial Corporation, China, and zinc oxide (ZnO) is industrially available products and used as received. HNTs were mined from Hubei Province, China, and purified accordingly to the previously reported procedure [Citation32].
2.2. Preparation of ENR cured by ZDF
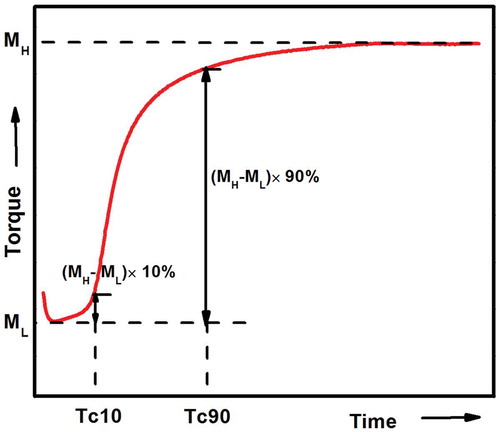
ENR, FA, ZnO, and HNTs were mixed in an open two-roll mill and compositions of the rubber compounds are listed in . Due to the formation of zinc ferulate by in situ reaction between ferulic acid and ZnO, molar ratios between FA and ZnO are set as 2:1. For all samples, the well mixed compounds were hot pressed at 160 °C with optimized curing time (Tc90) to cure the compounds. As shown in the below , Tc90 is determined as the time when the torque is equal to [ML+(MH–ML)*90%].
Table 1. Compositions of ENR and ENR/HNT composites cured by ZDF.
2.3. Characterizations
Curing behaviors of ENR and ENR/HNT composites cured by ZDF were determined at 160 °C using a U-CAN UR-2030 vulcameter (Taiwan). Tensile tests of composites at room temperature were measured accordingly to ISO 37-2005, using a UCAN UT-2060 machine (Taiwan). Tensile tests at elevated temperature were determined by a TA Q800 dynamic mechanical analyzer (USA). After equilibrium at the testing temperature, samples with thickness of ~0.5 mm were stretched with an extension rate of 0.5 N/min until the stress/strain limit reached.
X-ray diffraction (XRD) data were collected at ambient temperature on a Rigaku Dmax/III diffractometer, using Cu Kα radiation (λ = 1.54 Å) with an accelerating voltage and current of 40 kV and 30 mA, respectively. Samples were scanned from 4° to 60°, with a step length of 0.02° at 24 °C.
Dynamic mechanical analysis (DMA) was also performed with a TA Q800 dynamic mechanical analyzer (USA). The test was carried out at the tension condition with a frequency of 1 Hz. In each case, a rectangular sample (typical dimensions = 8 × 6 × 0.5 mm) was loaded under tension and an oscillatory deformation with an amplitude of 0.5% strain and a force track of 125% was applied. The scanning temperature was ranged from −50 to 100 °C at a heating rate of 3 °C/min.
The cryogenically fractured surfaces of the ENR/HNT composites were observed by field emission scanning electron microscopy (FESEM, Nova Nano SEM 430). Energy dispersive X-ray spectra (EDS) of Zn in the ZDA-cured ENR samples were collected using a Horiba EX-250 spectrometer.
Shape memory behaviors of ZDF-cured ENR and ENR/HNT nanocomposites were characterized by the thermomechanical cycle experiments and measured with a TA Q800 instrument. The test was carried out under controlled force mode. Prior to deformation, samples with dimension of 8 × 6 × 0.5 mm were heated to 30 °C above Tg, and equilibrated for 10 min. In step 1 (deformation step), samples were deformed with an extension rate of 0.5 N/min, from a preloaded value of 0.001 N to a designated value with a setting strain (). In step 2 (cooling step), samples were cooled to 40 °C below Tg (Tg-40 °C) at a rate of 3 °C/min under a constant force to prevent the recovery. In step 3 (unloading and shape fixing step), the force imposed on the samples was unloaded to the preloaded value of 0.001 N at a rate of 0.5 N/min. An additional 20 min isothermal step was followed to ensure shape fixing at Tg-40 °C. Upon unloading, part of the strain (
−
) was instantaneously recovered, leaving a fixing strain (
). In the final step (recovery step), samples were reheated to 30 °C above Tg at a rate of 3 °C/min and held for another 10 min to recover any possible residual strain. Then a permanent strain (
) was left. This four-step thermomechanical cycle was repeated three times for each sample.
The fixing ratios () and recovery ratios (
) were defined as:
3. Results and discussion
3.1. SME of ENR/ZDF network
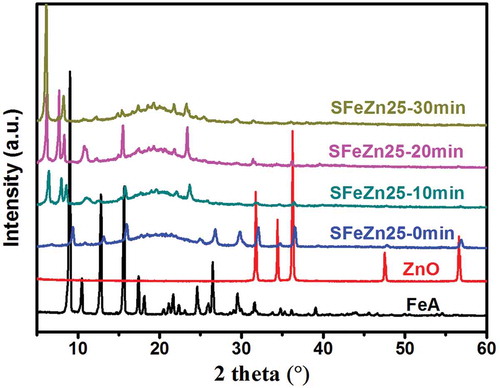
To verify to the reaction between ZnO and FeA and the formation of ZDF, XRD data was collected (). In the experiment, ZnO and FeA were incorporated into SBR, and the resulting FeA/ZnO/SBR compound was treated at 160 °C for different time. It should be noted that SBR is chosen as the rubber matrix to replace ENR, with the aim to avoid the consumption of the yielded ZDF. The samples are named as SFeZn25-xmin, where S represents SBR to distinguish with ENR. XRD patterns show that the peaks of SFeZn25-0 min are the superposition of peaks of FeA and ZnO. The heat treated samples (SFeZn25-10 min, SFeZn25-20 min, SFeZn25-30 min) exhibit a new peak at 6.2°, and the intensity increase monotonously with the increasing of curing time. What’s more, the intensity of peaks belonging to FeA and ZnO reduce greatly along the curing time. Both the appearance of a new peak and the reduction of peak intensity of FeA and ZnO provide convincing evidences for the formation of ZDF.
Figure 2. XRD patterns of FeA, ZnO, FeA/ZnO/SBR compound (SFeZn25-0 min) and heat treated FeA/ZnO/SBR compounds with different curing time (SFeZn25-10 min, SFeZn25-20 min, SFeZn25-30 min).
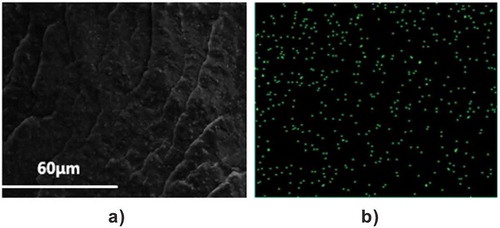
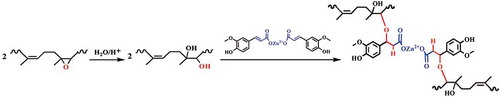
According to previous work, the crosslinking mechanism between ENR and ZDF is related to oxa-Michael reaction. shows the formula of the crosslinking reaction. Due to ring-opening of epoxy group in ENR, hydroxyl formed under the catalysis of Zn2+. Then the conjugated C=C in ZDF is attacked by the generated –OH, resulting in formation of ether bond as the crosslinks. The detailed molecular mechanism of this crosslinking reaction has been disclosed via the characterizations of model substance [Citation26]. To investigate the dispersion of ZDF, SEM and EDS spectra for EFeZn40 were recorded (). From the SEM photo ((a)), one can observe that, even though high content of FA and ZnO are added, the sample exhibits smooth fracture surface and no aggregated particles are found. The observed ridges are actually caused by the cryogenic fracture process. The dispersion of ZDF could further be evaluated by the zinc element mapping. From the EDS spectrum ((b)), it is clearly that zinc element is uniformly dispersed in the whole sample. The uniform dispersion of ZDF is realized by the conversion of inorganic ZnO particles into reactive ZDF molecules and the subsequent crosslinking of ENR by ZDF through oxa-Michael reaction.
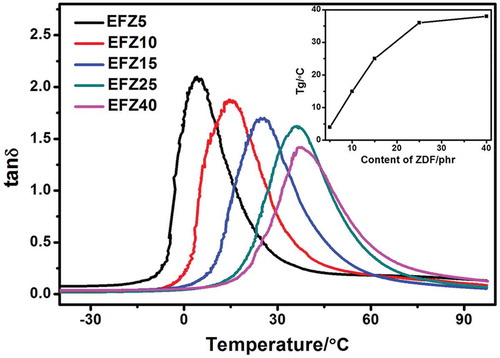
DMA measurement was conducted to study the influence of ZDF content on Tg of ENR. presents curves of tan δ of ZDF-cured ENR with various ZDF content. As revealed, with the increasing of ZDF content, peak values of tan δ decrease continuously. For example, when ZDF content increases from 5 phr to 40 phr, tan δ decreases by 33.3%. This decrease should be ascribed to the consistently increased confinement of chain motion arising from the increase in crosslink density. Regarding to Tg, along the increasing of curing agents, Tg exhibits an increasing trend (inset in ). The Tgs for all the samples are above 0 °C, which are quite high values for crosslinked diene-based rubbers. The increment in Tg is also ascribed to the strong confinement of segment motion due to the increased crosslink density. From these results, we may come to a conclusion that Tg of ZDF-cured ENR can be tuned in a wide range from 4 °C to 38 °C, just by changing curing agent concentration. Accordingly, these ZDF-cured ENR exhibits potential as SMPs.
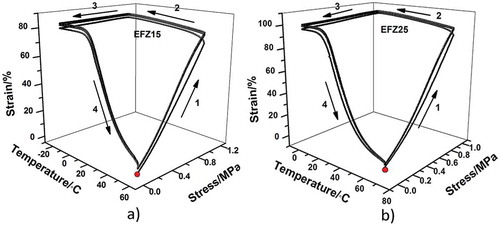
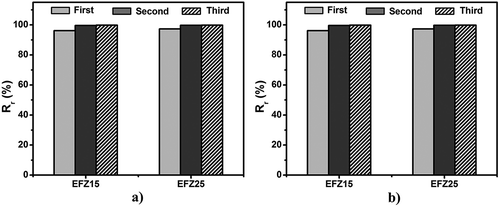
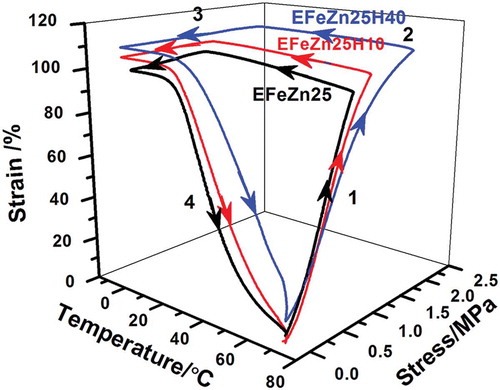
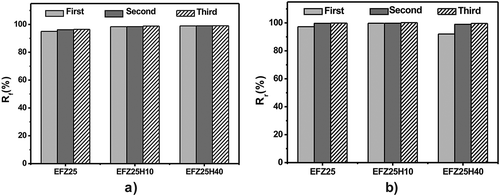
To evaluate the SME of ZDF-cured ENR, the thermomechanical cycling was performed under controlled force mode. The thermomechanical cycling for every sample is repeated for three times. As revealed in , the strain change during unloading (step 3) is minimal, indicating excellent fixity of temporary shape while the starting point is almost overlapped with the ending point, suggesting good recovery of the original shape. Although the first cycle shows a little difference from the latter two, the second and third cycles are completely overlapped, indicating that shape memory performances of both EFeZn15 and EFeZn25 show excellent repeatability. The fixing ratio (Rf) and recovery ratio (Rr) were calculated and compared in . Both Rf and Rr of EFeZn15 and EFeZn25 are more than 93%, suggesting that changes in ZDF concentration have a minimal effect on shape memory performance. It is notable that the Rf and Rr are improved to some degree with the increase in cycling times. The relatively poorer Rf and Rr of first cycle should be ascribed to the unrelaxed stress remained during processing. Besides, the slippage of excessive crystalline aggregates of ZDF may be another origin for the imperfect Rf and Rr in the first cycle. As to the following cycling, much better shape memory performance is realized due to the relaxation of the residual stress and rearrangement of the excessive ZDF aggregates during the first cycling. Similar behaviors have been found in other systems filled with small crystalline substance or inorganic filler [Citation25,Citation33].
3.2. Regulation of SME of ZDF-cured ENR by HNTs
HNTs are naturally occurring nanotubular clay. Compared to the more commonly used 2D clay such as montmorillonite, HNTs can be easily dispersed via conventional processing facilities [Citation34]. In virtue of the favorable interfacial interaction, such as hydrogen bonding, HNTs have been demonstrated to be very effective in reinforcing polar rubber without sophisticated treatment [Citation35]. In the present study, to further regulate the SME of ZDF-cured ENR, HNTs are incorporated as reinforcement for ZDF-cured ENR.
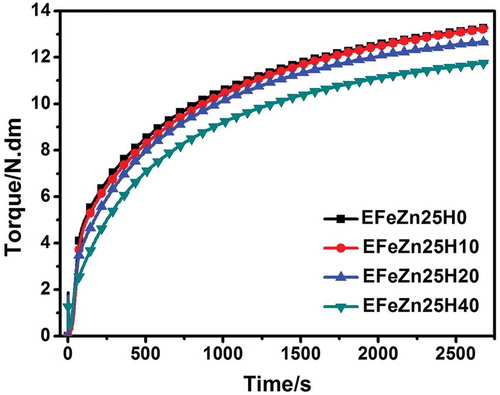
Curing curve measured using a vulcameter is a convenient method to monitor the curing process and reflect curing characteristics, such as scorch time, optimal curing time, maximum torque (MH), and curing rate. Generally the maximum torque is indicative for crosslink density. depicts curing profiles of ENR and ENR/HNT nanocomposites cured by ZDF. As shown, the MH and curing rate decrease continuously with the increment in HNT content, indicating the detrimental effect of HNTs on crosslinking of ZDF-cured ENR. This observation may be attributed to the partial consumption of ZDF by HNTs. As was reported, Zn2+ can catalyze the oxa-Michael reaction between ENR and ZDA. For one thing, ZDA can promote nucleophilic attack between –OH in ENR and –C=C– in ZDA due to formation of carbonyl-metal ion complexation [Citation36]. For the other thing, ZDA can act as nucleophilic cation as well as Lewis acid to catalyze the ring-opening reaction of ENR [Citation37,Citation38]. Similarly, the in situ formed ZDF also acts as catalyst and curing agent. However, with addition of plenty of HNTs, ZDF are partially adsorbed on the surface of HNTs due to hydrogen bonds and complexation interaction, which consequently suppresses the catalytic and crosslinking efficiencies. Therefore, although HNTs may improve torque via the hydromechanics effect, the resultant MH is unexpectedly decreased due to the suppressed crosslinking of the matrix.
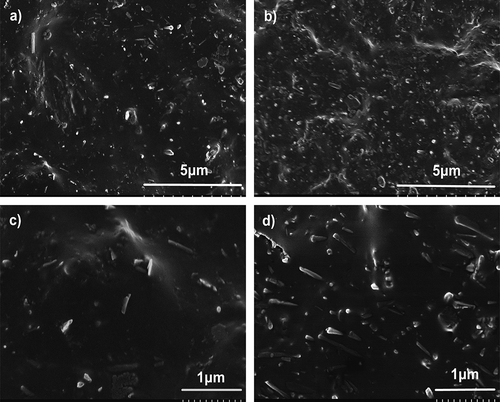
The dispersion state of HNTs in ZDF-cured ENR is evaluated by morphological observations. displays SEM images of ENR/HNT nanocomposites cured by ZDF with 10 phr and 40 phr HNTs, respectively. As shown, even though 40 phr HNTs are added, HNTs disperse uniformly and most of the tubes are individualized in the ENR matrix. Considering that existence of plenty of hydroxyls in the ZDF-cured ENR network, which could form hydrogen bonds with surface hydroxyls on the surface of HNTs, so the uniform dispersion of HNTs should be attributed to the hydrogen bonding between ENR and HNTs. In addition, the residual epoxy groups also make a contribution to the well dispersion due to the potential hydrogen bonds between epoxy groups and surface hydroxyls on HNTs. Similar interaction between carboxylated styrene-butadiene rubber and HNTs has been reported [Citation35]. As shown in , blurry interfaces are exhibited, which further suggests strong interfacial interaction.
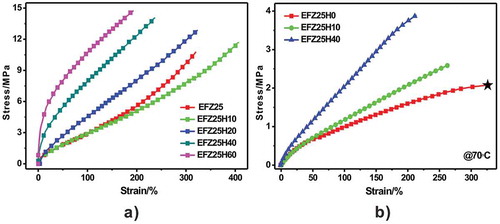
depicts stress-strain curves of ZDF-cured ENR/HNT nanocomposites with variable HNT loading at temperatures below and above Tg (at 70 °C). As indicated, with the increasing of HNT content, tensile strength and modulus increase continuously. Compared with neat ENR, modulus (at 100% strain) of EFeZn25H60 increases by 250% (from 3.0 to 10.6 MPa). The striking enhancements in moduli should originate from the strong interfacial adhesion and excellent compatibility between ENR and HNTs, which is consistent with the morphological observations. Meanwhile, elongation at break decreases sharply with increment of HNTs. The phenomenon should be ascribed to the inevitable aggregation of partial HNTs when high content of HNT is incorporated. These aggregated HNTs act as regions of stress concentration and results in the decrease in elongation at break. It is essential to evaluate the tensile properties at temperature above Tg since forces will be applied to samples at high temperature when thermomechanical cycling of SMPs are performed. displayed stress-stain curves of ZDF-cured ENR and ENR/HNT composites at 70 °C. An obviously enhanced tensile strength and modulus are exhibited at elevated temperature with the inclusion of HNTs. For example, compared with neat ENR, modulus (at 100% strain) of EFeZn25H40 increases by one-fold. In summary, the incorporation of HNTs effectively improves modulus of ZDF-cured ENR both at room temperature and elevated temperature.
Figure 9. Stress-strain curves of ENR/HNT nanocomposites cured by ZDF at room temperature (a) and at 70 °C (b). The black asterisk is used to illustrate that the sample is not fractured but the test is stopped due to reaching the elongation limit of DMA instruments.
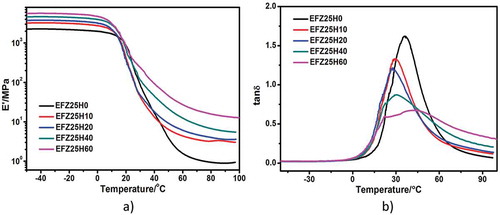
To investigate the effect of HNT content on viscoelasticity of ENR/HNT composites, DMA measurement was performed. The dependences of storage modulus (E’) and loss factor (tan δ) on temperature for neat ENR and ENR/HNT composites are show in and , respectively. All the samples exhibit glassy state with high modulus at low temperature and rubbery state with low modulus at high temperature. The sharp decrease in E’ around 30 °C is due to the glass transition. The transition favors the deformation at elevated temperature and fixity of temporary shape at lower temperature, ensuring the shape memory performance. Via the inclusion of HNTs, E’ of composite in both glassy region and rubbery region improves strikingly. For example, compared with neat ENR, E’ values for EFeZn25H40 at 0 °C and 70 °C increase by about one-fold and seven-fold, respectively. The dramatic reinforcement of HNTs toward ENR is ascribed to the uniform dispersion of HNTs facilitated by strong interfacial adhesion between HNTs and ENR, which guarantees efficient force transferring between HNTs and ENR. Theoretically, higher modulus in glassy state is beneficial to Rf. As is well known, temporary shape of SMPs are fixed at glassy state. For sample with higher modulus in glassy state, larger stress is required for the relaxation and creep of polymer chain, which results in a reduced retraction during fixing and an increased Rf. On the contrary, higher modulus in rubbery state is detrimental to shape recovery. The drive force for shape recovery is the entropy elasticity, which is regulated by temperature. For sample with higher modulus in rubbery state, larger recovery stress and higher entropy elasticity are required during shape recovery. Accordingly, under the same recovery temperature, lower Rr and recovery rate are exhibited. The peak value of tan δ decreases consistently with the increasing filler loading, indicating the continuous decrease of segment mobility caused by HNTs. This phenomenon is attributed to the formation of large interface region and strong interfacial interaction. Moreover, it is notable that the starting temperature of Tg transition is declined along the increasing of HNT loading, indicating a decreased crosslink degree of ENR, which is consistent with the results of curing curves (). As is discussed above, the partial absorption of ZDF by HNTs should be responsible for this phenomenon.
Figure 10. Storage modulus (a) and tan δ (b) of neat ENR and ENR/HNT composites as a function of temperature.
Thermomechanical cycles of ENR and ENR/HNT composites including variable filler contents under force controlled mode were conducted to investigate the effect of HNTs on shape memory performance. The setting strains are all similar value (approximately 100%) to more accurately study the effect. As shown in , all samples exhibit excellent shape fixing and recovery properties on the whole, as evidenced by little strain change during unloading (step 3) and the overlapping of the starting point and ending point of thermomechanical cycles. It is notable that with almost the same strain, compared with neat ENR, the corresponding stress (step 2) for EFeZn25H40 increases greatly by 138%. Therefore, the incorporation of inorganic nanotubes seems to be an efficient way to tune stress of the SMPs. summarizes Rf and Rr of ZDF-cured ENR and ENR/HNT composites. In , all samples exhibit a high Rf value and the Rf value is increasing with the increment of HNT content. By incorporating HNTs, interfacial interaction between HNTs and ENR confines the motion of ENR chain and retards the relaxation of polymer chain. Therefore, retraction during stretching and cooling is less and larger Rf is found for ENR/HNT composites. depicts the effect of HNT content on the Rr value for ENR/HNT composites, which shows a decreasing trend in the first cycle along the increase in HNT content. This unexpected result is related to the inevitable aggregate of nanotubes as well as unexpected slippage during stretching. On one hand, when HNTs are incorporated, excess HNTs tend to aggregate, which is detrimental to elasticity of the composites. On the other hand, the slippage between ENR chain and HNTs during stretching is inevitable and the corresponding deformation is permanent, which is detrimental to shape recovery. The two phenomena become apparent at higher HNT content. Therefore, with increasing HNT content, the Rr value decreases consistently. Moreover, the improvement of modulus caused by HNT incorporation discussed above also makes a contribution to the trend of Rf and Rr change. Notably, Rr of ENR/HNT composites could be tuned to high value by increasing the cycling times. Such increase in the Rr value along increasing cycling numbers is due to that the rearrangement of the fillers occur during thermomechanical cycles, which decreases the deformation caused by slipping between ENR chain and HNTs.
To demonstrate the shape memory process visually, shape evolution photos of EFeZn25 and EFeZn25H40 from a temporary spiral to permanent linear shape are taken and arranged in . The recovery process for EFeZn25 and EFeZn25H40 at 40 °C are displayed in and , respectively. One can observe that when 40 phr HNTs are incorporated, the recovery rate declines obviously under the same recovery temperature. For example, the recovery time of EFeZn25 is only 7 s while for EFeZn25H40, the recovery time reaches 20 s. However, when the recovery temperature of EFeZn25H40 is elevated to 60 °C ((c)), the recovery rate could be tuned to that achieved in EFeZn25, indicating the recovery rate is conveniently tuned. According to the reports by Kim et al. [Citation39,Citation40], the sharp transition from glassy state to rubbery state is crucial to the material for use as SMPs. A high elasticity ratio between glass-state modulus and rubbery-state modulus (Eg’/Er’) allows easy shaping at T > Tg and good resistance to deformation at T < Tg. For samples used to take evolution photos, the order of value of Eg’/Er’ is (c) > (a) > (b) (the values are 320, 246 and 131, respectively, calculated from corresponding DMA data), which is coincident with recovery rates displayed in . Therefore, we can simply regulate the recovery rate by changing elasticity ratio, which is controllable via changing filler content and recovery temperature. Considering the tunable Tg in a wide range by changing ZDF loading, tunable stress by changing HNT content and tunable recovery rate, this rubber-based SMP is highly controllable and is potential in extensive applications.
Figure 13. Evolution of (a) EFeZn25 at 45 °C, (b) EFeZn25H40 at 45 °C and (c) EFeZn25H40 at 60 °C from a temporary spiral to permanent linear shape.

At last, it is necessary to emphasize that the relative low triggering temperature may cause the question that the temporary shape of such SMPs cannot be readily fixed at room temperature, which is a widespread question for SMPs with relative low transition temperature. However, a cold condition for the storage of those SMPs is easy to achieve. On the other hand, SMPs with low transition temperature is also desired in some specific cases. For example, human tissues are soft and vulnerable, and body temperature, namely the triggering temperature for biological SMPs, is about 37°C, which requires low triggering temperatures and low moduli of SMPs when those materials enter into human body.
4. Conclusion
The viability of a kind of rubber-based SMPs fabricated with ZDF-cured ENR is demonstrated in utility temperature range. Excellent shape memory performance is achieved as evidenced by high Rf and Rr. The triggering temperature can be tuned in a wide range from 4 °C to 38 °C just by changing curing agent concentration. HNTs are incorporated to further regulate the shape memory effect. With the incorporation of HNTs, the stress and recovery rate of the SMPs are found to be tunable, which widens the application of this kind of SMPs. The main raw materials for the developed SMPs (NR, FA, and HNTs) are inexpensive and sustainable, endowing extra significance to this kind of SMPs. The combination of adoption of sustainable raw materials, and the excellent and tunable SME makes these SMPs potentially useful in many applications, such as various actuators and heat-shrinkable package materials.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- E. Gil and S. Hudson, Stimuli-reponsive polymers and their bioconjugates, Prog. Polym. Sci. 29 (2004), pp. 1173–1222. doi:10.1016/j.progpolymsci.2004.08.003
- C. Zeng, H. Seino, J. Ren, and N. Yoshie, Polymers with multishape memory controlled by local glass transition temperature, ACS Appl. Mater. Interfaces 6 (2014), pp. 2753–2758. doi:10.1021/am405287p
- Z. Tang, D. Sun, D. Yang, B. Guo, L. Zhang, and D. Jia, Vapor grown carbon nanofiber reinforced bio-based polyester for electroactive shape memory performance, Compos. Sci. Technol. 75 (2013), pp. 15–21. doi:10.1016/j.compscitech.2012.11.019
- A. Lendlein, H. Jiang, O. Jünger, and R. Langer, Light-induced shape-memory polymers, Nature 434 (2005), pp. 879–882. doi:10.1038/nature03496
- W.M. Huang, B. Yang, L. An, C. Li, and Y.S. Chan, Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism, Appl. Phys. Lett. 86 (2005), pp. 114105. doi:10.1063/1.1880448
- F. Li, X. Zhang, J. Hou, M. Xu, X. Luo, D. Ma, and B.K. Kim, Studies on thermally stimulated shape memory effect of segmented polyurethanes, J. Appl. Polym. Sci. 64 (1997), pp. 1511–1516. doi:10.1002/(ISSN)1097-4628
- J. Hao and R. Weiss, Mechanically tough, thermally activated shape memory hydrogels, ACS Macro. Lett. 2 (2013), pp. 86–89. doi:10.1021/mz3006389
- A. Lendlein and S. Kelch, Shape‐memory polymers, Angew. Chem. Int. Edit. 41 (2002), pp. 2034–2057. doi:10.1002/1521-3773(20020617)41:12<2034::AID-ANIE2034>3.0.CO;2-M
- M. Behl, J. Zotzmann, and A. Lendlein, Shape-memory polymers and shape-changing polymers, in Shape-Memory Polymers, A. Lendlein, eds., Vol. 226, Springer, Berlin, 2010, pp. 1–40.
- J. Kunzelman, T. Chung, P.T. Mather, and C. Weder, Shape memory polymers with built-in threshold temperature sensors, J. Mater. Chem. 18 (2008), pp. 1082–1086. doi:10.1039/b718445j
- A. Lendlein and R.S. Langer, Biodegradable Shape Memory Polymeric Sutures, United States Patent US8834522, 2012.
- C. Liu, H. Qin, and P. Mather, Review of progress in shape-memory polymers, J. Mater. Chem. 17 (2007), pp. 1543–1558. doi:10.1039/b615954k
- M.C. Serrano, L. Carbajal, and G.A. Ameer, Novel biodegradable shape‐memory elastomers with drug‐releasing capabilities, Adv. Mater. 23 (2011), pp. 2211–2215. doi:10.1002/adma.v23.19
- H. Zhang, H. Wang, W. Zhong, and Q. Du, A novel type of shape memory polymer blend and the shape memory mechanism, Polymer 50 (2009), pp. 1596–1601. doi:10.1016/j.polymer.2009.01.011
- R. Weiss, E. Izzo, and S. Mandelbaum, New design of shape memory polymers: Mixtures of an elastomeric ionomer and low molar mass fatty acids and their salts, Macromolecules 41 (2008), pp. 2978–2980. doi:10.1021/ma8001774
- Y.-W. Chang, J.K. Mishra, J.-H. Cheong, and D.-K. Kim, Thermomechanical properties and shape memory effect of epoxidized natural rubber crosslinked by 3-amino-1,2,4-triazole, Polym. Int. 56 (2007), pp. 694–698. doi:10.1002/(ISSN)1097-0126
- H. Kang, M. Li, Z. Tang, J. Xue, X. Hu, L. Zhang, and B. Guo, Synthesis and characterization of biobased isosorbide-containing copolyesters as shape memory polymers for biomedical applications, J. Mater. Chem. B 2 (2014), pp. 7877–7886. doi:10.1039/C4TB01304B
- Y. Liu, K. Yao, X. Chen, J. Wang, Z. Wang, H.J. Ploehn, C. Wang, F. Chu, and C. Tang, Sustainable thermoplastic elastomers derived from renewable cellulose, rosin and fatty acids, Polym. Chem. 5 (2014), pp. 3170–3181. doi:10.1039/c3py01260c
- S. Wang, S. Vajjala Kesava, E.D. Gomez, and M.L. Robertson, Sustainable thermoplastic elastomers derived from fatty acids, Macromolecules 46 (2013), pp. 7202–7212. doi:10.1021/ma4011846
- Y. Xia and R.C. Larock, Vegetable oil-based polymeric materials: Synthesis, properties, and applications, Green Chem. 12 (2010), pp. 1893–1909. doi:10.1039/c0gc00264j
- R. Bhardwaj and A.K. Mohanty, Advances in the properties of polylactides based materials: A review, J. Biobased Mater. Bioenergy 1 (2007), pp. 191–209. doi:10.1166/jbmb.2007.023
- P.A. Wilbon, F. Chu, and C. Tang, Progress in renewable polymers from natural terpenes, terpenoids, and rosin, Macromol. Rapid Commun. 34 (2013), pp. 8–37. doi:10.1002/marc.v34.1
- A. Gandini, The irruption of polymers from renewable resources on the scene of macromolecular science and technology, Green Chem. 13 (2011), pp. 1061–1083. doi:10.1039/c0gc00789g
- T. Lin, S. Ma, Y. Lu, and B. Guo, New design of shape memory polymers based on natural rubber crosslinked via oxa-Michael reaction, ACS Appl. Mater. Interfaces 6 (2014), pp. 5695–5703. doi:10.1021/am500236w
- T. Lin and B. Guo, Curing of rubber via oxa-Michael reaction toward significantly increased aging resistance, Ind. Eng. Chem. Res. 52 (2013), pp. 18123–18130. doi:10.1021/ie403485e
- T. Lin, X. Zhang, Z. Tang, and B. Guo, Renewable conjugated acids as curatives for high-performance rubber/silica composites, Green Chem. 17 (2015), pp. 3301–3305. doi:10.1039/C5GC00834D
- D.N. Krishnan, N. Prasanna, E.P. Sabina, and M. Rasool, Hepatoprotective and antioxidant potential of ferulic acid against acetaminophen-induced liver damage in mice, Comp. Clin. Path 22 (2012), pp. 1177–1181. doi:10.1007/s00580-012-1546-y
- N. Kumar and V. Pruthi, Potential applications of ferulic acid from natural sources, Biotechnol. Rep. 4 (2014), pp. 86–93. doi:10.1016/j.btre.2014.09.002
- A. Saija, A. Tomaino, R.L. Cascio, D. Trombetta, A. Proteggente, A. De Pasquale, N. Uccella, and F. Bonina, Ferulic and caffeic acids as potential protective agents against photooxidative skin damage, J. Sci. Food Agric. 79 (1999), pp. 476–480. doi:10.1002/(ISSN)1097-0010
- C. Mancuso and R. Santangelo, Ferulic acid: Pharmacological and toxicological aspects, Food Chem. Toxicol. 65 (2014), pp. 185–195. doi:10.1016/j.fct.2013.12.024
- E. Graf, Antioxidant potential of ferulic acid, Free Radic. Biol. Med. 13 (1992), pp. 435–448. doi:10.1016/0891-5849(92)90184-I
- D.G. Shchukin, G.B. Sukhorukov, R.R. Price, and Y.M. Lvov, Halloysite nanotubes as biomimetic nanoreactors, Small 1 (2005), pp. 510–513. doi:10.1002/(ISSN)1613-6829
- W.S. Guo, H.L. Kang, Y.W. Chen, B.C. Guo, and L.Q. Zhang, Stronger and faster degradable biobased poly(propylene sebacate) as shape memory polymer by incorporating boehmite nanoplatelets, ACS Appl. Mater Interfaces 4 (2012), pp. 4006–4014. doi:10.1021/am300828u
- M.L. Du, B.C. Guo, and D.M. Jia, Newly emerging applications of halloysite nanotubes: A review, Polym. Int.. 59 (2010), pp. 574–582.
- M. Du, B. Guo, Y. Lei, M. Liu, and D. Jia, Carboxylated butadiene–styrene rubber/halloysite nanotube nanocomposites: Interfacial interaction and performance, Polymer 49 (2008), pp. 4871–4876. doi:10.1016/j.polymer.2008.08.042
- Y.-M. Hong, Z.-L. Shen, X.-Q. Hu, W.-M. Mo, X.-F. He, B.-X. Hu, and N. Sun, Acid-catalyzed intramolecular oxa-Michael addition reactions under solvent-free and microwave irradiation conditions, Arkivoc. 14 (2009), pp. 146–155.
- M. Chini, P. Crotti, L.A. Flippin, F. Macchia, and M. Pineschi, Regiochemical control of the ring-opening of 1, 2-epoxides by means of chelating processes. 2. Synthesis and reactions of the cis-and trans-oxides of 4-[(benzyloxy) methyl] cyclohexene, 3-cyclohexenemethanol, and methyl 3-cyclohexenecarboxylate, J. Org. Chem. 57 (1992), pp. 1405–1412. doi:10.1021/jo00031a018
- I. Paterson and D.J. Berrisford, Meso epoxides in asymmetric synthesis: Enantioselective opening by nucleophiles in the presence of chiral Lewis acids, Angew. Chem. Int. Ed. Engl. 31 (1992), pp. 1179–1180. doi:10.1002/(ISSN)1521-3773
- B.K. Kim, S.Y. Lee, and M. Xu, Polyurethanes having shape memory effects, Polymer 37 (1996), pp. 5781–5793. doi:10.1016/S0032-3861(96)00442-9
- B.K. Kim, S.Y. Lee, J.S. Lee, S.H. Baek, Y.J. Choi, J.O. Lee, and M. Xu, Polyurethane ionomers having shape memory effects, Polymer 39 (1998), pp. 2803–2808. doi:10.1016/S0032-3861(97)00616-2