?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Transdermal drug delivery (TDD) has gained clinical approval over several decades, with extensive research dedicated to novel drug and device development. Despite notable research progress, the market adoption of TDD devices has not met anticipated levels, with oral administration and injection remaining predominant delivery methods. To maximize the potential of TDD, we identify bottlenecks hindering its widespread clinical application and propose promising research avenues. We begin by analyzing stringent demands necessary to truly benefit patients, addressing significant challenges in biomechanics, nanomedicine, and flexible electronics. Subsequently, we delve into skin anatomy, enhancement strategies, nano-carriers, and their underlining mechanisms, highlighting the importance and framework of quantitative modeling. Based on these discussions, we highlight the core strength of TDD, such as automatic precise administration based on feedback and high delivery efficiencies, especially applicable to localized conditions (e.g., central nervous system diseases, tumors). Finally, we envision the future of intelligent TDD device and its operation scenario, aiming to steer research efforts toward faster translation of laboratory innovations into widely used products for sufferers.
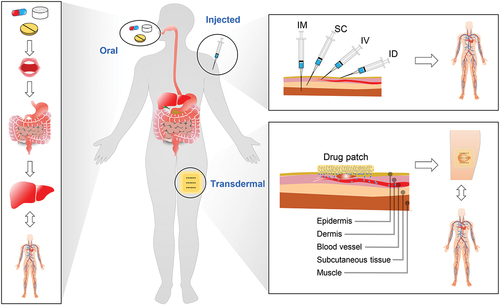
For thousands of years, drug therapy has served as a fundamental pillar of medical treatment [Citation1]. The predominant modalities for drug delivery today are oral administration and injection () [Citation2–5]. However, oral administration often exhibits diminished bioavailability owing to the first-pass effect, while injection involves trauma and often requires professional operation; both posing challenges in sustaining a sustained and stable blood drug concentration [Citation3,Citation6]. In order to overcome the above limitations, transdermal drug delivery (TDD), which involves drug absorption through the skin, has emerged as a viable approach for localized or systemic therapeutic interventions [Citation4]. Since the skin inherently acts as a barrier [Citation7] against external agents and lacks the absorptive properties of the gastrointestinal tract, physical or chemical enhancement techniques are indispensable for facilitating drug permeation through this barrier [Citation3,Citation4,Citation8]. Numerous investigations have been devoted to innovative physical or chemical enhancement strategies (e.g., sonophoresis [Citation9–13], iontophoresis [Citation14–18], microneedles [Citation19–27], and electroporation [Citation28–34]) and their synergistic combinations (e.g., iontophoresis & sonophoresis [Citation35,Citation36], and iontophoresis & microneedles [Citation37,Citation38]), as well as the application of various diseases (e.g., tumors [Citation39–42], heart disease [Citation43,Citation44], hypertension [Citation45,Citation46], diabetes [Citation21,Citation47–51], and arthritis [Citation52]).
Figure 1. Three strategies for drug delivery. Left: oral administration; from the mouth to the gastrointestinal tract, to the liver, and finally to the vascular system. Top right: injection administration including intramuscular (IM) injection, subcutaneous (SC) injection, intravenous (IV) injection, and intradermal (ID) injection; quickly reaching the vascular system. Bottom right: TDD; transporting locally for a period of time before reaching the vascular system.
Despite significant research achievements, the market adoption of TDD devices falls short of predicted levels. Up to now, oral administration and injection remain the dominant drug delivery methods [Citation2,Citation3]. The primary reasons for this discrepancy can be summarized as follows. On the one hand, for most drugs, the rate of drug delivery with existing commercial devices often fails to reach levels that ensure effective treatment without causing pain and tissue damage [Citation3,Citation4]. On the other hand, the existing commercial devices are often bulky and not portable [Citation4,Citation53]. These limitations highlight the considerable distance these devices have yet to cover to meet the stringent demands required for optimal patient care. An ideal TDD device should fulfill the following criteria. 1) Sufficient rate of drug delivery. The delivery rate should be adequate for effective treatment. 2) Low skin damage. Dermal cells should not undergo extensive apoptosis, with minor skin damage healing within a few days to avoid infection. 3) Pain-free. Painless treatment can improve the patient compliance. 4) Wearable capability. Devices wearability is crucial for improving patients’ quality of life and treatment efficacy. 5) Intelligent management capability. Intelligent management capability is essential for accurately controlling drug concentration according to physiological indexes and reducing the burden of patients. 6) Low cost. The above requirements should be implemented within an acceptable cost range. Addressing these scientific and technological challenges and meeting these requirements necessitates collaborative efforts from researchers in the fields of biomechanics, flexible electronics, and nanomedicine are essential. For example, transforming the skin from a barrier that blocks external substances into an efficient drug absorption pathway, without causing tissue damage, requires an in-depth understanding of the biomechanics of TDD [Citation54]. This understanding becomes even more critical when considering the bio-chemo-physical coupling phenomena [Citation55]. To avoid the toxicity of certain drugs to the skin and improve the drug delivery efficiency, nanomedicine technology, involving encapsulating nanoscale drug particles in a specially designed shell and releasing them upon reaching the target site [Citation56–58], is frequently necessary. Wearable capability and intelligence require the integration and improvement of the existing flexible electronic technologies.
In this review, we aim to address the challenges associated with TDD and propose promising research routes. As a basis for understanding the process of TDD, we discuss the detailed anatomy of the skin and various types of pathways for drug absorption from the skin. We then delve into a discussion of different enhancement strategies, nano-carriers and elucidate the underlying mechanisms, based on which we further discuss the quantitative modeling of the process of TDD. The pharmacokinetic model is also discussed here to construct a complete theory from transdermal absorption to metabolism of the drugs. We highlight its efficacy in treating systemic diseases such as diabetes and hypertension, as well as its potential for addressing localized conditions like central nervous system disorders and tumors across different anatomical regions. Based on the above discussion, we point out the core competence of TDD, that is, high delivery efficiencies (especially for localized disease) and automatic precise administration based on feedback. We then discuss recent developments in wearable TDD devices and biosensors, which collectively contribute to the development of intelligent and wearable drug delivery systems. Finally, we predict the future intelligent TDD device and its working scenario, and summarize the achievements and trends into a technology roadmap. Ultimately, we hope to steer and to accelerate research efforts toward faster translation of laboratory innovations into widely used products.
Anatomy of the skin and pathways of drug absorption
To understand the mechanisms of drug absorption through the skin, we begin by delineating the intricate anatomy of the skin and the diverse pathways involved in drug uptake. The human skin, encompassing approximately 1.5 − 2.0 m2, serves as the body’s largest organ and plays a crucial role in protecting the body from external environmental influences [Citation59]. As shown in , the outermost layer of human skin is the epidermis with a total thickness of approximately 50–100 µm [Citation60]. The constituent cells of the epidermis include keratinocytes, melanocytes, Langerhans cells, and Merkel cells. Keratinocytes are the main constituent cells of the epidermis, accounting for over 80% of the total number of epidermal cells. According to the differentiation stage, keratinocytes can be divided into five layers, which are the stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum (SC) from inside to outside.
The SC serves as the major barrier against external substances [Citation61]. It is composed of 15–30 layers of corneocyte cells with a total thickness of 10–20 µm. [Citation62,Citation63] The main components of the SC are keratin proteins derived from dead keratinocyte cells in the deeper layers [Citation64] and lipids including ceramides (30–40%) [Citation65,Citation66], cholesterols, cholesterols esters, free fatty acids, squalene, wax esters and triglycerides [Citation67]. The corneocyte cells are interconnected by corneodesmosomes, ensuring the structural integrity of the SC [Citation68]. Beneath the SC, stratum lucidum is a clear and thin layer that consists of 2–3 layers of keratinocyte cells, primarily present in palms, soles and digits [Citation69,Citation70]. The subsequent layer is the stratum granulosum, where the cells have a thicker membrane compared to the first two layers. Below this layer is the stratum spinosum, consisting of 5–10 layers of keratinocytes [Citation59], and hosting Langerhans cells, accounting for 3–5% of epidermal cells [Citation71]. Langerhans cells, characterized by dendritic morphology, are an important type of immune cells with phagocytic function (engulfing bacteria, exogenous particles and damaged cells), capable of recognizing, processing, and presenting antigens [Citation72]. The stratum basale directly contacts the dermis via interconnecting collagen fibers. In the stratum basale, the cells proliferate and become primary cells for keratinocytes that are present in the upper epidermal layers [Citation73]. Epidermal turnover occurs about 28 days, with new cells from the stratum basale migrating upwards to replace cells in the stratum corneum, which are subsequently shed. Merkel cells are considered to be a type of neuroendocrine cell that forms a cellular axonal complex with sensory nerve fibers [Citation74,Citation75], enabling the perception of pain.
The dermis, located beneath the epidermis and intricately linked to the epidermal stratum basale via the basal membrane, plays a supporting role for the epidermis [Citation4]. It is mainly composed of fibrous protein (collagen fibers, elastic fibers, and reticular fibers), matrix and cells. The dermis contains several types of functional cells, among which fibroblasts play a pivotal role in synthesizing collagen, elastic, and reticular fibers, thereby contributing significantly to the skin’s elasticity and tensile strength [Citation59]. The matrix is an amorphous substance that fills the gaps between fiber bundles such as collagen fibers and elastic fibers, playing an important role in the connection, nutrition, and protection. It contains mucopolysaccharides such as hyaluronic acid that can bind with water to prevent water loss. There are two layers in the dermis, namely the papillary layer and the reticular layer [Citation59]. The papillary layer is shallow and thin, hosting fine fibers, abundant capillaries, lymphatic vessels, nerve endings and tactile bodies. In contrast, the reticular layer is deep and thick, with interwoven dense fibers, and larger blood vessels, lymphatic vessels and nerves [Citation76]. Due to the presence of phagocytes, fibroblasts, leucocytes, and mast cells, the dermis plays a crucial role in immune function [Citation77]. In addition, there are numerous hair follicles, sebaceous glands, and sweat glands in the dermis for sweating and sebum secretion [Citation78]. The layer below the dermis is the subcutaneous layer, which serves to connect the skin, muscles, and bones [Citation79].
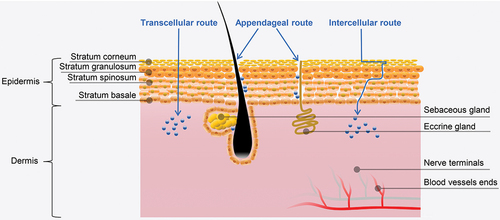
The possible pathways for drugs pass through the skin can be divided into transcellular route, intercellular route and appendageal route [Citation80]. In the transcellular route, drugs navigate across membranes composed of lipid bilayers [Citation81]. This route is mainly taken by hydrophobic drugs because of the hydrophobic properties of lipid complex in the cell membranes of the SC [Citation29]. In the intercellular route, the drugs diffuse through the intercellular space of residing keratinocytes in the SC [Citation82]. The intercellular route is the predominant pathway for drug absorption, particularly for hydrophilic compounds [Citation83,Citation84]. Several models have been developed to predict permeability of the stratum corneum to hydrophobic drugs [Citation85–87]. For example, one equation formulated by Potts and Guy [Citation87] correlates skin permeability (P) with solute molecular mass (M) and octanol – water partition coefficient (Ko/w):
The appendageal route refers to drug delivery through hair follicles or sweat ducts within the skin [Citation80]. This route is particularly advantageous for transport of large macromolecules that are difficult to penetrate the epidermal cells [Citation29]. Nevertheless, the effectiveness of this route is somewhat limited due to the smaller absorption area (~0.1% of skin area) [Citation53]. Overall, the skin is originally a barrier that protects against external substances and does not have a good absorption effect like the gastric mucosa or intestinal mucosa. Therefore, the utilization of physical or chemical enhancement techniques becomes necessary to facilitate drug permeation through this barrier.
Enhancement strategies and underlying mechanisms
The physical or chemical enhancement strategies for TDD include chemical enhancers [Citation88–94], iontophoresis [Citation14–18], microneedles [Citation19–27], sonophoresis [Citation9–13,Citation95], electroporation [Citation28–34], laser radiation [Citation96–103], etc., each with different underlying mechanisms. For example, chemical enhancers disrupt the structure of lipid bilayer of the stratum corneum by extracting lipids using solvents and surfactants to generate nanoscale lipid stacking defects [Citation3]. Iontophoresis provides a motive force for drug molecule transport through sustained low electrical current (0.5 to 20 mA [Citation104,Citation105]), as shown in . Anionic drugs are placed beneath the cathode, while cationic drugs are placed beneath the anode. In the skin, anions move toward the anode, while cations move oppositely. Iontophoresis is mainly used for ionized drugs delivery, although it can also deliver weak charged or neutral molecules through electroosmosis [Citation3]. The factors that affect efficiency include physicochemical characteristics of the drugs, current magnitude, duration, and physiology of the application site. The molecular weight of the drug is suggested to be less than 10,000 Da, [Citation106] and the examples include nonsteroidal anti-inflammatory drugs (ibuprofen, aspirin, etc.) [Citation107], granisetron [Citation108], almotriptan [Citation109], donepezil [Citation110], sodium nonivamide acetate [Citation111] and insulin [Citation112]. Microneedle technology utilizes very short needles to penetrate the stratum corneum and deliver drugs into the skin in a minimally invasive manner (). Microneedles are typically made of biocompatible substances or biodegradable polymers [Citation26]. The factors that affect efficiency include physicochemical characteristics of the drugs, size and density of microneedles. Microneedle technology can promote the delivery of both small and large molecules. It has been shown to be useful in the delivery of corona virus 2019 (Covid-19) vaccines [Citation25,Citation27,Citation113,Citation114], long-acting antiretroviral drugs for human immunodeficiency virus (HIV) (e.g., rilpivirine [Citation115,Citation116]) and contraceptives [Citation23] (e.g., estradiol, levonorgestrel [Citation117–119], ethynyl estradiol, and etonogestrel [Citation24]).
Figure 3. Schematic illustrations of the underlying mechanisms of typical enhancement strategies. a) Iontophoresis; b) microneedle; c) sonophoresis; d) electroporation.
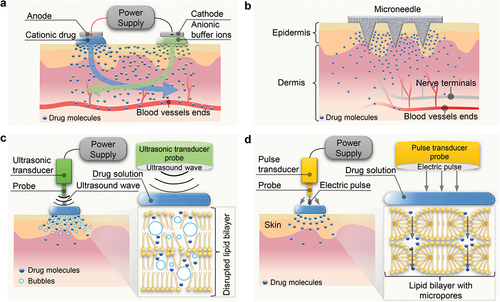
Sonophoresis, also known as phonophoresis, utilizes low-frequency (LFS, 20 − 100 kHz) or high-frequency (HFS, ≥0.7 MHz) ultrasound based on the cavitation effect (). For long-term use without damage to organs, the United States Food and Drug Administration (US FDA) recommends a maximum mechanical index (MI) of 1.9, which is calculated by dividing the peak pressure (MPa) measured in water by the square root of frequency (MHz).[Citation120,Citation121] The cavitation of LFS mainly occurs on the surface of the skin, and the expected mechanism is the oscillation and collapse of bubbles on the skin surface, resulting in local shock waves and liquid micro jets toward the stratum corneum, increasing skin permeability temporarily. HFS cavitation mainly occurs in skin, and bubbles are generated in the cavities among keratinocytes in the stratum corneum. Oscillating cavitation bubbles can cause disruption of the lipid bilayer in the stratum corneum, thereby increasing skin permeability [Citation95]. The thermal effect may also play nonnegligible roles in some cases [Citation9,Citation10,Citation95,Citation122,Citation123]. Factors affecting efficiency include the frequency, intensity, application time, coupling medium, and distance between the ultrasonic probe and the skin [Citation124–126]. Sonophoresis can promote the delivery of hydrophobic and hydrophilic drugs, as well as for the delivery of small and large molecules, such as ketoprofen [Citation126], vancomycin [Citation127], fuocinolone acetonide [Citation11], gemcitabine hydrochloride [Citation128], and proteins (e.g., insulin, interferon γ, and erythropoietin) [Citation129]. Ultrasound can not only generate microbubbles in situ, but also on-demand stimulate injected phase-changing nanodroplets loaded with drugs, transforming them into microbubbles and generating cavitation [Citation130–139]. This cavitation can apply forces on cell connections in biological barrier membranes (such as the blood-brain barrier), triggering a transient and reversible increase in permeability. Electroporation creates aqueous pores in the lipid bilayer of the SC by applying high voltage (10–1000 V) for a brief duration (less than a few hundred milliseconds), enabling deeper drug penetration [Citation33] (). Efficiency factors include the voltage magnitude, length of pulse, number of pulses and the physicochemical properties of the drug [Citation33]. Electroporation can promote the delivery of both small and large molecules, such as alniditan [Citation140], tetracaine [Citation141], fentanyl [Citation142], timolol [Citation143], insulin [Citation144], calcein [Citation34], dextran and doxorubicin [Citation145], yet high voltage poses a risk of cell damage [Citation146,Citation147]. Laser radiation can achieve controlled destruction and ablation of the SC with depth from tens to hundreds of micrometers, thereby accelerating drug delivery [Citation96–100,Citation102]. Efficiency factors include the laser type, intensity, length of pulse, number of pulses and the physicochemical properties of the drug [Citation101,Citation103]. Researchers have already demonstrated through animal experiments that laser radiation can facilitate the delivery of both small and large molecules, such as diclofenac, morphine, nalbuphine, buprenorphine, methotrexate, prednisone, and vaccines [Citation96–103]. However, the laser equipment is typically expensive, limiting its potential applications [Citation101]. The comparison of the advantages and disadvantages of the above typical enhancement strategies is shown in .
Although the utilization of aforementioned strategies can improve the rate of drug delivery, it is still challenging for numerous drugs to achieve an effective therapeutic dose. Simply increasing the intensity under a single strategy to increase drug delivery rate can lead to significant cellular stimulation, toxicity, and even cause serious skin damage. For example, traditional chemical enhancers and iontophoresis lack the precision to confine their effects to the stratum corneum, as high intensity can cause cytotoxicity and tissue damage. Sonophoresis and microneedles, while more adept at disrupting the stratum corneum for enhanced TDD without compromising deeper tissues, can still pose risks such as dermal damage at elevated intensities or larger pore sizes. To address these challenges, a judicious integration of multiple strategies in a coherent sequence without surpassing their safety thresholds can substantially boost drug delivery rates while upholding safety standards. In recent years, researchers in the field of biomedicine have conducted many experimental explorations in the combination of TDD strategies, confirming the advantages of multiple-strategy combinations over a single strategy [Citation38], such as the combination of iontophoresis and sonophoresis, and the combination of iontophoresis and microneedles. Qualitative explanations for these combinations have also been proposed: iontophoresis can provide a motive force for drug molecule transport, while sonophoresis or microneedles can disrupt a part of the stratum corneum to improve the permeability, leading to a synergistic effect between these mechanisms.
Table 1. Comparison of advantages and disadvantages of typical enhancement strategies.
Quantitative modeling of the process of TDD
The development of a quantitative theory to access the efficacy of various combinations of drug delivery strategies are of great guiding significance in advancing TDD technology. The key factors involved in the aforementioned strategies include stress fields, electric fields, chemical reactions, and biological feedback, operating molecular, cellular, and macroscopic organizational scales [Citation55,Citation148–154]. However, quantitative models for such multi-physical field coupling are still relatively limited. The existing theoretical models for TDD mainly include continuum mechanics models and equivalent circuit models. For example, Mitragotri et al. [Citation54] proposed a continuum mechanics model for sonophoresis, in which the skin is simplified as a homogeneous block to calculate the strain and the damaged pore caused by ultrasound induced bubbles or micro-jets (). Chizmadzhev et al. [Citation155] established an equivalent circuit model for electroporation, as depicted in . The resistance of bulk solution Rb is in series with the skin resistance, which includes two parallel branches: The resistance Rx refers to the main pathways of background electrolyte going through the appendages and stratum corneum; The resistance Rc corresponds to the inner part of each compartment and Rl to lipid bilayers, forming boundaries between the compartments. In addition, numerical simulations combining microneedles and iontophoresis often employ the Nernst-Planck equation from electrochemistry as a control equation [Citation156,Citation157]. While these models offer insights for single-strategy TDD, they fall short in guiding combinations of multiple strategies. Simple superposition is inadequate due to differing spatial scales and strong coupling effects among strategies.
Figure 4. Existing theoretical models for TDD. a) Theoretical model for sonophoresis [Citation54]. Left: three possible modes through which inertial cavitation may enhance the permeability of stratum corneum. Right: a schematic illustration of a spherical shock wave propagating through the surface of stratum corneum. b) Theoretical model for electroporation [Citation155]. Left: structure and transport model of stratum corneum where the corneocytes are shown by the shaded area and are surrounded by the lipid domain. The lipid and lipid-corneocyte routes of ion transport are denominated as t and s, respectively. The inset shows the multi-lamellar lipid domain after formation of electropores. Right: the equivalent electrical scheme of stratum corneum. U, Us and Ul are the voltage drop on the corresponding resistances.
![Figure 4. Existing theoretical models for TDD. a) Theoretical model for sonophoresis [Citation54]. Left: three possible modes through which inertial cavitation may enhance the permeability of stratum corneum. Right: a schematic illustration of a spherical shock wave propagating through the surface of stratum corneum. b) Theoretical model for electroporation [Citation155]. Left: structure and transport model of stratum corneum where the corneocytes are shown by the shaded area and are surrounded by the lipid domain. The lipid and lipid-corneocyte routes of ion transport are denominated as t and s, respectively. The inset shows the multi-lamellar lipid domain after formation of electropores. Right: the equivalent electrical scheme of stratum corneum. U, Us and Ul are the voltage drop on the corresponding resistances.](/cms/asset/413dec35-e922-47a9-aaca-7395c38d1549/tsnm_a_2366210_f0004_c.jpg)
On the other hand, due to the large molecular weight, the transmembrane transport of nanomedicine especially including nano-carriers involves endocytosis and exocytosis, which needs to be considered and modeled on a smaller scale. This process is significantly different from the transport of ions and small molecules. Over the past few decades, the forms of nano-carriers have evolved from liposomes [Citation158,Citation159] to polymer nanoparticles [Citation160–167] and metal nanoparticles [Citation168–171], then to lipid nanoparticles [Citation172–174], nanostructured lipid carriers [Citation175–180] and phase-changing nanodroplets [Citation130–139], and recently to metal-organic frameworks [Citation181–183] and biomimetic nanocarriers [Citation184] (extracellular vesicles inspired [Citation185–187], viruses inspired [Citation188], bacteria inspired [Citation189,Citation190], etc.), as shown in . The size, shape, stiffness, surface chemical properties, and biological functions of the nano-carriers can all affect the performance of transmembrane transport. There are many models describing the transmembrane transport of nanomedicine. For example, Deserno proposed a model for the elastic deformation of a fluid membrane upon colloid binding, and found a line of continuous binding transitions and a second line of discontinuous envelopment transitions, which meet at an unusual triple point () [Citation191]. Gao et al. established a model for receptor-mediated endocytosis, showing that the particles in the size range of tens to hundreds of nanometers can enter or exit cells via wrapping even in the absence of clathrin or caveolin coats, and an optimal particles size exists for the smallest wrapping time, which broadly agrees with experimental observations [Citation192]. Afterward, Yi et al. investigated the adhesive wrapping of a soft elastic vesicle by a lipid membrane, showing that there exist a maximum of five distinct wrapping phases, which depend on the vesicle size, adhesion energy, surface tension of the membrane, and bending rigidity ratio between vesicle and membrane [Citation193]. Wang et al. presented a theoretical analysis regarding the cooperative entry of nanoparticles into cells, demonstrating that oblate ellipsoidal nanoparticles exhibit greater energy compensation during cooperative entry compared to individual nanoparticles or spherical nanoparticles () [Citation194]. Yi et al. further investigated the incorporation of soft particles into lipid vesicles, considering the global deformation of the vesicles () [Citation195]. However, the above models rarely consider the important role of the biological functions of nanomedicine and cells in endocytosis and exocytosis, and cannot explain the advantages of the biomimetic carriers found in the experiments.
Figure 5. Structures and mechanical models of nano-carriers. a) Schematic diagram of the structures of various nano-carriers: from the traditional to the most advanced. b) the model for elastic deformation of a fluid membrane upon colloid binding [Citation191]. c) the model for cooperative entry of nanoparticles into the cell [Citation194]. d) the model for incorporation of soft particles into lipid vesicles [Citation195].
![Figure 5. Structures and mechanical models of nano-carriers. a) Schematic diagram of the structures of various nano-carriers: from the traditional to the most advanced. b) the model for elastic deformation of a fluid membrane upon colloid binding [Citation191]. c) the model for cooperative entry of nanoparticles into the cell [Citation194]. d) the model for incorporation of soft particles into lipid vesicles [Citation195].](/cms/asset/c0280e6d-d193-419f-bc66-90b06daba35c/tsnm_a_2366210_f0005_c.jpg)
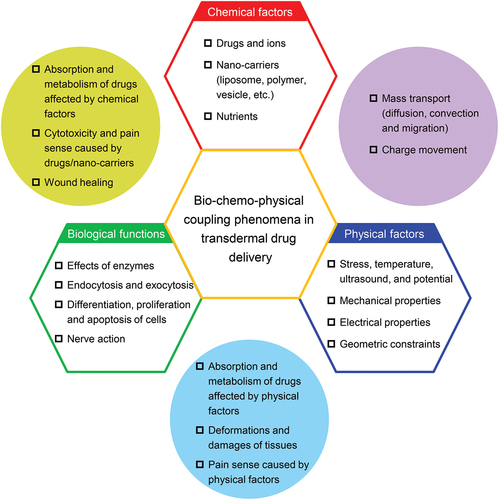
Now we consider the bio-chemo-physical coupling phenomena in TDD (), which could inspire a new theoretical framework for quantitative TDD. Chemical factors include concentrations, molecular weights, ionizability and hydrophilicity of the delivered drugs, types, and concentrations of other ions, pH value, hydrophilicity and other chemical properties of the nano-carriers (liposomes, polymers, vesicles, etc.), and concentrations of nutrients (blood glucose, blood oxygen, etc.). Physical factors include stress field, temperature field, and potential field inside the skin, ultrasound field inside and outside the skin, mechanical and electrical properties of the skin, stiffnesses, sizes, and shapes of the nano-carriers, and geometric constraints provided by the devices. Biological functions include effects of enzymes, endocytosis and exocytosis, differentiation, proliferation and apoptosis of cells, and nerve action. The mutual coupling relationships between them are mainly reflected in the following aspects. (1) Absorption and metabolism of drugs are affected by chemical factors (drugs, enhancers, nano-carriers, skin tissue); Drugs/nano-carriers can cause cytotoxicity and pain perception; The rate of wound healing is influenced by nutrition. (2) Absorption and metabolism of drugs are affected by environmental physical factors (stress, temperature, etc.) and physical factors of drugs/nano-carriers (stiffness, size, shape, etc.); Physical factors (excessive stress, temperature, etc.) can cause damages of tissues; Physical factors (excessive stress, temperature, etc.) can also cause pain sense. (3) The mass transport and charge movement of drug molecules are coupled, involving electrochemical reactions, convection, and diffusion. For specific application situations, some of the above coupling relationships can be reasonably selected for theoretical modeling. Detailed process of the theoretical modeling can refer to some models that have been developed to describe bio-chemo-mechanical processes at the molecular, cellular, and tissular levels [Citation55,Citation148–154]. According to different mathematical methods, they can be roughly divided into three categories: continuum mechanics models, discrete models, and multi-scale models [Citation55]. The continuum field equations maintain unity in mathematical description and convenience in numerical solution [Citation149,Citation196]. For discrete models, some detailed physical mechanisms at the cellular and subcellular scales can be considered [Citation148,Citation197]. Multi-scale models facilitate the understanding of how macroscopic mechanical properties and biological functions depend on complex physical mechanisms at different length and time scales [Citation198,Citation199]. By combining hierarchical structures with various models, multi-scale models can share the advantages of different modeling methods and reflect the synergistic effects of different levels of size.
Pharmacokinetic models
To construct a comprehensive theory from transdermal absorption to metabolism of the drugs, it’s essential to incorporate the pharmacokinetic models. These models aim to study the changes in the amount of substances with time during the absorption, distribution, metabolism, and excretion of drugs through various pathways [Citation200]. To facilitate research and application, complex physiological systems are commonly simplified, and the established pharmacokinetic models include compartment models [Citation201,Citation202], noncompartment models based on statistical moment principle [Citation203–205], physiologically based pharmacokinetic models [Citation206,Citation207], etc. Among them, the compartment models are the most classic pharmacokinetic models, which were first proposed by Widmark and Tandberg [Citation208] on the basis of the kinetic study of Michaelis and Menten [Citation209], and developed by Teorell et al. [Citation201,Citation202] The compartment model treats the body as a system, dividing it into several compartments according to the drug transport rate characteristics, and assuming that the drugs entering the compartments can be evenly distributed throughout the compartments in an instant [Citation210]. The mathematical modeling process of single-compartment models and two-compartment models for intravenous administration and extravascular administration is discussed below.
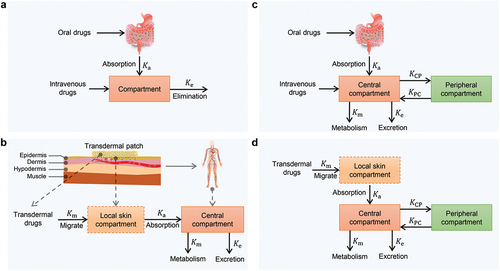
If the drugs are rapidly distributed in the body after intravenous administration, the single-compartment model can be used to describe this process, as shown in . The elimination of drugs is assumed to obey first-order rate kinetics, that is, the elimination rate is proportional to the amount of drugs in the body at this time. Then, the differential equation about the amount of drugs can be established as
where is the amount of drugs in the body at time
, and
is the first-order elimination rate constant. Compared with intravascular administration, there is an additional absorption process for extravascular administration, such as oral administration () and TDD (). When the drugs deliver through the skin, the absorption site (the local skin) can be considered as a ‘compartment.’ The processes of absorption and elimination for extravascular administration also typically obey first-order rate kinetics. Based on this, the differential equations for the amount of drugs at the absorption site and the amount of drugs in the body can be established respectively:
Figure 7. Existing pharmacokinetic models (the compartment models). a) one-compartment model for oral and injection. b) two-compartment model for oral and injection. c) two-compartment model for TDD. d) three-compartment model for TDD.
where is the amount of drugs in absorption site at time
, and
is the first-order absorption rate constant.
Upon absorption, many drugs are rapidly distributed across various tissues, organs, and body fluids. Approximately, these components together with the blood system form a compartment, constitute what is known as the central compartment. Conversely, areas where drug distribution occurs at a slower pace are referred to as peripheral compartments. Thus, a two-compartment model can be formed, as shown in . When administered intravenously, there is no absorption process. The drugs first enter the system through the central compartment. While being eliminated in the central compartment, the drugs undergo reversible transport between the central compartment and the peripheral compartment. Generally, the processes of elimination and transport obey first-order rate kinetics, based on which the differential equations for the amount of drugs in each compartment are established as:
where is the amount of drugs in the central compartment at time
;
is the amount of drugs in the peripheral compartment at time
;
is the first-order rate constant for transport from the peripheral compartment to the central compartment;
is the first-order rate constant for transport from the central compartment to the peripheral compartment;
is the first-order rate constant for drug elimination from the central compartment. When the drugs deliver through the skin, there is an additional absorption process (). Assuming the absorption and the elimination of the drugs obey first-order rate kinetics, the amount of drugs in each compartment satisfies the following relationship.
The aforementioned models can be employed to calculate pharmacokinetic parameters such as rate constant, biological half-life, apparent volume of distribution, clearance rate, area under the curve, etc. These parameters are valuable for guiding the development of new drugs and facilitating rational clinical drug use.
By combining the above pharmacokinetic models with the TDD models mentioned in the previous section, we can assess the delivery efficiency across various drug delivery methods. illustrates the transport pathways of drug transport within the body. For the oral administration, drugs are delivered from the mouth to the gastrointestinal tract, then to the liver, and eventually to the vascular system. For the intravenous injection, drugs quickly reach the vascular system. For the TDD, drugs are transported locally for a period of time before reaching the vascular system. Correspondingly, drug concentration distribution in the body can be calculated after some simplification. Here, we present a schematic diagram only for qualitative explanation (). Due to the first-pass effect, the drug concentration under oral administration is usually lower than that under intravenous injection. In fact, the drug efficiency under intravenous injection is still not high enough for local diseases. A recent work reveals that the typical delivery efficiency of anti-tumor drugs through intravenous injection is only about one out of one million () [Citation211]. After the drugs are administered intravenously, they have to transport through the bloodstream to reach the final target site. Many of the drugs are taken up by the liver, spleen, and other reticuloendothelial organs. After entering the solid tumor, they have to cross the blood vessel, extracellular matrix, and other non-tumor cells before reaching the tumor cells. Then the drugs have to cross the cell membrane, vesicles, and other subcellular structures before reaching the target in the nucleus. However, for the TDD, the drug concentration in the body near the patch is much higher than that in other locations, and the drug concentration in other locations could be close to that of intravenous injection conditions. This distinguishing feature makes TDD more efficient in treating superficial local diseases compared to intravenous injection, and far more effective than oral administration.
Figure 8. Delivery efficiency under different methods of drug delivery. a) Illustration of the drug transport pathways in the body under different methods of drug delivery. b) Schematic diagram of the distribution of drug concentration under different methods of drug delivery. c) Typical delivery efficiency (about one out of one million) of anti-tumor drugs through intravenous injection [Citation211].
![Figure 8. Delivery efficiency under different methods of drug delivery. a) Illustration of the drug transport pathways in the body under different methods of drug delivery. b) Schematic diagram of the distribution of drug concentration under different methods of drug delivery. c) Typical delivery efficiency (about one out of one million) of anti-tumor drugs through intravenous injection [Citation211].](/cms/asset/2ee71e3c-7087-4166-b54a-b0e68e353700/tsnm_a_2366210_f0008_c.jpg)
Application of TDD in diseases of different body parts
After years of development, TDD technology has been applied to treat various diseases of different body parts. These diseases generally fall into two categories: systemic diseases and localized diseases, as shown in and . Systemic diseases treated by TDD mainly include heart disease, hypertension, diabetes, etc. Nitroglycerin patches were approved by the US FDA for the treatment of angina in 1981 [Citation4]. Clonidine patches were approved by the US FDA for the treatment of hypertension in 1990 [Citation4]. These nitroglycerin and clonidine patches exhibit fewer adverse effects than conventional oral dosage forms. Insulin patches have been widely studied for the treatment of diabetes since 1991 [Citation49], and there are still a lot of new progress in recent years.[Citation21,Citation47,Citation48,Citation50,Citation51,Citation212,Citation213] TDD also has great potential in providing long-acting contraceptives, such as the sustained release of levonorgestrel (one month to one year).[Citation23,Citation117,Citation119,Citation214] The main advantage of TDD for systemic diseases is to achieve programmable and precise administration based on disease feedback or in a fixed manner. Localized diseases treated by TDD mainly include central nervous system diseases (e.g., Parkinson’s disease [Citation215], Alzheimer’s disease [Citation216,Citation217], depression [Citation218,Citation219]), glioblastoma [Citation220], breast cancer [Citation221], superficial tumors (e.g., melanoma [Citation219,Citation220], papilloma [Citation222,Citation223], T-cell lymphoma [Citation224]), dermatosis [Citation225], arthritis [Citation52], etc. Transdermal delivery offers not only programmable and precise administration but also achieves high delivery efficiencies, making it a promising therapeutic approach.
Figure 9. The application of TDD in diseases of different body parts. a) Systemic diseases. b) Localized diseases.
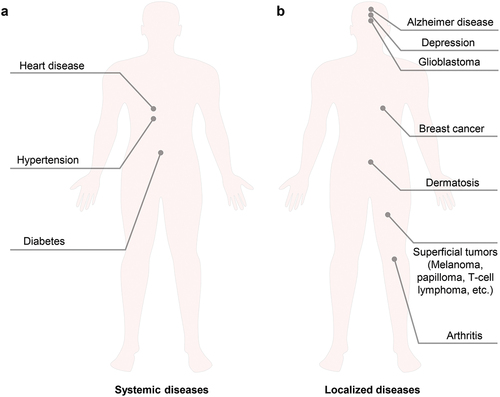
Table 2. Application of TDD in systemic diseases and localized diseases.
Here we focus on discussing central nervous system diseases and superficial tumors. The delivery efficiency of drugs for central nervous system diseases is typically low, both orally and intravenously, due to the presence of the blood-brain barrier and blood-tumor barrier [Citation226]. Kim et al. [Citation216] prepared microneedles containing donepezil hydrochloride using a micro-molding method based on a hydroxypropyl methylcellulose-ethanol/water mixture and a carboxymethyl cellulose water solution, and administered them through the back skin of mice. Pharmacokinetic studies have shown that, at a dosage of 692 μg·kg−1, the bioavailability of microneedles is 2.4 times that of oral administration, and the peak concentration (Cmax) of the same dose of microneedle administration is 4 times that of oral administration. Similarly, Zhao et al. [Citation217] prepared microneedles based on methacryl gelatin for the delivery of donepezil hydrochloride. After the microneedles puncture into the subcutaneous area, the polyvinyl alcohol in the backing layer dissolves and wraps around the needle body to achieve controlled drug release. Courtenay et al. [Citation218] constructed hydrogel microneedles for transdermal delivery of esketamine. Experimental results have shown that microneedles can continuously release esketamine for more than 24 hours in rats, and the blood drug concentration within 24 hours is higher than 0.15–0.3 μg·mL−1. In addition, Kumar et al. [Citation219] prepared a transdermal patch composed of duloxetine and sulfobutyl ether-β-cyclodextrin. After transdermal delivery of the patch to rats, autonomous activity was measured using a digital photoelectric velocimeter, showing a notable antidepressant effect. Bali et al. [Citation215] developed a kind of polymer nanoparticles loaded with Silagilan (SGN-PNP) for the transdermal treatment of Parkinson’s disease. The brain drug concentration of the SGN-PNP after intravenous injection reaches 7.7 times that of only the SGN, confirming the targeting ability of the SGN-PNP; Meanwhile, compared to intravenous injection of the SGN-PNP, transdermal delivery of the SGN-PNP prolongs the time to peak (Tmax) of brain drug from 0.5 h to 36 h, and increases the concentration of brain drug from 1401.3 ng·h·mL−1 to 25,451.6 ng·h·mL−1, demonstrating excellent ability of sustained-release drug delivery. A recent study [Citation220] found that the meningeal lymphatic vessels can connect to the deep cervical lymph nodes, so the drugs can directly flow into the meningeal lymphatic vessels through the deep cervical lymph nodes, which can be used for the treatment of brain diseases.
For tumor treatment, the efficiency of oral and intravenous delivery is also very low due to the absorption of drugs by other organs in the body, while the combination of transdermal delivery and nanomedicine holds promise for improving delivery efficiency and reducing toxic side effects. There are three main types of drug therapy for tumors: chemotherapy, gene therapy, and immunotherapy. Chemotherapy drugs widely used in clinical superficial tumors include doxorubicin, cisplatin, paclitaxel, etc. Ahmed et al. [Citation227] used liposomes loaded with doxorubicin and celecoxib for transdermal melanoma treatment, demonstrating significant anti-tumor effects. Bhatnagar et al. [Citation221] delivered doxorubicin hydrochloride and docetaxel, resulting in a notable improvement in survival rates compared to intratumoral chemotherapy drugs. Lan et al. [Citation39] delivered cisplatin encapsulated with lipid nanoparticles, leading to significantly increased tumor cell apoptosis without serum platinum or organ toxicity in vivo. Huang et al. [Citation228] achieved sustained release of doxorubicin and trametinib, overcoming P-glycoprotein-mediated multidrug resistance and reducing systemic toxic side effects.
Abnormal gene expression influences the occurrence and progression of tumors. Gene-based therapies primarily involve delivering nucleic acids into the body, such as plasmid DNA (pDNA), small interfering RNA (siRNA), and oligonucleotides. These nucleic acids are usually encapsulated in polymers, liposomes, nanoparticles, or cell penetrating peptides for transdermal delivery. Li et al. [Citation40] developed pH-responsive polycaprolactone microneedles loaded with the p53 expression plasmid (p53 DNA), which demonstrated an efficiency of 90.1% in inhibiting subcutaneous tumors. Ruan et al. [Citation229] delivered a nanocomposite consisting of cell-penetrating peptide (octaarginine) and B-Raf proto-oncogene serine/threonine protein kinase siRNA for the treatment of melanoma, inducing tumor cell apoptosis and inhibiting tumor cell proliferation. The combination of transdermal delivery and gene therapy exhibits significant tissue permeability and minimal side effects, along with advantages in enhancing gene stability and promoting gene expression [Citation230–232].
Immunotherapy, which stimulates the innate immune system of the body to recognize and eliminate tumors, possesses the characteristics of high specificity, high efficacy, and long-lasting effects. As a result, it has emerged as a first-line treatment for melanoma and lung cancer [Citation233]. The primary approach involves delivering antigen vaccines, immune checkpoint inhibitors, genetic vaccines, antibodies, cytokines, etc [Citation41,Citation234]. The skin, being a robust immune organ, harbors a substantial population of dermal dendritic cells and Langerhans cells, which contribute significantly to immune defense mechanisms. When the microenvironment is disturbed, these dendritic cells become activated, initiating a series of immune reactions and activating the immune system. However, the stratum corneum greatly hinders the transdermal delivery of macromolecules such as antigens and antibodies. Therefore, immunotherapy based on the technology of TDD has shown great advantages [Citation235]. Chen et al. [Citation236] developed chitosan microneedles for continuous delivery of the model antigen ovalbumin. After delivering low-dose ovalbumin through microneedles, antibody levels can persist for 18 weeks, which is 2.5 times higher than the full dose of ovalbumin injected into muscles. Additionally, Ye et al. [Citation42] developed a B16-F10 melanoma vaccine patch, which transdermally delivers tumor lysates bound to melanin, directly targeting antigen-presenting cells, and releasing the drug under near-infrared light irradiation, thereby promoting antigen uptake. The release of local cytokines greatly improves the survival rate of mice and has a significant inhibitory effect on both in situ and distal tumors. However, the translation of skin tumor immunotherapy from animals to clinical application still faces significant hurdles, such as limited vaccines immunogenicity, insufficient skin dendritic cell antigenicity, immune suppression, immune evasion, etc. A comprehensive understanding of skin immune physiology and tumor immune mechanisms is imperative. Designing efficient and safe TDD is essential to realize the clinical potential of these therapies.
Wearable TDD devices and biosensors
Wearable TDD devices and biosensors together are fundamental to the wearability and intelligence of the systems, a domain that has experienced rapid development in recent years. Up to now, enhancement strategies for wearable TDD devices have included sonophoresis [Citation237,Citation238], iontophoresis [Citation38,Citation157], microneedles [Citation23–25,Citation27,Citation113–119], and electroporation [Citation237], as shown in . For instance, Li et al. [Citation238] presented a stretchable electronic facial mask (SEFM) for sonophoresis (). This work addresses the technical challenges associated with sonophoresis on large and complex human facial surfaces by introducing a single-side structure for piezoelectric components, ensuring low bending stiffness and high bendability to conform comfortably to the human face. Additionally, they introduced the single-side soft pressing (SSSP) technique for encapsulation. The animal experiments and human facial experiments have demonstrated the enormous potential of the developed SEFM in facial healthcare applications. Afterward, Yu et al. developed a conformable ultrasound patch (cUSP) utilizing intermediate-frequency sonophoresis (IFS) for effective cavitation-enhanced transdermal delivery of cosmeceuticals () [Citation239]. This study demonstrated the cUSP’s suitability for short-exposure, targeted transdermal delivery for patients and consumers suffering from skin conditions. Iontophoresis and microneedles are frequently combined to enhance delivery efficiency in the wearable TDD devices. The primary structural difference lies in the design of microneedles, such as solid polymer-based ion-conductive porous microneedles () [Citation38] and animal masticatory system bio-inspired microneedles () [Citation157]. More recently, the electroporation mechanism has also been applied to stretchable electronic facial mask to achieve cost-effective TDD () [Citation237].
Table 3. Representative wearable TDD devices.
Figure 10. Representative applications of wearable TDD devices and biosensors. a) a stretchable electronic facial mask for sonophoresis [Citation238]. b) a conformable ultrasound patch for cavitation-enhanced transdermal cosmeceutical delivery [Citation239]. c) Solid polymer-based ion-conductive porous microneedles for improving iontophoresis [Citation38]. d) a biomimetic microneedle theranostic platform for intelligent and precise management of diabetes [Citation157]. e) a stretchable electronic facial mask for skin electroporation [Citation237]. f) Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis [Citation245]. Top: photograph of the wearable device on a subject’s wrist, integrating the sensor array and the wireless flexible printed circuit board (FPCB). Bottom: schematic of the sensor array (including glucose, lactate, potassium, sodium and temperature sensors). g) Wearable ultrasound systems. Top: a fully integrated wearable ultrasound system to monitor deep tissues in moving subjects [Citation242]. Bottom: a wearable cardiac ultrasound imager [Citation241]. h) Skin-interfaced biosensors for wireless physiological monitoring in neonatal and pediatric intensive-care units [Citation244]. Top: placement of a soft, wireless limb unit on a neonate at the ankle-to-base of the foot. Bottom: exploded-view illustration of the limb unit. IC: integrated circuit; Li-po: lithium polymer battery; LMS: low-modulus silicone; PCB: printed circuit board.
![Figure 10. Representative applications of wearable TDD devices and biosensors. a) a stretchable electronic facial mask for sonophoresis [Citation238]. b) a conformable ultrasound patch for cavitation-enhanced transdermal cosmeceutical delivery [Citation239]. c) Solid polymer-based ion-conductive porous microneedles for improving iontophoresis [Citation38]. d) a biomimetic microneedle theranostic platform for intelligent and precise management of diabetes [Citation157]. e) a stretchable electronic facial mask for skin electroporation [Citation237]. f) Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis [Citation245]. Top: photograph of the wearable device on a subject’s wrist, integrating the sensor array and the wireless flexible printed circuit board (FPCB). Bottom: schematic of the sensor array (including glucose, lactate, potassium, sodium and temperature sensors). g) Wearable ultrasound systems. Top: a fully integrated wearable ultrasound system to monitor deep tissues in moving subjects [Citation242]. Bottom: a wearable cardiac ultrasound imager [Citation241]. h) Skin-interfaced biosensors for wireless physiological monitoring in neonatal and pediatric intensive-care units [Citation244]. Top: placement of a soft, wireless limb unit on a neonate at the ankle-to-base of the foot. Bottom: exploded-view illustration of the limb unit. IC: integrated circuit; Li-po: lithium polymer battery; LMS: low-modulus silicone; PCB: printed circuit board.](/cms/asset/94dfbf99-7015-4622-9128-70b0ee726608/tsnm_a_2366210_f0010_c.jpg)
Physiological data of the body is crucial for precise medical treatment. Up to now, various wearable biosensors including wearable ultrasound systems [Citation240–243], multifunctional intensive-care biosensors [Citation244], multi-channel sweat-analysis sensors [Citation245], strain sensors [Citation246–250], pressure sensors [Citation251,Citation252], temperature sensors [Citation253,Citation254], pulse sensors [Citation254,Citation255], blood oxygen sensors [Citation256,Citation257], etc. have been developed, as shown in . For instance, Gao et al. [Citation245] proposed fully integrated wearable sensor arrays for multi-channel in situ sweat analysis, providing monitoring of glucose, lactate, potassium, sodium and temperature (). Wearable ultrasound systems are quite helpful for measuring physiological data of deep tissues [Citation240–243] (). On the basis of their previous work on a wearable cardiac ultrasound imager [Citation241], Xu et al. developed a fully integrated autonomous wearable ultrasound system [Citation242], which can monitor physiological signals (central blood pressure, heart rate, cardiac output, tidal volume, etc.) of deep tissues in real time during exercise, demonstrating huge application prospects. Rogers et al. [Citation244] developed noninvasive skin-interfaced biosensors for wireless physiological monitoring in neonatal and pediatric intensive-care units (). These sensors not only match existing clinical standards for heart rate, respiration rate, blood oxygenation and temperature, but also provides additional important features, such as tracking movements and body orientation, capturing acoustic signatures of cardiac activity, and recording tonality characteristics of crying, which demonstrates their potential to greatly enhance the quality of neonatal and pediatric critical care. Pulse sensors are usually composed of highly sensitive strain sensors [Citation246–250] or pressure sensors [Citation251,Citation252,Citation254,Citation255]. Bao et al. [Citation255] developed a bioinspired microhairy pressure sensor, enabling ultraconformability on nonflat surfaces and significant enhancement in the signal-to-noise ratio. The sensor can measure weak pulsations of internal jugular venous pulses stemming from a human neck, which is beneficial for rapid diagnosis of cardiovascular and cardiac diseases.
Table 4. Representative wearable biosensors.
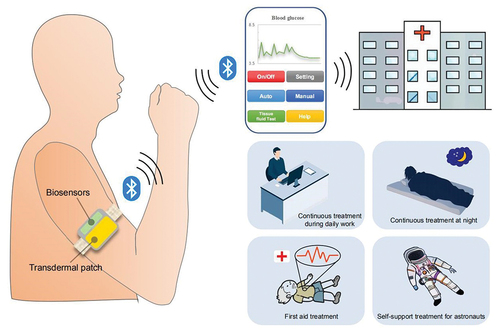
Future intelligent TDD device
As illustrated in , the future intelligent TDD device should consist of wearable transdermal patches, wearable biosensors, an integrated micro-circuit for local computing and storage, and a wireless communication micro-module. Firstly, the biosensors monitor the necessary physiological signals, after which the local micro-circuit computes an appropriate dosage or wirelessly transmits this data to a smartphone for calculation. Subsequently, the drug release from the transdermal patch is regulated through the calculation results. Doctors can oversee and intervene in the treatment process using the cloud application. This approach enables precise spatiotemporal control and feedback regulation of drug delivery, which is crucial for precision medicine. Such a drug delivery system is versatile, being applicable not only to systemic diseases like diabetes and hypertension for automatic precise administration based on feedback, but also to localized diseases such as central nervous system diseases (Parkinson’s disease, Alzheimer’s disease, etc.), superficial tumors (melanoma, papilloma, etc.), glioblastoma, breast cancer and dermatosis, ensuring automatic precise administration and high delivery efficiencies. We also illustrated several typical application scenarios of TDD, depicted in the bottom right corner of . It is suitable for continuous treatment during daily work without interruption and allows for automatic continuous treatment without the need for personnel on duty at night. Furthermore, it is appropriate for emergency treatment during the onset of cardiovascular and cerebrovascular diseases, particularly as patients may lose consciousness. At such critical moments, the drug delivery rate can be increased while disregarding potential skin damage. Additionally, it is well-suited for self-support treatment for astronauts, given the limited availability of space medical resources.
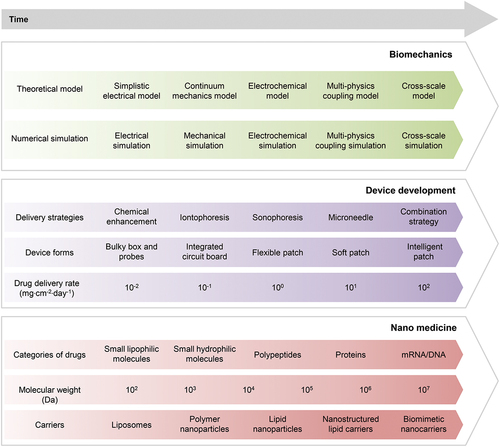
To achieve the above goals, it is necessary to overcome the three major challenges of science and technology, mainly involving biomechanics, nanomedicine, and flexible electronics. We summarize the achievements and trends of development into a technology roadmap to guide development strategies, as depicted in . In terms of biomechanics, theoretical models and numerical simulations have evolved from early circuit equivalence related to iontophoresis and electroporation to continuum mechanics models and electrochemical models. At the current stage, multi-physics coupling models are urgently needed to guide the processes of drug delivery. In the future, it will be necessary to establish multi-scale models to thoroughly understand these processes and achieve more efficient drug delivery. For the development of devices, the enhanced delivery strategy has gradually evolved from chemical enhancement to iontophoresis, sonophoresis, and microneedles, and more recently to combination strategy. The form of commercial devices has gradually evolved from bulky boxes and probes in the early days to integrated and portable circuit boards. Flexible patches, even soft patches, have been developed in the laboratory, and they will continue to evolve into intelligent patches in the future. The upper rate limit of drug delivery for typical drugs (medium molecular weight) has developed from 0.01 mg·cm−2·day−1 to 10 mg·cm−2·day−1, and in the future, it needs to reach hundreds of mg·cm−2·day−1. Finally, we summarize the technological progress and prospects in nanomedicine. Initially, only small lipophilic molecules comprised the drug category. Subsequently, small hydrophilic molecules were added to the range, followed by polypeptides and proteins, and most recently, mRNA and DNA. Transdermal delivery of mRNA and DNA is still in its early stages. Correspondingly, the molecular weight has increased from the order of 102 Da to the order of 107 Da. The design of nanocarriers is crucial for improving delivery efficiency, which has evolved from liposomes to polymer nanoparticles, nanoemulsions, lipid nanoparticles, and nanostructured lipid carriers, and most recently to biomimetic nanocarriers [Citation184] (extracellular vesicles inspired [Citation185–187], viruses inspired [Citation188], bacteria inspired [Citation189,Citation190], etc.). We hope that collaborative efforts among researchers in biomechanics, nanomedicine, and flexible electronics will drive forward relevant research and pave the way for widespread clinical applications of TDD in the near future.
Conclusion and perspective
In summary, TDD has gained clinical approval over several decades, with extensive research dedicated to novel drug and device development. The developed enhancement strategies include sonophoresis, iontophoresis, microneedles, and electroporation, and their synergistic combinations (e.g., iontophoresis & sonophoresis, and iontophoresis & microneedles). The diseases treated by TDD mainly include heart disease, hypertension, diabetes, central nervous system diseases (e.g., Parkinson’s disease, Alzheimer’s disease, depression), glioblastoma, breast cancer, superficial tumors (e.g., melanoma, papilloma, T-cell lymphoma), dermatosis, arthritis, etc. Despite notable research progress, the market adoption of TDD devices has not met anticipated levels, with oral administration and injection remaining predominant delivery methods. The bottlenecks hindering its widespread clinical application mainly lies in two aspects: 1) Current technology has not yet resolved the contradiction between drug delivery rate and tissue damage; 2) Most existing demonstration scenarios cannot fully leverage its pivotal strengths: feedback-based automatic precise administration and high delivery efficiency (especially applicable to localized diseases).
Based on these bottlenecks, we identify three major challenges in science and technology, primarily involving biomechanics, nanomedicine, and flexible electronics, and propose specific research routes to address these challenges. The most lacking and crucial aspect currently is the bio-chemo-mechanical coupling modeling and related experiments, aiming to find methods to improve drug delivery rate without damaging deep tissues and ensuring rapid recovery of skin damage. Researchers in the field of flexible electronics can develop advanced wearable TDD devices with combinations of enhancement strategies based on these methods. Another primary goal for researchers in the field of flexible electronics is to identify the most suitable application scenarios, especially prioritizing localized diseases. Focus on these application scenarios, specific technical requirements need to be refined to reverse-engineer device structures, circuits, and low-cost fabrication processes. Unfortunately, much of the current research is merely demonstrations aimed at showcasing delivery enhancement effects. For researchers in the field of nanomedicine, there is a need to develop more nanomedicines suitable for TDD, considering the differences between crossing the barrier of the skin and the barrier to target tissues from blood vessels. We anticipate that collaborative efforts among researchers in biomechanics, nanomedicine, and flexible electronics will propel relevant research and ultimately lead to widespread clinical applications of TDD in the near future.
Author contributions
S.L. and X.F. conceived the topic and proposed the structure of this review. S.L. carried out the data curation, produced the figures, and wrote the manuscript in consultation with X.F. J.W. provided outstanding technical support for the production of the figures. X.P. participated in the discussion. X.F. supervised the project.
Acknowledgments
X.F. gratefully acknowledges the support from the National Natural Science Foundation of China (Grants Nos. 12032014 and 11921002). S.L. thanks Youfu Cui for sharing his interesting experience on medication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sneader W. Drug discovery: A history. Chichester: John Wiley and Sons; 2005:1–468.
- Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–672. doi: 10.1038/nrd4363
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504
- Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–124. doi: 10.1038/nrd1304
- Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5(9):951–967. doi: 10.1038/s41551-021-00698-w
- Goodman LS, Gilman A, Hardman JG, et al. Goodman & Gilman’s the pharmacological basis of therapeutics. 9th ed. (NY): McGraw-Hill, Health Professions Division; 1996; p. xxi, 1905 p.
- Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x
- Lasagna L, Greenblatt DJ. More than skin deep: transdermal drug-delivery systems. N Engl J Med. 1986;314(25):1638–1639. doi: 10.1056/NEJM198606193142509
- Bommannan D, Menon GK, Okuyama H, et al. 2. Examination of the mechanism(s) of ultrasound-enhanced transdermal drug delivery. Pharmaceut Res. 1992;9(8):1043–1047. doi: 10.1023/A:1015806528336
- Bommannan D, Okuyama H, Stauffer P, et al. 1. The use of high-frequency ultrasound to enhance transdermal drug delivery. Pharmaceut Res. 1992;9(4):559–564. doi: 10.1023/A:1015808917491
- McElnay JC, Kennedy TA, Harland R. The influence of ultrasound on the percutaneous absorption of fluocinolone acetonide. Int J Pharm. 1987;40(1–2):105–110. doi: 10.1016/0378-5173(87)90054-8
- Mitragotri S, Blankschtein D, Langer R. Transdermal drug delivery using low-frequency sonophoresis. Pharmaceut Res. 1996;13(3):411–420. doi: 10.1023/A:1016096626810
- Mitragotri S. Sonophoresis: a 50-year journey. Drug Discovery Today. 2004;9(17):735–736. doi: 10.1016/S1359-6446(04)03209-X
- Kalia YN, Naik A, Garrison J, et al. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–658. doi: 10.1016/j.addr.2003.10.026
- Jacoby A. The treatment of pelvic inflammation by iontophoresis of acetyl beta methylcholine chloride. Am J Obstet Gynecol. 1936;31(1):93–100. doi: 10.1016/S0002-9378(36)90959-6
- Kovacs J. The iontophoresis of acetyl-beta-methylcholine chloride in the treatment of chronic arthritis and peripheral vascular disease. The Am J Med Sci. 1934;188(1):32–36. doi: 10.1097/00000441-193407000-00004
- Martin L, Ruland H, Ruland L. Studies on the local and systematic effects of acetyl beta-methylcholine administered by iontophoresis. N Engl J Med. 1937;217(6):202–205. doi: 10.1056/NEJM193708052170602
- Murdan S. Electro-responsive drug delivery from hydrogels. J Control Release. 2003;92(1–2):1–17. doi: 10.1016/S0168-3659(03)00303-1
- Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023
- Henry S, McAllister DV, Allen MG, et al. Microfabricated microneedles: A novel approach to transdermal drug delivery. J Pharmaceut sci. 1998;87:922–925.
- Lee H, Song C, Hong YS, et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv. 2017;3(3):e1601314. doi: 10.1126/sciadv.1601314
- Amani H, Shahbazi MA, D’Amico C, et al. Microneedles for painless transdermal immunotherapeutic applications. J Control Release. 2021;330:185–217. doi: 10.1016/j.jconrel.2020.12.019
- Donnelly RF, Larraneta E. Slowly dissolving intradermal microneedles. Nat Biomed Eng. 2019;3(3):169–170. doi: 10.1038/s41551-019-0369-4
- He ML, Yang GZ, Zhang SH, et al. Dissolving microneedles loaded with etonogestrel microcrystal particles for intradermal sustained delivery. J Pharmaceut sci. 2018;107(4):1037–1045. doi: 10.1016/j.xphs.2017.11.013
- Kim E, Erdos G, Huang SH, et al. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;0:102743. doi: 10.1016/j.ebiom.2020.102743
- Larrañeta E, Lutton REM, Woolfson AD, et al. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater Sci Eng R-Rep. 2016;104:1–32. doi: 10.1016/j.mser.2016.03.001
- Vander Straeten A, Sarmadi M, Daristotle JL, et al. A microneedle vaccine printer for thermostable COVID-19 mRNA vaccines. Nat Biotechnol. 2024;42:510–517. doi: 10.1038/s41587-023-01774-z
- Alexander A, Dwivedi S, Ajazuddin, et al. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164(1):26–40. doi: 10.1016/j.jconrel.2012.09.017
- Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14(2):101–114. doi: 10.1016/S0928-0987(01)00167-1
- Denet AR, Vanbever R, Préat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56(5):659–674. doi: 10.1016/j.addr.2003.10.027
- Prausnitz MR, Bose VG, Langer R, et al. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. In: Proceedings of the National Academy of Sciences of the United States of America; 1993, 90. p. 10504–10508.
- Yarmush ML, Golberg A, Sersa G, et al. Electroporation-based technologies for medicine: principles, applications, and challenges. In: Yarmush ML, editor. Annual review of biomedical engineering, Vol. 16. 2014. p. 295–320. doi: 10.1146/annurev-bioeng-071813-104622.
- Charoo NA, Rahman Z, Repka MA, et al. Electroporation: an avenue for transdermal drug delivery. Curr Drug Deliv. 2010;7(2):125–136. doi: 10.2174/156720110791011765
- Zorec B, Becker S, Rebersek M, et al. Skin electroporation for transdermal drug delivery: the influence of the order of different square wave electric pulses. Int J Pharm. 2013;457(1):214–223. doi: 10.1016/j.ijpharm.2013.09.020
- Park J, Lee H, Lim GS, et al. Enhanced transdermal drug delivery by Sonophoresis and simultaneous application of Sonophoresis and iontophoresis. AAPS Pharm Sci Tech. 2019;20(3):96. doi: 10.1208/s12249-019-1309-z
- Watanabe S, Takagi S, Ga K, et al. Enhanced transdermal drug penetration by the simultaneous application of iontophoresis and sonophoresis. J Drug Delivery Sci Technol. 2009;19(3):185–189. doi: 10.1016/S1773-2247(09)50034-2
- Donnelly RF, Singh TRR, Garland MJ, et al. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22(23):4879–4890. doi: 10.1002/adfm.201200864
- Kusama S, Sato K, Matsui Y, et al. Transdermal electroosmotic flow generated by a porous microneedle array patch. Nat Commun. 2021;12(1):658. doi: 10.1038/s41467-021-20948-4
- Lan XM, She JC, Lin DA, et al. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe cancer therapy. ACS Appl Mater Inter. 2018;10(39):33060–33069. doi: 10.1021/acsami.8b12926
- Li XF, Xu Q, Zhang P, et al. Cutaneous microenvironment responsive microneedle patch for rapid gene release to treat subdermal tumor. J Control Release. 2019;314:72–80. doi: 10.1016/j.jconrel.2019.10.016
- Li DD, Hu DD, Xu HX, et al. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials. 2021;264:120410. doi: 10.1016/j.biomaterials.2020.120410
- Ye YQ, Wang C, Zhang XD, et al. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2(17):eaan5692. doi: 10.1126/sciimmunol.aan5692
- Ammar HO, Ghorab M, El-Nahhas SA, et al. Design of a transdermal delivery system for aspirin as an antithrombotic drug. Int J Pharm. 2006;327(1–2):81–88. doi: 10.1016/j.ijpharm.2006.07.054
- Kanr M, Ita KB, Popova IE, et al. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur J Pharm Biopharm. 2014;86(2):284–291. doi: 10.1016/j.ejpb.2013.10.007
- Ahad A, Al-Jenoobi FI, Al-Mohizea AM, et al. Systemic delivery of β-blockers via transdermal route for hypertension. Saudi Pharm J. 2015;23(6):587–602. doi: 10.1016/j.jsps.2013.12.019
- Kim KJ, Hwang MJ, Shim WG, et al. Sustained drug release behavior of captopril-incorporated chitosan/carboxymethyl cellulose biomaterials for antihypertensive therapy. Int j biol macromol. 2024;255:128087. doi: 10.1016/j.ijbiomac.2023.128087
- Jin X, Zhu DD, Chen BZ, et al. Insulin delivery systems combined with microneedle technology. Adv Drug Deliv Rev. 2018;127:119–137. doi: 10.1016/j.addr.2018.03.011
- Mo R, Jiang TY, Di J, et al. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43(10):3595–3629. doi: 10.1039/c3cs60436e
- Tachibana K, Tachibana S. Transdermal delivery of insulin by ultrasonic vibration. J Pharm Pharmacol. 1991;43(4):270–271. doi: 10.1111/j.2042-7158.1991.tb06681.x
- Yu JC, Wang JQ, Zhang YQ, et al. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat Biomed Eng. 2020;4(5):499–506. doi: 10.1038/s41551-019-0508-y
- Zhang YQ, Yu JC, Kahkoska AR, et al. Advances in transdermal insulin delivery. Adv Drug Deliv Rev. 2019;139:51–70. doi: 10.1016/j.addr.2018.12.006
- Qindeel M, Ullah MH, Fakhar Ud D, et al. Recent trends, challenges and future outlook of transdermal drug delivery systems for rheumatoid arthritis therapy. J Control Release. 2020;327:595–615. doi: 10.1016/j.jconrel.2020.09.016
- Alkilani AZ, McCrudden MTC, Donnelly RF. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–470. doi: 10.3390/pharmaceutics7040438
- Tezel A, Mitragotri S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys J. 2003;85(6):3502–3512. doi: 10.1016/S0006-3495(03)74770-5
- Sun S-Y, Zhang H, Fang W, et al. Chapter Three – Bio-chemo-mechanical coupling models of soft biological materials: a review. In: Bordas SPA, editor. Advances in Applied Mechanics. Vol. 55. San Diego: Elsevier; 2022. p. 309–392.
- Chan WCW. Nanomedicine 2.0. accounts of chemical research. Acc Chem Res. 2017;50(3):627–632. doi: 10.1021/acs.accounts.6b00629
- Kim BYS, Rutka JT, Chan WCW. Current concepts: Nanomedicine. N Engl J Med. 2010;363(25):2434–2443. doi: 10.1056/NEJMra0912273
- Wagner V, Dullaart A, Bock AK, et al. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–1217. doi: 10.1038/nbt1006-1211
- Kolarsick PAJ, Kolarsick MA, Goodwin C. Anatomy and physiology of the skin. J Dermatol Nurses’ Assoc. 2011;3:203–213. doi: 10.1097/JDN.0b013e3182274a98
- Sandby-Moller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm-Venereol. 2003;83(6):410–413. doi: 10.1080/00015550310015419
- Madison KC. Barrier function of the skin: “La raison d’Être” of the epidermis. J Invest Dermatol. 2003;121(2):231–241. doi: 10.1046/j.1523-1747.2003.12359.x
- Böhling A, Bielfeldt S, Himmelmann A, et al. Comparison of the stratum corneum thickness measured in vivo with confocal raman spectroscopy and confocal reflectance microscopy. Skin Res Technol. 2014;20(1):50–57. doi: 10.1111/srt.12082
- Russell LM, Wiedersberg S, Delgado-Charro MB. The determination of stratum corneum thickness an alternative approach. Eur J Pharm Biopharm. 2008;69(3):861–870. doi: 10.1016/j.ejpb.2008.02.002
- Eckhart L, Lippens S, Tschachler E, et al. Cell death by cornification. BBA-Mol Cell Res. 2013;1833(12):3471–3480. doi: 10.1016/j.bbamcr.2013.06.010
- Goldstein AM, Abramovits W. Ceramides and the stratum corneum: structure, function, and new methods to promote repair. Int J Dermatol. 2003;42(4):256–259. doi: 10.1046/j.1365-4362.2003.01507.x
- Hamanaka S, Nishio M, Hara H, et al. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J Invest Dermatol. 2002;119(2):416–423. doi: 10.1046/j.1523-1747.2002.01836.x
- Boer M, Duchnik E, Maleszka R, et al. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postep Derm Alergol. 2016;33:1–5. doi: 10.5114/pdia.2015.48037
- McGrath JA, Eady RAJ, Pope FM. Anatomy and Organization of Human Skin. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. Oxford; Malden (MA): Blackwell Science; 2004 p. 45–128.
- Corcuff P, Bertrand C, Leveque JL. Morphometry of human epidermis in vivo by real-time confocal microscopy. Arch Dermatol Res. 1993;285(8):475–481. doi: 10.1007/BF00376820
- Limat A, Stockhammer E, Breitkreutz D, et al. Endogeneously regulated site-specific differentiation of human palmar skin keratinocytes in organotypic cocultures and in nude mouse transplants. Eur J Cell Biol. 1996;69(3):245–258.
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–947. doi: 10.1038/nri2455
- Clayton K, Vallejo AF, Davies J, et al. Langerhans cells-programmed by the epidermis. Front Immunol. 2017;8:1676. doi: 10.3389/fimmu.2017.01676
- Matsui T, Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol. 2015;27(6):269–280. doi: 10.1093/intimm/dxv013
- Abraham J, Mathew S. Merkel cells: A collective review of current concepts. Int J Appl Basic Med. 2019;9(1):9–13. doi: 10.4103/ijabmr.IJABMR_34_18
- Cichorek M, Wachulska M, Stasiewicz A, et al. Skin melanocytes: biology and development. Postep Derm Alergol. 2013;30:30–41. doi: 10.5114/pdia.2013.33376
- Shirshin EA, Gurfinkel YI, Priezzhev AV, et al. Two-photon autofluorescence lifetime imaging of human skin papillary dermis: assessment of blood capillaries and structural proteins localization. Sci Rep-UK. 2017;7(1):7. doi: 10.1038/s41598-017-01238-w
- Nguyen AV, Soulika AM. The Dynamics of the skin’s immune system. Int J Mol Sci. 2019;20(8):20. doi: 10.3390/ijms20081811
- Lovaszi M, Szegedi A, Zouboulis CC, et al. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermatoendocrinol. 2017;9(1):e1375636. doi: 10.1080/19381980.2017.1375636
- Wong R, Geyer S, Weninger W, et al. The dynamic anatomy and patterning of skin. Exp Dermatol. 2016;25(2):92–98. doi: 10.1111/exd.12832
- Ramadon D, McCrudden MTC, Courtenay AJ, et al. Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Deliv Transl Res. 2022;12(4):758–791. doi: 10.1007/s13346-021-00909-6
- Ruela ALM, Perissinato AG, Lino MED, et al. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz J Pharm Sci. 2016;52(3):527–544. doi: 10.1590/s1984-82502016000300018
- Zhang A, Jung EC, Zhu HJ, et al. Vehicle effects on human stratum corneum absorption and skin penetration. Toxicol Ind Health. 2017;33(5):416–425. doi: 10.1177/0748233716656119
- Sznitowska M, Janicki S, Williams AC. Intracellular or intercellular localization of the polar pathway of penetration across stratum corneum. J Pharmaceut sci. 1998;87(9):1109–1114. doi: 10.1021/js980018w
- N’Da DD. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules. 2014;19(12):20780–20807. doi: 10.3390/molecules191220780
- Fiserovabergerova V, Pierce JT, Droz PO. Dermal absorption potential of industrial-chemicals - criteria for skin notation. Am J Ind Med. 1990;17(5):617–635. doi: 10.1002/ajim.4700170507
- McKone TE, Howd RA. Estimating dermal uptake of nonionic organic chemicals from water and soil: I. Unified fugacity-based models for risk assessments. Risk Anal. 1992;12(4):543–557. doi: 10.1111/j.1539-6924.1992.tb00711.x
- Potts RO, Guy RH. Predicting skin permeability. Pharmaceut Res. 1992;9(5):663–669. doi: 10.1023/A:1015810312465
- Karande P, Jain A, Ergun K, et al. Design principles of chemical penetration enhancers for transdermal drug delivery. In: Proceedings of the National Academy of Sciences of the United States of America; 2005, 102. p. 4688–4693.
- Aqil M, Ahad A, Sultana V, et al. Status of terpenes as skin penetration enhancers. Drug Discovery Today. 2007;12(23–24):1061–1067. doi: 10.1016/j.drudis.2007.09.001
- Kanikkannan N, Kandimalla K, Lamba SS, et al. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr Med Chem. 2000;7(6):593–608. doi: 10.2174/0929867003374840
- Kovácik A, Kopecná M, Vávrová K. Permeation enhancers in transdermal drug delivery: benefits and limitations. Expert Opin Drug Delivery. 2020;17(2):145–156. doi: 10.1080/17425247.2020.1713087
- Moser K, Kriwet K, Naik A, et al. Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm. 2001;52(2):103–112. doi: 10.1016/S0939-6411(01)00166-7
- Pathan IB, Setty CM. Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pharm Res. 2009;8(2):173–179. doi: 10.4314/tjpr.v8i2.44527
- Williams AC, Barry BW. Skin absorption enhancers. Crit Rev Ther Drug Carrier Syst. 1992;9(3–4):305–353.
- Polat BE, Hart D, Langer R, et al. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release. 2011;152(3):330–348. doi: 10.1016/j.jconrel.2011.01.006
- Bachhav YG, Summer S, Heinrich A, et al. Effect of controlled laser microporation on drug transport kinetics into and across the skin. J Control Release. 2010;146(1):31–36. doi: 10.1016/j.jconrel.2010.05.025
- Gou S, Del Río-Sancho S, Laubach HJ, et al. YAG fractional laser ablation improves cutaneous delivery of pentoxifylline from different topical dosage forms. Int J Pharm. 2022;628:122259. doi: 10.1016/j.ijpharm.2022.122259
- Joshi D, Gala RP, Uddin MN, et al. Novel ablative laser mediated transdermal immunization for microparticulate measles vaccine. Int J Pharm. 2021;606:120882. doi: 10.1016/j.ijpharm.2021.120882
- Lee WR, Shen SC, Wang KH, et al. The effect of laser treatment on skin to enhance and control transdermal delivery of 5-fluorouracil. J Pharmaceut Sci. 2002;91(7):1613–1626. doi: 10.1002/jps.10142
- Lin CH, Aljuffali IA, Fang JY. Lasers as an approach for promoting drug delivery via skin. Expert Opin Drug Delivery. 2014;11(4):599–614. doi: 10.1517/17425247.2014.885501
- Sklar LR, Burnett CT, Waibel JS, et al. Laser assisted drug delivery: A review of an evolving technology. Lasers Surg Med. 2014;46(4):249–262. doi: 10.1002/lsm.22227
- Vora D, Kim Y, Banga AK. Development and evaluation of a heparin gel for transdermal delivery via laser-generated micropores. Ther Deliv. 2021;12(2):133–144. doi: 10.4155/tde-2020-0024
- Zhao YW, Voyer J, Li YB, et al. Laser microporation facilitates topical drug delivery: a comprehensive review about preclinical development and clinical application. Expert Opin Drug Delivery. 2023;20(1):31–54. doi: 10.1080/17425247.2023.2152002
- Kassan DG, Lynch AM, Stiller MJ. Physical enhancement of dermatologic drug delivery: iontophoresis and phonophoresis. J Am Acad Dermatol. 1996;34(4):657–666. doi: 10.1016/S0190-9622(96)80069-7
- Gangarosa LP, Hill JM. Modern Iontophoresis for local-drug delivery. Int J Pharm. 1995;123(2):159–171. doi: 10.1016/0378-5173(95)00047-M
- Vranic E. Iontophoresis: fundamentals, developments and application. Bosn J Basic Med Sci. 2003;3(3):54–58. doi: 10.17305/bjbms.2003.3530
- Zuo J, Du LN, Li M, et al. Transdermal enhancement effect and mechanism of iontophoresis for non-steroidal anti-inflammatory drugs. Int J Pharm. 2014;466(1–2):76–82. doi: 10.1016/j.ijpharm.2014.03.013
- Cázares-Delgadillo J, Ganem-Rondero A, Quintanar-Guerrero D, et al. Using transdermal iontophoresis to increase granisetron delivery across skin in vitro and in vivo: effect of experimental conditions and a comparison with other enhancement strategies. Eur J Pharm Sci. 2010;39(5):387–393. doi: 10.1016/j.ejps.2010.01.008
- Calatayud-Pascual MA, Balaguer-Fernández C, Serna-Jiménez CE, et al. Effect of iontophoresis on in vitro transdermal absorption of almotriptan. Int J Pharm. 2011;416(1):189–194. doi: 10.1016/j.ijpharm.2011.06.039
- Saluja S, Kasha PC, Paturi J, et al. A novel electronic skin patch for delivery and pharmacokinetic evaluation of donepezil following transdermal iontophoresis. Int J Pharm. 2013;453(2):395–399. doi: 10.1016/j.ijpharm.2013.05.029
- Fang JY, Huang YB, Wu PC, et al. Transdermal iontophoresis of sodium nonivamide acetate I. consideration of electrical and chemical factors. Int J Pharm. 1996;143(1):47–58. doi: 10.1016/S0378-5173(96)04681-9
- Pillai O, Borkute SD, Sivaprasad N, et al. Transdermal iontophoresis of insulin – II. Physicochemical considerations. Int J Pharm. 2003;254(2):271–280. doi: 10.1016/S0378-5173(03)00034-6
- Shin MD, Shukla S, Chung YH, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotech. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y
- Chakhalian D, Shultz RB, Miles CE, et al. Opportunities for biomaterials to address the challenges of COVID-19. J Biomed Mater Res Part A. 2020;108(10):1974–1990. doi: 10.1002/jbm.a.37059
- Mc Crudden MTC, Larrañeta E, Clark A, et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J Control Release. 2018;292:119–129. doi: 10.1016/j.jconrel.2018.11.002
- Mc Crudden MTC, Larrañeta E, Clark A, et al. Formulation, and evaluation of novel dissolving microarray patches containing rilpivirine for intravaginal delivery. Adv Healthcare Mater. 2019;8(9):1801510. doi: 10.1002/adhm.201801510
- Yavuz B, Chambre L, Harrington K, et al. Silk fibroin microneedle patches for the sustained release of levonorgestrel. ACS Appl Bio Mater. 2020;3(8):5375–5382. doi: 10.1021/acsabm.0c00671
- Li W, Tang J, Terry RN, et al. Long-acting reversible contraception by effervescent microneedle patch. Sci Adv. 2019;5(11):eaaw8145. doi: 10.1126/sciadv.aaw8145
- Li W, Terry RN, Tang J, et al. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat Biomed Eng. 2019;3(3):220–229. doi: 10.1038/s41551-018-0337-4
- Barnett SB, Ter Haar GR, Ziskin MC, et al. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med Biol. 2000;26(3):355–366. doi: 10.1016/S0301-5629(00)00204-0
- Ghanem MA, Maxwell AD, Wang YN, et al. Noninvasive acoustic manipulation of objects in a living body. In: Proceedings of the National Academy of Sciences of the United States of America; 2020, 117. p. 16848–16855.
- Brucks R, Nanavaty M, Jung D, et al. The effect of ultrasound on the in vitro penetration of ibuprofen through human-epidermis. Pharmaceut Res. 1989;6(8):697–701. doi: 10.1023/A:1015938522673
- Levy D, Kost J, Meshulam Y, et al. Effect of ultrasound on transdermal drug delivery to rats and guinea pigs. J Clin Investig. 1989;83(6):2074–2078. doi: 10.1172/JCI114119
- Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. Int J Pharm. 2002;243(1–2):1–15. doi: 10.1016/S0378-5173(02)00299-5
- Ita K. Recent progress in transdermal sonophoresis. Pharm Dev Technol. 2017;22(4):458–466. doi: 10.3109/10837450.2015.1116566
- Herwadkar A, Sachdeva V, Taylor LF, et al. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int J Pharm. 2012;423(2):289–296. doi: 10.1016/j.ijpharm.2011.11.041
- Argenziano M, Banche G, Luganini A, et al. Vancomycin-loaded nanobubbles: a new platform for controlled antibiotic delivery against methicillin-resistant staphylococcus aureus infections. Int J Pharm. 2017;523(1):176–188. doi: 10.1016/j.ijpharm.2017.03.033
- Baji S, Hegde AR, Kulkarni M, et al. Skin permeation of gemcitabine hydrochloride by passive diffusion, iontophoresis and sonophoresis: In vitro and in vivo evaluations. J Drug Delivery Sci Technol. 2018;47:49–54. doi: 10.1016/j.jddst.2018.06.019
- Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science. 1995;269(5225):850–853. doi: 10.1126/science.7638603
- Ji YW, Zheng J, Geng ZL, et al. Controllable formation of bulk perfluorohexane nanodroplets by solvent exchange. Soft Matter. 2022;18(2):425–433. doi: 10.1039/D1SM01457A
- Jin J, Yang L, Chen F, et al. Drug delivery system based on nanobubbles. Interdiscip Mater. 2022;1(4):471–494. doi: 10.1002/idm2.12050
- Lea-Banks H, Hynynen K. Sub-millimetre precision of drug delivery in the brain from ultrasound-triggered nanodroplets. J Control Release. 2021;338:731–741. doi: 10.1016/j.jconrel.2021.09.014
- Ma XT, Yao MN, Shi JY, et al. High intensity focused ultrasound-responsive and ultrastable cerasomal perfluorocarbon nanodroplets for alleviating tumor multidrug resistance and epithelial–mesenchymal transition. ACS Nano. 2020;14(11):15904–15918. doi: 10.1021/acsnano.0c07287
- Spatarelu CP, Jandhyala S, Luke GP. Dual-drug loaded ultrasound-responsive nanodroplets for on-demand combination chemotherapy. Ultrasonics. 2023;133:107056. doi: 10.1016/j.ultras.2023.107056
- Vlatakis S, Zhang WQ, Thomas S, et al. Effect of phase-change nanodroplets and ultrasound on blood–brain barrier permeability in vitro. Pharmaceutics. 2024;16(1):51. doi: 10.3390/pharmaceutics16010051
- Xiao H, Li XX, Li B, et al. Sono-promoted drug penetration and extracellular matrix modulation potentiate sonodynamic therapy of pancreatic ductal adenocarcinoma. Acta Biomaterialia. 2023;161:265–274. doi: 10.1016/j.actbio.2023.02.038
- Yin TH, Wang P, Li JG, et al. Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials. 2013;34(18):4532–4543. doi: 10.1016/j.biomaterials.2013.02.067
- Zhang WQ, Shi YH, Abd Shukor S, et al. Phase-shift nanodroplets as an emerging sonoresponsive nanomaterial for imaging and drug delivery applications. Nanoscale. 2022;14(8):2943–2965. doi: 10.1039/D1NR07882H
- Zhao YZ, Du LN, Lu CT, et al. Potential and problems in ultrasound-responsive drug delivery systems. Int J Nanomed. 2013;8:1621–1633. doi: 10.2147/IJN.S43589
- Jadoul A, Lecouturier N, Mesens J, et al. Transdermal alniditan delivery by skin electroporation. J Control Release. 1998;54(3):265–272. doi: 10.1016/S0168-3659(97)00195-8
- Wu QH, Liang WQ, Bao JL, et al. Enhanced transdermal delivery of tetracaine by electroporation. Int J Pharm. 2000;202(1–2):121–124. doi: 10.1016/S0378-5173(00)00432-4
- Vanbever R, Langers G, Montmayeur S, et al. Transdermal delivery of fentanyl: rapid onset of analgesia using skin electroporation. J Control Release. 1998;50(1–3):225–235. doi: 10.1016/S0168-3659(97)00147-8
- Denet AR, Préat V. Transdermal delivery of timolol by electroporation through human skin. J Control Release. 2003;88(2):253–262. doi: 10.1016/S0168-3659(03)00010-5
- Sen A, Daly ME, Hui SW. Transdermal insulin delivery using lipid enhanced electroporation. Biochim Biophys Acta, Biomembr. 2002;1564(1):5–8. doi: 10.1016/S0005-2736(02)00453-4
- Blagus T, Markelc B, Cemazar M, et al. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J Control Release. 2013;172(3):862–871. doi: 10.1016/j.jconrel.2013.09.030
- Escobar-Chávez JJ, Bonilla-Martínez D, Villegas-González MA, et al. The use of sonophoresis in the administration of drugs throughout the skin. J Pharm Pharm Sci. 2009;12(1):88–115. doi: 10.18433/J3C30D
- Murthy SN, Sen A, Zhao YL, et al. Temperature influences the postelectroporation permeability state of the skin. J Pharmaceut sci. 2004;93(4):908–915. doi: 10.1002/jps.20016
- Lin SZ, Li B, Lan GH, et al. Activation and synchronization of the oscillatory morphodynamics in multicellular monolayer. In: Proceedings of the National Academy of Sciences of the United States of America; 2017, 114. p. 8157–8162.
- Xue SL, Li B, Feng XQ, et al. Biochemomechanical poroelastic theory of avascular tumor growth. J Mech Phys Solids. 2016;94:409–432. doi: 10.1016/j.jmps.2016.05.011
- Xue SL, Li B, Feng XQ, et al. A non-equilibrium thermodynamic model for tumor extracellular matrix with enzymatic degradation. J Mech Phys Solids. 2017;104:32–56. doi: 10.1016/j.jmps.2017.04.002
- Xue SL, Yin SF, Li B, et al. Biochemomechanical modeling of vascular collapse in growing tumors. J Mech Phys Solids. 2018;121:463–479. doi: 10.1016/j.jmps.2018.08.009
- Yin SF, Xue SL, Li B, et al. Bio-chemo-mechanical modeling of growing biological tissues: finite element method. Int J Non Linear Mech. 2019;108:46–54. doi: 10.1016/j.ijnonlinmec.2018.10.004
- Chen X, Li Y, Guo M, et al. Polymerization force-regulated actin filament-Arp2/3 complex interaction dominates self-adaptive cell migrations. In: Proceedings of the National Academy of Sciences of the United States of America; 2023, 120. p. e2306512120.
- Lin SZ, Ye S, Xu GK, et al. Dynamic migration modes of collective cells. Biophys J. 2018;115(9):1826–1835. doi: 10.1016/j.bpj.2018.09.010
- Chizmadzhev YA, Zarnitsin VG, Weaver JC, et al. Mechanism of electroinduced ionic species transport through a multilamellar lipid system. Biophys J. 1995;68(3):749–765. doi: 10.1016/S0006-3495(95)80250-X
- Li XL, Huang XS, Mo JS, et al. A fully integrated closed-loop system based on mesoporous microneedles-iontophoresis for diabetes treatment. Adv Sci. 2021;8(16):2100827. doi: 10.1002/advs.202100827
- Yang JB, Zheng ST, Ma DY, et al. Masticatory system–inspired microneedle theranostic platform for intelligent and precise diabetic management. Sci Adv. 2022;8(50):eabo6900. doi: 10.1126/sciadv.abo6900
- Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44(10):1094–1104. doi: 10.1021/ar200105p
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861
- Cabane E, Zhang XY, Langowska K, et al. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases. 2012;7(1):9. doi: 10.1007/s13758-011-0009-3
- Casalini T, Rossi F, Castrovinci A, et al. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front Bioeng Biotechnol. 2019;7:259. doi: 10.3389/fbioe.2019.00259
- Feng SS. New-concept chemotherapy by nanoparticles of biodegradable polymers: where are we now? Nanomedicine. 2006;1(3):297–309. doi: 10.2217/17435889.1.3.297
- Jochum FD, Theato P. Temperature- and light-responsive smart polymer materials. Chem Soc Rev. 2013;42(17):7468–7483. doi: 10.1039/C2CS35191A
- Khandare J, Calderón M, Dagia NM, et al. Multifunctional dendritic polymers in nanomedicine: opportunities and challenges. Chem Soc Rev. 2012;41(7):2824–2848. doi: 10.1039/C1CS15242D
- Lian HY, Hu M, Liu CH, et al. Highly biocompatible, hollow coordination polymer nanoparticles as cisplatin carriers for efficient intracellular drug delivery. Chem Comm. 2012;48(42):5151–5153. doi: 10.1039/c2cc31708g
- Liu YT, Li K, Pan J, et al. Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials. 2010;31(2):330–338. doi: 10.1016/j.biomaterials.2009.09.036
- Zhang XD, Dong YC, Zeng XW, et al. The effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatment. Biomaterials. 2014;35(6):1932–1943. doi: 10.1016/j.biomaterials.2013.10.034
- Arvizo R, Bhattacharya R, Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin Drug Delivery. 2010;7(6):753–763. doi: 10.1517/17425241003777010
- Bhumkar DR, Joshi HM, Sastry M, et al. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24(8):1415–1426. doi: 10.1007/s11095-007-9257-9
- Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38(6):1759–1782. doi: 10.1039/b806051g
- Kumar A, Zhang X, Liang XJ. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol Adv. 2013;31(5):593–606. doi: 10.1016/j.biotechadv.2012.10.002
- Kulkarni JA, Darjuan MM, Mercer JE, et al. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano. 2018;12(5):4787–4795. doi: 10.1021/acsnano.8b01516
- Tenchov R, Bird R, Curtze AE, et al. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi: 10.1021/acsnano.1c04996
- Thi TTH, Suys EJA, Lee JS, et al. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines. 2021;9(4):359. doi: 10.3390/vaccines9040359
- Elnaggar YSR, El-Massik MA, Abdallah OY. Fabrication, appraisal, and transdermal permeation of sildenafil citrate-loaded nanostructured lipid carriers versus solid lipid nanoparticles. Int J Nanomed. 2011;6:3195–3205. doi: 10.2147/IJN.S25825
- Han YQ, Zhang Y, Li DN, et al. Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int J Nanomed. 2014;9:4107–4115. doi: 10.2147/IJN.S67770
- Rizwanullah M, Ahmad J, Amin S. Nanostructured lipid carriers: a novel platform for chemotherapeutics. Curr Drug Deliv. 2016;13(1):4–26. doi: 10.2174/1567201812666150817124133
- Shao ZY, Shao JY, Tan BX, et al. Targeted lung cancer therapy: preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int J Nanomed. 2015;10:1223–1233. doi: 10.2147/IJN.S77837
- Taratula O, Kuzmov A, Shah M, et al. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171(3):349–357. doi: 10.1016/j.jconrel.2013.04.018
- Zhao X, Tang DY, Yang T, et al. Facile preparation of biocompatible nanostructured lipid carrier with ultra-small size as a tumor-penetration delivery system. Colloids Surf B Biointerfaces. 2018;170:355–363. doi: 10.1016/j.colsurfb.2018.06.017
- Chen WH, Luo GF, Vázquez-González M, et al. Glucose-responsive metal–organic-framework nanoparticles act as “Smart” Sense-and-Treat Carriers. ACS Nano. 2018;12(8):7538–7545. doi: 10.1021/acsnano.8b03417
- He CB, Liu DM, Lin WB. Nanomedicine applications of hybrid nanomaterials built from metal–ligand coordination bonds: nanoscale metal–organic frameworks and nanoscale coordination polymers. Chem Rev. 2015;115(19):11079–11108. doi: 10.1021/acs.chemrev.5b00125
- Simon-Yarza T, Mielcarek A, Couvreur P, et al. Nanoparticles of metal-organic frameworks: on the road to in vivo efficacy in biomedicine. Adv Mater. 2018;30(37):1707365. doi: 10.1002/adma.201707365
- Chen ZW, Wang ZJ, Gu ZB, et al. Accounts of chemical research. Acc Chem Res. 2019;52(5):1255–1264. doi: 10.1021/acs.accounts.9b00079
- Palivan CG, Goers R, Najer A, et al. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem Soc Rev. 2016;45(2):377–411. doi: 10.1039/C5CS00569H
- Tai WY, Mo R, Di J, et al. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules. 2014;15(10):3495–3502. doi: 10.1021/bm500364a
- Yu JC, Zhang YQ, Ye YQ, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. In: Proceedings of the National Academy of Sciences of the United States of America; 2015, 112. p. 8260–8265.
- Segel M, Lash B, Song JW, et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science. 2021;373(6557):882–889. doi: 10.1126/science.abg6155
- Kreitz J, Friedrich MJ, Guru A, et al. Programmable protein delivery with a bacterial contractile injection system. Nature. 2023;616(7956):357–364. doi: 10.1038/s41586-023-05870-7
- Shen C, Li YC, Zeng ZA, et al. Systemic administration with bacteria-inspired nanosystems for targeted oncolytic therapy and antitumor immunomodulation. ACS Nano. 2023;17(24):25638–25655. doi: 10.1021/acsnano.3c10302
- Deserno M. Elastic deformation of a fluid membrane upon colloid binding. Phys Rev E. 2004;69(3):031903. doi: 10.1103/PhysRevE.69.031903
- Gao HJ, Shi WD, Freund LB. Mechanics of receptor-mediated endocytosis. In: Proceedings of the National Academy of Sciences of the United States of America; 2005, 102. p. 9469–9474.
- Yi X, Shi XH, Gao HJ. Cellular uptake of elastic nanoparticles. Phys Rev Lett. 2011;107(9):098101. doi: 10.1103/PhysRevLett.107.098101
- Wang JL, Yao HM, Shi XH. Cooperative entry of nanoparticles into the cell. J Mech Phys Solids. 2014;73:151–165. doi: 10.1016/j.jmps.2014.09.006
- Yi X, Gao HJ. Incorporation of soft particles into lipid vesicles: effects of particle size and elasticity. Langmuir. 2016;32(49):13252–13260. doi: 10.1021/acs.langmuir.6b03184
- Garikipati K, Arruda EM, Grosh K, et al. A continuum treatment of growth in biological tissue: the coupling of mass transport and mechanics. J Mech Phys Solids. 2004;52(7):1595–1625. doi: 10.1016/j.jmps.2004.01.004
- Alt S, Ganguly P, Salbreux G. Vertex models: from cell mechanics to tissue morphogenesis. Philos Trans R Soc London, Ser B. 2017;372(1720):20150520. doi: 10.1098/rstb.2015.0520
- Gao Y, Xue SL, Meng QH, et al. Multiscale fracture mechanics model for the dorsal closure in Drosophila embryogenesis. J Mech Phys Solids. 2019;127:154–166. doi: 10.1016/j.jmps.2019.03.012
- Bellomo N, Bellouquid A, Nieto J, et al. Multiscale biological tissue models and flux-limited chemotaxis for multicellular growing systems. Math Models & Methods In Appl Sci. 2010;20(7):1179–1207. doi: 10.1142/S0218202510004568
- Wagner JG. History of pharmacokinetics. Pharmacol Therapeut. 1981;12(3):537–562. doi: 10.1016/0163-7258(81)90097-8
- Teorell T. Kinetics of distribution of substances administered to the body I the extravascular modes of administration. Archives Int De Pharmacodyn Et De Ther. 1937;57:205–225.
- Teorell T. Kinetics of distribution of substances administered to the body II the intravascular modes of administration. Archives Int De Pharmacodyn Et De Ther. 1937;57:226–240.
- Cutler DJ. Theory of the mean absorption time, an adjunct to conventional bioavailability studies. J Pharm Pharmacol. 1978;30(1):476–478. doi: 10.1111/j.2042-7158.1978.tb13296.x
- Perl W, Samuel P. Input-output analysis for total input rate and total traced mass of body cholesterol in man. Circ Res. 1969;25(2):191–199. doi: 10.1161/01.RES.25.2.191
- Riegelman S, Collier P. The application of statistical moment theory to the evaluation ofin vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980;8(5):509–534. doi: 10.1007/BF01059549
- Bellman R, Jacquez JA, Kalaba R. Some mathematical aspects of chemotherapy: I. One-organ models. Bull Math Biophys. 1960;22(2):181–198. doi: 10.1007/BF02478005
- Himmelstein KJ, Lutz RJ. A review of the applications of physiologically based pharmacokinetic modeling. J Pharmacokinet Biopharm. 1979;7(2):127–145. doi: 10.1007/BF01059734
- Widmark E, Tandberg J. Uber die bedingungen fur die akkumulation indifferenter narkoliken theoretische bereckerunger. Biochem Z. 1924;147:358–369.
- Michaelis L, Menten ML. Die kinetik der invertinwirkung. Biochem Z. 1913;49:352.
- Hedaya MA. Basic pharmacokinetics. 2nd ed. Boca Raton: Taylor & Francis/CRC Press; 2012; p xxxiii, 561 p.
- Poon W, Kingston BR, Ouyang B, et al. A framework for designing delivery systems. Nat Nanotech. 2020;15(10):819–829. doi: 10.1038/s41565-020-0759-5
- Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28. doi: 10.1016/j.jconrel.2014.03.053
- Ye YQ, Yu JC, Wen D, et al. Polymeric microneedles for transdermal protein delivery. Adv Drug Deliv Rev. 2018;127:106–118. doi: 10.1016/j.addr.2018.01.015
- Li W, Tang J, Terry RN, et al. Long-acting reversible contraception by effervescent microneedle patch. Sci Adv. 2019;5(11):5. doi: 10.1126/sciadv.aaw8145
- Bali NR, Salve PS. Selegiline nanoparticle embedded transdermal film: an alternative approach for brain targeting in Parkinson’s disease. J Drug Delivery Sci Technol. 2019;54:101299. doi: 10.1016/j.jddst.2019.101299
- Kim JY, Han MR, Kim YH, et al. Tip-loaded dissolving microneedles for transdermal delivery of donepezil hydrochloride for treatment of Alzheimer’s disease. Eur J Pharm Biopharm. 2016;105:148–155. doi: 10.1016/j.ejpb.2016.06.006
- Zhao ZQ, Liang L, Hu LF, et al. Subcutaneous implantable microneedle system for the treatment of Alzheimer’s disease by delivering donepezil. Biomacromolecules. 2022;23(12):5330–5339. doi: 10.1021/acs.biomac.2c01155
- Courtenay AJ, McAlister E, McCrudden MTC, et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J Control Release. 2020;322:177–186. doi: 10.1016/j.jconrel.2020.03.026
- Kumar R, Sinha VR, Dahiya L, et al. Transdermal delivery of duloxetine-sulfobutylether-β-cyclodextrin complex for effective management of depression. Int J Pharm. 2021;594:120129. doi: 10.1016/j.ijpharm.2020.120129
- Zhao PF, Le ZC, Liu LX, et al. Therapeutic delivery to the brain via the lymphatic vasculature. Nano Lett. 2020;20(7):5415–5420. doi: 10.1021/acs.nanolett.0c01806
- Bhatnagar S, Bankar NG, Kulkarni MV, et al. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model. Int J Pharm. 2019;556:263–275. doi: 10.1016/j.ijpharm.2018.12.022
- El Moussaoui S, Fernández-Campos F, Alonso C, et al. Topical mucoadhesive alginate-based hydrogel loading ketorolac for pain management after pharmacotherapy, ablation, or surgical removal in condyloma acuminata. Gels. 2021;7(1):8. doi: 10.3390/gels7010008
- Sahu P, Kashaw SK, Sau S, et al. Discovering pH triggered charge rebound surface modulated topical nanotherapy against aggressive skin papilloma. Mater Sci Eng C-Mater Biol Appl. 2020;107:110263. doi: 10.1016/j.msec.2019.110263
- Dai WW, Wang CH, Yu CH, et al. Preparation of a mixed-matrix hydrogel of vorinostat for topical administration on the rats as experimental model. Eur J Pharm Sci. 2015;78:255–263. doi: 10.1016/j.ejps.2015.07.019
- Dong WJ, Ye J, Wang WJ, et al. Self-assembled lecithin/chitosan nanoparticles based on phospholipid complex: a feasible strategy to improve entrapment efficiency and transdermal delivery of poorly lipophilic drug. Int J Nanomed 2020, 15, 5629–5643. doi: 10.2147/IJN.S261162
- Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41. doi: 10.1038/s41568-019-0205-x
- Ahmed KS, Shan XT, Mao J, et al. Derma roller® microneedles-mediated transdermal delivery of doxorubicin and celecoxib co-loaded liposomes for enhancing the anticancer effect. Mater Sci Eng C-Mater Biol Appl. 2019;99:1448–1458. doi: 10.1016/j.msec.2019.02.095
- Huang SH, Liu HL, Huang SS, et al. Dextran methacrylate hydrogel microneedles loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma. Carbohydr Polym. 2020;246:116650. doi: 10.1016/j.carbpol.2020.116650
- Ruan WY, Zhai YH, Yu KY, et al. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int J Pharm. 2018;553(1–2):298–309. doi: 10.1016/j.ijpharm.2018.10.043
- Pan JT, Ruan WY, Qin MY, et al. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci Rep-UK. 2018;8(1):1117. doi: 10.1038/s41598-018-19463-2
- Zhang YJ, Davis DA, AboulFotouh K, et al. Novel formulations and drug delivery systems to administer biological solids. Adv Drug Deliv Rev. 2021;172:183–210. doi: 10.1016/j.addr.2021.02.011
- Pearton M, Saller V, Coulman SA, et al. Microneedle delivery of plasmid DNA to living human skin: formulation coating, skin insertion and gene expression. J Control Release. 2012;160(3):561–569. doi: 10.1016/j.jconrel.2012.04.005
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017
- Chen GJ, Chen ZT, Wen D, et al. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. In: Proceedings of the National Academy of Sciences of the United States of America; 2020, 117. p. 3687–3692.
- Kim NW, Kim SY, Lee JE, et al. Enhanced cancer vaccination by in situ nanomicelle-generating dissolving microneedles. ACS Nano. 2018;12(10):9702–9713. doi: 10.1021/acsnano.8b04146
- Chen MC, Lai KY, Ling MH, et al. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomaterialia. 2018;65:66–75. doi: 10.1016/j.actbio.2017.11.004
- Xu XK, Guo L, Liu H, et al. Stretchable electronic facial masks for skin electroporation. Adv Funct Mater. 2024;34:2311144. doi: 10.1002/adfm.202311144
- Li S, Xu JW, Li R, et al. Stretchable electronic facial masks for sonophoresis. ACS Nano. 2022;16(4):5961–5974. doi: 10.1021/acsnano.1c11181
- Yu CC, Shah AS, Amiri N, et al. A Conformable ultrasound patch for cavitation-enhanced transdermal cosmeceutical delivery. Adv Mater. 2023;35(23):2300066. doi: 10.1002/adma.202300066
- Hu HJ, Zhu X, Wang CH, et al. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci Adv. 2018;4(3):eaar3979. doi: 10.1126/sciadv.aar3979
- Hu HJ, Huang H, Li MH, et al. A wearable cardiac ultrasound imager. Nature. 2023;613(7945):667–675. doi: 10.1038/s41586-022-05498-z
- Lin MY, Zhang ZY, Gao XX, et al. A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects. Nat Biotechnol. 2024;42(3):448–457. doi: 10.1038/s41587-023-01800-0
- Wang CH, Chen XY, Wang L, et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science. 2022;377(6605):517–523. doi: 10.1126/science.abo2542
- Chung HU, Rwei AY, Hourlier-Fargette A, et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nature Med. 2020;26(3):418–429. doi: 10.1038/s41591-020-0792-9
- Gao W, Emaminejad S, Nyein HYY, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529(7587):509–514. doi: 10.1038/nature16521
- Li S, Liu GD, Li R, et al. Contact-resistance-free stretchable strain sensors with high repeatability and linearity. ACS Nano. 2022;16(1):541–553. doi: 10.1021/acsnano.1c07645
- Bai HD, Li S, Barreiros J, et al. Stretchable distributed fiber-optic sensors. Science. 2020;370(6518):848–852. doi: 10.1126/science.aba5504
- Mickle AD, Won SM, Noh KN, et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature. 2019;565(7739):361–365. doi: 10.1038/s41586-018-0823-6
- Niu SM, Matsuhisa N, Beker L, et al. A Wireless body area sensor network based on stretchable passive tags. Nat Electron. 2019;2(8):361–368. doi: 10.1038/s41928-019-0286-2
- Zhou ZH, Chen K, Li XS, et al. Sign-to-speech translation using machine-learning-assisted stretchable sensor arrays. Nat Electron. 2020;3(9):571–578. doi: 10.1038/s41928-020-0428-6
- Bai NN, Wang L, Wang Q, et al. Graded intrafillable architecture-based iontronic pressure sensor with ultra-broad-range high sensitivity. Nat Commun. 2020;11(1):209. doi: 10.1038/s41467-019-14054-9
- Mannsfeld SCB, Tee BCK, Stoltenberg RM, et al. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat Mater. 2010;9(10):859–864. doi: 10.1038/nmat2834
- Matsuhisa N, Kaltenbrunner M, Yokota T, et al. Printable elastic conductors with a high conductivity for electronic textile applications. Nat Commun. 2015;6(1):7461. doi: 10.1038/ncomms8461
- Zhang F, Zang Y, Huang D, et al. Flexible and self-powered temperature–pressure dual-parameter sensors using microstructure-frame-supported organic thermoelectric materials. Nat Commun. 2015;6(1):8356. doi: 10.1038/ncomms9356
- Pang C, Koo JH, Nguyen A, et al. Highly skin-conformal microhairy sensor for pulse signal amplification. Adv Mater. 2015;27(4):634–640. doi: 10.1002/adma.201403807
- Khan Y, Han D, Pierre A, et al. A flexible organic reflectance oximeter array. In: Proceedings of the National Academy of Sciences of the United States of America; 2018, 115. p. E11015–E11024.
- Schwartz G, Tee BC, Mei J, et al. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Flexible polymer transistors with high pressure sensitivity for applications in electron skin and health monitoring. Nat Commun. 2013;4(1):1859. doi: 10.1038/ncomms2832