Abstract
The antioxidant activity of aqueous extract, ethanol extract, and hydroethanolic pod extract of a Cameroonian spice Dichrostachys glomerata (D. glomerata) was investigated. When compared with the two other extracts, the aqueous extract exhibited the lowest phenolic content, AEABTS (Antiradical Efficiency) and AEDPPH values whereas the ethanol extract had the highest phenolic content, AEABTS and AEDPPH values. The DPPH (α, α-diphenyl-β-picrylhydrazyl) radical and ABTS (2, 2′ -azinobis (3-ethylbenzothiazoline-6-sulfonic acid) cation radical scavenging activities were proven and correlated with the reductive potential and phenolic content of the extracts with r 2 greater than 0.9. All extracts had effective, superoxide anion radical, hydroxyl radical and nitric oxide scavenging activity, at all tested concentrations in a concentration-dependent manner. Each extract showed a concentration-dependent effect on chelating activity and α-linoleic acid oxidation inhibition activity. When compared with the controls, each extract significantly decreased malondialdehyde and lipid hydroperoxides formation in low-density lipoprotein (LDL). The hydroethanolic extract exhibited the highest inhibition of LDL oxidation. These results suggest that pods from D. glomerata can be good source of natural antioxidants.
Se estudió la actividad antioxidante de los extractos acuoso, etanólico e hidroetanólico de vaina de una especia camerunense Dichrostacrys glomerata (D. glomerata). Comparado con los otros dos, el extracto acuoso mostró el contenido en fenólicos más bajo, valores AEABTS (Eficiencia de Antirradical) y AEDPPH, mientras el extracto de etanol tuvo el contenido en fenólicos más alto, valores AEABTS y AEDPPH. Los poderes de captación del radical DPPH (α, α-diphenyl-β-picrylhydrazyl) y del catión radical ABTS (2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) fueron probados y correlacionados con el potencial reductivo y contenido en fenólico de los extractos con r 2 mayor de 0.9. Todos los extractos tuvieron eficacia, poder de captación de radical anión superóxido, radical hidroxilo y óxido nítrico, en todas las concentraciones probadas en una manera de dependiente de la concentración. Cada extracto mostró un efecto dependiente de la concentración tanto en la actividad quelante como en la actividad de inhibición de oxidación de ácido α-linoleico. Comparado con controles, cada extracto redujo significativamente la formación de malondialdehído y hiperóxido lípido en lipoproteína de baja densidad (LDL). El extracto hidroetanólico mostró la mayor inhibición de oxidación LDL. Estos resultados sugieren que las vainas de D. glomerata pueden ser una buena fuente de antioxidantes naturales.
Introduction
Strikingly, there are some common risk factors and pathophysiological conditions that affect most diseases grouped under the category of modern chronic diseases: cardiovascular diseases, hypertension, diabetes mellitus, and some forms of cancer. Oxidative stress is a central risk factor for chronic diseases. Oxidative stress, the consequence of an imbalance of pro-oxidants and antioxidants in the organism, is increasingly being recognized as a key phenomenon in chronic diseases (Moskovitz, Yim, & Chock, Citation2002). Free-radicals in the form of reactive oxygen species (ROS) and reactive nitrogen species are generated continuously in the body because of metabolism and disease (Yeum, Aldini, Chung, Krinsky, & Russell, Citation2003). Their action is opposed by a balanced system of antioxidant defenses, including antioxidant compounds (C and E vitamins, carotene, uric acid) and enzymes (catalase, superoxide dismutase, glutathione peroxidase/reductase); yet, these defense systems are not sufficient in critical situations (oxidative stress, contamination, UV exposure, etc.) where the production of free radicals significantly increases (Mondon, Leclercq, & Lintner, Citation1999). However, this natural antioxidant mechanism can be inefficient, and hence dietary intake of antioxidant compounds is important. Recent reports indicated that there is an inverse relationship between dietary intake of antioxidant rich foods and the incidence of human diseases (Pérez-Jiménez et al., Citation2008).
The antioxidant mechanisms of action include the scavenging reactive oxygen and the nitrogen free radical species, decreasing the localized oxygen concentration thereby reducing the molecular oxygen's oxidation potential, metabolizing lipid peroxides to non-radical products and the chelating metal ions to prevent the generation of free radicals. In this way, antioxidants limit free radicals from oxidizing low-density lipoprotein (LDL) cholesterol. Oxidized LDL may increase the risk of atherosclerosis, promoting platelet adhesion, which can lead to thrombosis thereby increasing the risk of heart disease or stroke, damaging the cell's DNA, which may lead to cancer, blocking the normal endothelial cell function and vasodilatation in response to nitric oxide (NO), a potential mechanism for heart disease and cancer, triggering inflammation and impairing immune function (Lakenbrink, Lapczynski, Maiwald, & Engelhardt, Citation2000).
Nowadays, there is a growing interest in natural antioxidants present in medicinal and dietary plants that might help attenuate oxidative damage. These natural antioxidants can protect the human body from free radicals and ROS related effects and retard the progress of many chronic diseases as well as lipid oxidative rancidity in foods (Silva, Souza, Rogez, Rees, & Larondelle, Citation2007). Not only for their scavenging properties, the interest in antioxidants has been increasing because of their high capacity in scavenging free radicals related to various diseases (Silva et al., Citation2007). Numerous studies have shown the strong antioxidant activity and the powerful scavenger activity against free radicals of aromatic or spicy and medicinal plants (Kumaran & Karunakaran, Citation2007; Vellosa et al., Citation2006). The use of these plant materials as natural antioxidants for food, cosmetics and other applications becomes necessary not only for food safety issues, but also because they are natural, non-synthetic products and are more readily acceptable to the consumers.
Spices are natural food additives that contribute immensely to the taste and flavor of our foods. These esoteric food adjuncts have been in use for thousands of years. Spices have also been recognized to possess several medicinal properties and have been effectively used in the indigenous systems of medicine in India and also in other countries (Srinivasan, Citation2005). Many spices possess potent antioxidant activity and examples include piper species (Agbor, Oben, Ngogang, Xinxing, & Vinson, Citation2007). Herbs are also found to be potent sources of natural antioxidants as well as retarding lipid oxidative rancidity in foods. Dichrostachys glomerata (D. glomerata) (Forssk.) Chiov. (Leguminosae-mimosoideae) is a deciduous tree of closed secondary jungle and fringing forest from Senegal to Western Cameroon, and extending across Africa to Sudan, Uganda and Zaïre and also found in Asia and Australia (Mbuya, Citation1994). Fruit and seeds that grow on D. glomerata are edible. Fruits dry dehiscent constricted pods which are commonly used as spices in a traditional soup of the western provinces of Cameroon called “Nah po”, eaten along with taro (Tchiégang & Mbougueng, Citation2005). In medicine, the bark is used to alleviate headache, toothache, dysentery and elephantiasis, and root infusions are consumed to treat leprosy, syphilis, coughs and, as an anthelmintic, purgative and strong diuretic. The leaves are particularly useful and can be eaten to treat epilepsy and can also be taken as a diuretic and laxative, and its powder can be used in the massage of fractures (Mbuya, Citation1994). Even though D. glomerata pods are increasingly consumed as human food, the beneficial effects of their bioactive compounds remain unexplored. Therefore, the current study was aimed at evaluating their phenolic constituents, antioxidant potential, free radical scavenging capacity of aqueous, hydroethanolic, and ethanol extracts of D. glomerata pods.
Methods and materials
Sample preparation and determination of polyphenol concentrations
Pods of D. glomerata were purchased from a local market in Bafoussam Cameroon. Dried D. glomerata pods were grounded and extracted in our laboratory using deionized water, hydroalcoholic solvent (50%), and ethanol (in 1:10 ratio). The extracts were filtered through Whatman No. 2 filter paper (Whatman International Limited, Kent, England) using a funnel and concentrated to about 10% of the original volume by a rotary evaporator before drying in the oven at 50°C. The amount of total phenolic content in the D. glomerata pod extracts was determined according to the procedure of Singleton and Rossi (Citation1965) with some modifications, using 1 mL of Folin-Ciocalteu's phenol reagent 0.2 N and 30 μL to develop a pigment whose absorbance was determined at 750 nm. The results were expressed as catechin equivalent.
Ferric reducing antioxidant power
The ferric reducing activity of the plant extracts was estimated based on the Ferric Reducing Ability “as a measure of antioxidant power”. Ferric reducing antioxidant power (FRAP) assay was developed by Benzie and Strain (Citation1996). The solutions for this assay consisted of 300 mmol/L acetate buffer pH 3.6, 10 mmol/L TPTZ (2, 4, 6-tripyridyl-s-triazine) in 400 mmol/L of HCl and 20 mmol/L Ferric chloride. The reagent for this assay was prepared fresh by mixing 10 parts of acetate buffer with one part of TPTZ solution and one part of ferric chloride. The assay was performed as follows: 2000 μL of freshly prepared FRAP reagent was mixed with 75 μL of sample, water, ethanol, or hydroalcoholic solvent as appropriate for the blank reagent. The absorbance was read at 593 nm using Spectronic Genesys 20 (Thermo Electron Corporation) after 30 min of incubation. The results were expressed as catechin and ascorbic acid equivalent.
Free radical scavenging activity on DPPH
The antioxidant activity of D. glomerata seed extracts, catechin and ascorbic acid were measured in terms of hydrogen donating or radical scavenging ability, using the stable radical, α, α-diphenyl-β-picrylhydrazyl (DPPH) method (Brand-Williams, Cuvelier, & Berset, Citation1995) modified by Sánchez-Moreno, Larrauri, and Saura-Calixto (Citation1998) to determine the kinetic parameters. A solution (20 μL) of the sample extracts at various concentrations was added to 1000 μL of DPPH solution. The decrease in absorbance at 517 nm was determined continuously at every minute with a Spectronic Genesys 20 (Thermo Electron Corporation) spectrophotometer until the reaction reached a plateau. The percentage of DPPH inhibition was calculated as follows. DPPH Scavenging Effect (%) = ((A 0−A 1)/ A 0)100), where A 0 was the absorbance of the DPPH solution control and A 1 was the absorbance in the presence of the sample DPPH assay at different time intervals until the steady-state. The parameter EC50 (Efficient Concentration 50), which reflects 50% depletion of the free radical, is expressed in terms of grams of dry extract/g of DPPH. It was calculated using the percentage inhibition of all concentrations at steady state, and then the linear or logarithmic regression was applied. The time taken to reach the steady state at EC50 (tEC50) and the antiradical efficiency (AE = 1/EC50tEC50) were also determined.
ABTS decolorization assay
The spectrophotometric analysis of 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid, ABTS•+ radical cation scavenging activity was determined according to the method described by Re et al. (Citation1999) with some modifications. The ABTS•+ cation radical was produced by the reaction between 7 mM ABTS in water (10 mL) and 4.9 mM potassium permanganate (10 mL), stored in the dark at room temperature for 12 h. Before usage, the ABTS•+ solution was diluted (about 1:10 v/v) with phosphate buffer saline (0.1 M, pH 7.4, NaCl 150 mM) to get an absorbance of 2.000 ± 0.025 at 734 nm. Then, 1 mL of ABTS•+ solution was added to 20 μL solution of different concentrations of the plant extracts (10–20 mg/mL). ABTS assays were expressed kinetically as described by Pérez-Jiménez and Saura-Calixto (Citation2008) who modified the original method so as to determine kinetic parameters; the percentage inhibition at 734 nm was calculated for each concentration relative to a blank absorbance (ethanol or water) at different time intervals until the reaction reached a plateau. EC50, tEC50 and AE were calculated as in the DPPH assay.
Antioxidant activity in a linoleic acid system
The antioxidant activity of extracts from D. glomerata was determined by the ferric thiocyanate method (Mitsuda, Yuasumoto, & Iwami, Citation1996). Each sample (25–100 μg/mL) in 0.5 mL of distilled water or ethanol and 2 mL phosphate buffer (0.04 M, pH 7.0), was mixed with linoleic acid emulsion (2.5 mL 0.04 M, pH 7.0) in a glass flask and stood, in darkness, at 37°C, to accelerate the oxidation. Therefore, 50 mL of linoleic acid emulsion contained 175 μg Tween-20, 155 μL linoleic acid, and 0.04 M potassium phosphate buffer (pH 7.0). On the other hand, 5 mL of solution composed of 2.5 mL of linoleic acid emulsion and 2.5 mL, 0.04 M potassium phosphate buffer (pH 7.0) without the samples served as control. Aliquots of 0.1 mL were taken at several intervals during incubation. The degree of oxidation was measured according to the thiocyanate method by sequentially adding ethanol (4.7 mL, 75%), ammonium thiocyanate (0.1 mL, 30%), sample solution (0.1 mL), and ferrous chloride (0.1 mL, 0.02 M in 3.5% HCl). After the mixture had rested for 3 min, the peroxide value was determined by monitoring absorbance at 500 nm using a spectrophotometer (Spectronic Genesys 20). During the linoleic acid oxidation, peroxides are formed and that leads to oxidation of Fe2+ to Fe3+. The latter ions form a complex with thiocyanate and this complex has a maximum absorbance at 500 nm. The degree of oxidation was measured for every 12 h until a day after the absorbance of the control reached its maximum. The lipid peroxidation inhibition (LPI) percentage was calculated as:
All analyses were run in triplicates; the absorbance caused by the extract was deducted and mean values were calculated.
Superoxide anion radical (O−2) scavenging activity
The superoxide radical scavenging activity was measured based on the method by Siddhuraju and Becker (Citation2007) with some modifications. The reaction mixture contained, 1 mL of each of the following solutions: 150 μM nitroblue tetrazolium (NBT), 60 μM phenazine methosulfate (PMS), 468 μM NADH, prepared in 0.1 M phosphate buffer pH 7.4 and different concentrations of the plant extracts (0–1000 μg/mL), added in that sequence. The mixture was incubated in the dark for 10 min at 25°C and the absorbance was later read at 560 nm. Decreased absorbance of reaction mixture indicates increased superoxide anion scavenging activity. Catechin was used as the positive control and the results were expressed as percentage inhibition of the superoxide radical. All determinations were performed in triplicate and the absorbance caused by the extract was removed. The scavenging activity on superoxide anion (SASA) radicals was expressed as:
Nitric oxide radical scavenging assay
The interaction of extracts of D. glomerata with NO was assessed by the nitrite detection method as described by Sreejayan and Rao (Citation1997). NO was generated from sodium nitroprusside previously bubbled with nitrogen and measured by the Greiss reaction. Sodium nitroprusside (0.25 mL, 10 mM) in phosphate-buffered saline (PBS) was mixed with 0.25 mL of different concentrations (50–300 μg/mL) of extracts dissolved in the suitable solvent system and incubated at 30°C in the dark for 180 min. The control was run as above but the sample was replaced with the same amount of water. After the incubation period, 0.25 mL of Griess reagent A (1% sulfanilamide in 5% phosphoric acid) was added, and kept at 30°C for 10 min. After incubation, 0.25 mL of Griess reagent B (0.1% N-1-naphthylethylene diamine dihydrochloride) was added mixed and incubated at 30°C for 20 min. The absorbance of the chromophore formed during the diazotization of nitrite with sulfanilamide and subsequent coupling with napthylethylenediamine was read at 546 nm. The same reaction mixture without the extract but with an equivalent quantity of distilled water served as a control. Catechin was used as reference standard. All analyses were run in a triplicate and the absorbance caused by the extract was removed. The percentage of inhibition was calculated as was done with superoxide anion radical scavenging activity.
Scavenging of hydroxyl radical
The hydroxyl radical (OH) scavenging activity of extracts was determined using a modification of the method of Halliwell, Gutteridge, and Aruoma (Citation1987). The reaction mixture consisted of FeCl3 (Ferric chloride) (300 μM), EDTA (780 μM), 2-deoxiribose (2.8 mM), ascorbic acid (300 μM), and H2O2 (4 mM) in potassium phosphate buffer (20 mM, pH 7.4). The final reaction volume (1 mL), which included different concentrations of extracts (250–1000 μg/mL), was incubated at 37°C for 1 h. After incubation, 1 mL of trichloroacetic acid (2.8% wt/vol) and 1 mL of thiobarbituric acid (TBA) (1% wt/vol) were added and further incubated at 100°C for 20 min. The reaction mixture was then allowed to cool at room temperature, and the absorbance read at 532 nm. The reaction mixture not containing the test sample was used as control. All determinations were performed in triplicate and the absorbance due to the extract was removed. The Hydroxyl radical scavenging activity (HRSA) was expressed as:
Ferrous metal ion chelating activity
The chelation of ferrous ions by D. glomerata and standard molecule was estimated by the method of Dinis, Madeira, and Almeida (Citation1994). Briefly, extracts (250–2000 μg/mL) in 0.5 mL were added to a solution of 2 mM FeCl2 (Ferrous chloride) (0.05 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL). Then, the mixture was shaken vigorously and left at room temperature for 10 min. Absorbance of the solution was measured spectrophotometricaly at 562 nm. All determinations were performed in triplicate and the absorbance due to the extract was removed. The percentage inhibition of ferrozine–Fe2+ complex formation was calculated by using the formula given below:
In vitro copper-induced oxidation of human low-density lipoprotein assay
LDL preparation and oxidation
Low-density lipoprotein (LDL) was obtained from our laboratory. The plasma was collected from a patient with hypercholesterolemia and LDL isolated according to the dual precipitation procedure based on the method developed by Garcia-Parra, Mejiade, Gardia, and Weigandt, (1977) as modified by Nerurkar and Taskar (Citation1985). Briefly, 3 mL of plasma was diluted to 6 mL with tris buffer (pH 7.0 (0.05 M) in 0.15 M NaCl). The sample was centrifuged for 1 h at 20,000g in refrigerated conditions. The supernatant 1 mL was removed for chylomicrons. Supernatant (4 mL) were taken for separation of Lp (a), VLDL and LDL by progressively raising the concentration to 20, 50, 60%, saturation of ammonium sulfate followed by centrifugation. The crude LDL precipitate fraction obtained at 60% saturation was dissolved in 2 mL Tris-HCl buffer pH 7.0 (0.05 M) in 0.15 M NaCl. Then, the 200 μL of a solution containing 14 mM sodium phosphotungstate and 2 mM magnesium chloride in distilled water was added and centrifuged to obtain a precipitate of pure LDL. The precipitate was dissolved in 0.15 M sodium chloride solution, made alkaline with sodium carbonate (10% w/v) and dialyzed in the dark for 24 h at 4°C against three changes of 1 L each of 0.01 M PBS 0.15 M NaCl, pH 7.4 before oxidation experiments. Protein was measured by the method of Lowry, Rosebrough, Farr, and Randall (Citation1951), using bovine serum albumin as standard and the final solution was adjusted to 700 μg protein/mL with 0.01 M, pH 7.4 PBS. Dialyzed LDL (70 μg of protein/mL) was oxidized in PBS at 37°C for 6 h in the presence of 25 μM CuSO4. The oxidation of LDL was performed in the presence and in the absence of different concentrations of extracts (0.1 to 1 μM of catechin equivalent) in a final volume of 400 μL. Ten microliters of 10 mM EDTA was added to negative control tube and refrigerated. The extract was replaced by equivalent amount of PBS in the control.
Assay of lipid peroxidation product as thiobarbituric acid reactive substances
After incubation, 10 μL of 10 mM EDTA was added to the control and test tubes to stop the reaction. Then, 1 mL of 10% trichloroacetic and 1 mL of clear saturation solution of TBA were added simultaneously and incubated at 90°C for 30 min. After centrifugation, the absorbance of the supernatant was measured at 532 nm using a spectrophotometer. The percentage inhibition was calculated as follows:
The thiobarbituric acid reactive substances (TBARS) content was also calculated using Malonedialdehyde extinction coefficient (0.156 × 106 M−1 cm−1) (Rubin, Merzlyak, & Juferova, Citation1976).
Measurement of lipid hydroperoxides (lipid-OOH)
The measurement of lipid hydroperoxides was determined using the FOX2 method by Miyazawa (Citation1989) with minor modifications (Harma, Harma, & Erel, Citation2003). The FOX2 test system is based on the oxidation of ferrous iron to ferric iron by the various types of peroxides contained in the samples, in the presence of xylenol orange which produces a colored ferric-xylenol orange complex whose absorbance can be measured. The FOX2 reagent was prepared by dissolving ferrous sulfate (6.75 mg) in 250 mM H2SO4 (10 mL) to give a final concentration of 250 μM ferrous iron in acid. This solution was then added to 90 mL HPLC-grade methanol containing 79.2 mg of butylated hydroxytoluene (BHT). Finally, 7.6 mg of xylenol orange was added, with stirring, to make the working reagent (250 μM ammonium ferrous sulfate, 100 μM xylenol orange, 25 mM H2SO4, and 4 mM BHT, in methanol in a final volume of 100 mL). The blank reagent contained all the components of the solution except ferrous sulfate. Sample (200 μL) of incubated control and tests were mixed with 1.8 mL FOX2 reagent. After incubation at room temperature for 30 min, the vials were centrifuged for 10 min. The absorbance of the supernatant was then determined at 560 nm. The lipid hydroperoxides content of the vials was determined as a function of the difference in absorbance between the test and blank samples using a solution of H2O2 as standard.
Statistical analysis
Experimental results were expressed as mean of triplicate ± standard deviation. Statistical analysis of the result was performed using SPSS 10.1 for Windows (SPSS, Chicago, IL, USA). Comparison of variance was carried out using Levene test. ANOVA one way, followed up by Duncan's Multiple Range tests (or Welch followed up by C-Dunnet test when variances were not equal) was performed. Correlations between one method and the other were established using the Pearson Product Moment Correlation. p values <0.05 were regarded as significant.
Results and discussion
Phenolic content
Phenolic compounds are the principal antioxidant constituents of natural products and are composed of phenolic acids and flavonoids. Phenolic compounds or polyphenols constitute one of the most numerous and widely distributed groups of substances in the plant kingdom with more than 8000 phenolic structures currently known. They are potent free radical terminators. They donate hydrogen to free radicals and thus break the reaction of lipid oxidation at the first initiation step. The high potential of polyphenols to scavenge free radicals may be because of their many phenolic hydroxyl groups. In the present study, the amount of phenolic compounds was determined as the catechin equivalent. The content of phenolic compound of D. glomerata extracts varied between 307.25 and 493.48 mg Catechin equivalent per gram of the extract (). The total phenolic content of the ethanol extract was significantly (p < 0.05) the highest. Many phenolics have been shown to contain high levels of antioxidant activity (Rice-Evans, Miller, & Paganga, Citation1996). Several parameters can influence the phenolic yield during the extraction process and this includes extraction temperature, solvent type, and solvent concentration. A preliminary study conducted in our laboratory demonstrated that the extraction with ethanol or with ethanol and water (50/50) at room temperature yielded the highest total phenolic content and antioxidant activity according to plants. A comparison of the total phenolic content of our samples with that of several tropical spices demonstrated a much higher total phenolic content in D. glomerata than in most tropical spices (Agbor, Oben, Ngogang, Xinxing, & Vinson, Citation2005; Wong, Leong, & Koh, Citation2006). Furthermore, the ethanol extract of our plant had a higher total phenolic content than the methanol extract of pepper, a spice commonly consumed in the sub-Saharan, oriental and western countries and known to possess high antioxidant activities (Agbor et al., Citation2007). This suggests the potential health benefit of D. glomerata to be utilized as a source of nutritional phenolics.
Table 1. Phenolic content expressed as catechin equivalents and ferric reducing antioxidant power (FRAP) in three fractions of the fruit of D. glomerata.
Tabla 1. Contenido fenólico expresado como catequinas equivalentes y Poder Antioxidante Reductor de Férrico (FRAP) en tres fracciones del fruto de D. glomerata.
Antioxidant activity
The antioxidant potential of different plant extracts and pure compounds can be measured using numerous in vitro assays. Each of these tests is based on one feature of the antioxidant activity, such as the ability to scavenge free radicals, or the inhibition of lipid peroxidation. However, a single method is not recommended for the evaluation of the antioxidant activity of different plant products, due to their complex composition (Shahidi, Citation2008). Therefore, the antioxidant effects of plant products must be evaluated by combining two or more different in vitro assays to get relevant data. Several methods have been developed to measure the antioxidant activity. Various mechanisms, such as free radical-scavenging (by acting as a hydrogen/electron donor or direct reaction with them), reducing capacity, metal ion chelation (thus preventing the formation of free radicals via the Fenton reactions), inhibition of radical-producing enzymes such as cyclooxygenase and lipoxygenase or increase the expression of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase and inhibition of both lipid peroxidation and LDL oxidation (Hollman & Katan, Citation1999), have been studied to explain the protective effects of nutritional antioxidants in health. In this study, the ability of antioxidants in the fruits of D. glomerata to scavenge free radicals, to reduce ferric ions, to chelate metal ions was investigated.
The ferric reducing capacity of the aqueous extract, ethanol extract, and hydroethanolic extract of D. glomerata was assessed. The FRAP assay measures the antioxidant effect of any substance in the reaction medium as reducing ability. Antioxidant potential of the fruit extracts of this spice was estimated from their ability to reduce TPTZ-Fe(III) complex to TPTZ-Fe(II) complex. The antioxidant capacity of the three extracts of D. glomerata fruits varied significantly (p < 0.05) () although both ET and HE values could be considered high. The FRAP antioxidant activity of the ethanol extract was the highest. The order of FRAP activity of the respective fruit samples extract was as follows: ethanol extract > hydroethanolic extract > aqueous extract. The ethanol extract was higher when compared with several Cameroonian spices like Scorodophloeus zenkeri, a spice of the same family (Agbor et al., Citation2005) supporting the antioxidative potential of this plant. The reductive ability of the samples assessed in this study suggests that the extracts were able to donate electrons; hence they should be able to donate electrons to free radicals in actual biological or food systems, making the radicals stable and unreactive. Many authors have reported that the reducing power of bioactive compounds (mainly phenolic acids and polyphenols), extracted from spices, herbs and medicinal plants, was associated with antioxidant activity, specifically scavenging of free radicals (Jiménez-Escrig, Jiménez-Jiménez, Pulido, & Saura-Calixto, Citation2001; Siddhuraju, Mohan, & Becker, Citation2002).
For ABTS•+ and DPPH• free radical scavenging capacity, the values of the parameters EC50, TEC50, and AE are shown in and , respectively. Strikingly, ethanol and hydroethanolic extracts of D. glomerata tested had exceptionally high scavenging activity expressed as AE when compared with the aqueous extract, catechin and ascorbic acid standards which presented lower AE. In our study, we used another way to express ABTS and DPPH antioxidant capacity results by considering kinetic parameters. The DPPH assay was performed as modified by Sánchez-Moreno et al. (Citation1998). ABTS assay was also performed with similar modification as recently reported for by Pérez-Jiménez and Saura-Calixto (Citation2008) to introduce this kind of parameters; by testing different initial concentrations of the test sample, and the percentage of inhibition taken at a steady state for each concentration, we established the EC50, that was the amount of sample needed to scavenge 50% of the original concentration of the free radical. depicts an example (ethanol extract DPPH kinetic scavenging activity) of kinetic behaviors of extracts at different concentrations as measured by ABTS and DPPH. Considering ethanol and hydroethanolic extracts, we realized that the time needed to reach the equilibrium was almost longer for lower than higher concentrations. For the aqueous extract, this time did not depend on the concentration. This may be because ethanol and hydroethanolic extracts contained higher antioxidant capacity. We also established the tEC50 or the time taken by the EC50 concentration to reach equilibrium; and AE that was the inverse of the product of EC50 and tEC50. With these parameters, it was advantageous to gain more comprehensive information on the sample's antioxidant capacity, taking into account not only their activity (defined by EC50) but also whether the antioxidant acts quickly or slowly (tEC50) and both simultaneously expressed by AE. This approach has been successfully used by many other researchers in quite different samples (Sánchez-Moreno, Larraurin, & Saura-Calixto, Citation1999; Vergara-Valencia et al., Citation2007). and show the kinetic parameters of ABTS and DPPH assays on different extracts and standards. With the exception of the aqueous extract, where the average time taken by antioxidants to react with the ABTS radical was shorter than the time taken to react with the DPPH radical, this time was almost the same for ethanol and hydroethanolic extract.
Figure 1. Time-related changes in percentage inhibition during incubation of ABTS•+ with different concentrations of ethanol extract. Ascorbic acid and catechin (60 mg/g of ABTS) were used as standard. The results are the mean from three replications.
Figura 1. Cambios de porcentaje de inhibición relacionados con tiempo durante la incubación de ABTS•+ con diferentes concentraciones de extracto de etanol. Ácido ascórbico y catequinas (60 mg/g de ABTS) fueron usados como estándar. Los resultados son el promedio de tres repeticiones.
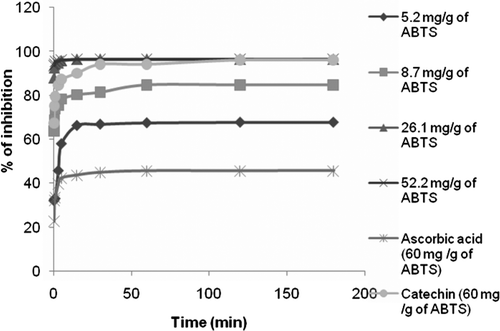
Table 2. Results of EC50 (expressed as mg or g of extract/g of free radical), TEC50 (min) and antiradical efficiency (AE) of ABTS•+ assay measured at a steady state, for different standards and samples of D. glomerata, obtained by ABTS•+ kinetic assay.
Tabla 2. Resultados de EC50 (expresados como mg o g de extracto/g de radical libre), TEC50 (min) y eficiencia antirradical (AE) de ABTS•+ ensayo medido en un estado fijo, para diferentes estándares y muestras de D. glomerata, obtenidos con ensayo cinético ABTS•+.
Table 3. Results of EC50 (expressed as mg or g of extract/g of free radical), TEC50 (min) and antiradical efficiency (AE) of DPPH• assay measured at a steady state, for different standards and samples of D. glomerata, obtained by DPPH• kinetic assay.
Tabla 3. Resultados de EC50 (expresado como mg o g de extracto/g de radical libre), TEC50 (min) y eficiencia antirradical (AE) de DPPH• ensayo medido en estado fijo, para diferentes estándares y muestras de D. glomerata, obtained por ensayo cinético de DPPH•.
The correlation between phenolic content and antioxidant capacity measured by FRAP, DPPH, and ABTS on one hand and between FRAP, DPPH, and ABTS on the other hand was tested. Linear regression analyses of the polyphenol content and scavenging of DPPH and ABTS by extracts showed a statistically significant correlation with r 2 of 0.979 and 0.929 (p < 0.01) (not shown) between AE values and estimated phenol content by Folin-Ciocalteu, respectively for DPPH and ABTS. Similar correlation was observed between phenol content and FRAP (r 2 = 0.932) (not shown) between DPPH and ABTS (r 2 = 0.964) (not shown), between FRAP and DPPH (r 2 = 0.95) (not shown), between FRAP and ABTS (r 2 = 0.967) (not shown). A linear correlation between radical scavenging activity and polyphenolic extract has been reported in an extensive range of spices, vegetables, fruits and beverages (Agbor et al., Citation2005; Saint-Cricq de Gaulejac, Glories, & Vivas, 1999). This correlation suggests that the contribution of phenolic compounds in this model is high.
The ferric thiocyanate method measures the amount of peroxide produced during the initial stages of oxidation, which is the primary product of oxidation. Antioxidant activity of D. glomerata and catechin standard was determined by the ferric thiocyanate method in the linoleic acid system. shows the yields and antioxidant activity of aqueous, hydroethanolic, and ethanol extracts of D. glomerata. All extracts and catechin exhibited effective levels of inhibitory activity toward lipid peroxidation at all concentrations. The effects of various concentrations of extracts of D. glomerata (25–100 μg/mL) on peroxidation in linoleic acid emulsion are shown in the table. The antioxidant activity of D. glomerata extracts increased with increasing concentrations. The highest concentration (100 μg/mL) of hydroethanolic and ethanol extracts of D. glomerata showed higher antioxidant activities than that of aqueous extract and was equal to that of 100 μg /mL concentration of catechin.
Table 4. Inhibition of lipid peroxidation (%) of different concentrations of three extracts from D. glomerata and catechin.
Tabla 4. Inhibición de la peroxidación lípida (%) de diferentes concentraciones de tres extractos de D. glomerata y catequinas.
Superoxide anion is a reduced form of molecular oxygen created by receiving one electron. Superoxide anion is an initial free radical formed from mitochondrial electron transport systems. Mitochondria generate energy using four-electron chain reactions, reducing oxygen to water. Some of the electrons escaping from the mitochondrial chain reaction directly react with oxygen and form superoxide anion. Endogenously, superoxides could be produced in large amounts by various metabolic and physiological processes. The formation of superoxide radical leads to a cascade formation of other ROS in the cell such as hydrogen peroxide, hydroxyl radical, peroxy nitrite, or singlet oxygen in living systems (Lee, Koo, & Min, Citation2004). Superoxide radical decreases the activity of other antioxidant defense enzymes such as catalase and glutathione peroxidase. In the PMS/NADH-NBT system, superoxide anion derived from dissolved oxygen by a PMS/NADH coupling reaction reduces NBT (yellow dye) to blue colored product called formazon. The decrease in absorbance at 560 nm with antioxidants thus indicates the consumption of superoxide anion in the reaction mixture. In this study, the effects of aqueous, hydroethanolic, and ethanol extracts of D. glomerata and catechin on superoxide radical were determined by the PMS-NADH superoxide generating system and the results shown in . All of the extracts had a scavenging activity on the superoxide radicals in a dose dependent manner (0.25–1 mg/mL). However, the highest scavenging ability was exhibited at the dose 1 mg/mL by the ethanol extract followed by catechin and the hydroethanolic extract. The aqueous extract had lowest scavenging activity.
Table 5. Percentage inhibition of superoxide anion radicals in the presence of different concentration of the extracts of D. glomerata.
Tabla 5. Porcentaje de inhibición del anión radical superóxido en presencia de diferentes concentraciones de los extractos de D. glomerata.
The chelating of ferrous ions by the extracts of D. glomerata was estimated by the method of Dinis et al. (Citation1994) and the results are shown in . The production of highly ROS such as superoxide anion radicals, hydrogen peroxide, and hydroxyl radicals is also catalyzed by free iron through Haber–Weiss reaction, . Among the transition metals, iron is known as the most important lipid oxidation pro-oxidant because of its high reactivity. The ferrous state of iron can stimulate lipid peroxidation by the Fenton reaction (H2O2 + Fe2+ → Fe3+ + OH− + OH•), and also accelerates peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals that can themselves abstract hydrogen and perpetuate the chain reaction of lipid peroxidation. Fe3+ ion also produces radicals from peroxides although the rate is 10-fold less than that of Fe2+ ion. Fe2+ ion is the most powerful pro-oxidant among various species of metal ions (Halliwell & Gutteridge, Citation1984). Ferrozine can quantitatively form complexes with Fe2+. In the presence of chelating agents, the complex formation is disrupted, thereby impeding the formation of the red color imparted by the complex as well. Measurement of color reduction, therefore, allows the estimating of the metal chelating activity of the coexisting chelators. In this assay, both extracts of D. glomerata and catechin standard interfered with the formation of ferrous and ferrozine complex, suggesting that they have chelating activity and are able to capture ferrous ion before ferrozine. As shown in , the formation of the Fe2+–ferrozine complex is not complete in the presence of catechin, aqueous, hydroethanolic and ethanol extracts of D. glomerata, indicating that all extracts of D. glomerata chelate with the iron. The absorbance of Fe2+–ferrozine complex was linearly decreased dose dependently (from 0.25 to 2 mg/mL). The difference between both hydroethanolic and ethanol extracts of D. glomerata and the aqueous extract and catechin was statistically significant (p < 0.05). The percentages of metal scavenging capacity of 2 mg/mL concentration of catechin standard, aqueous, hydroethanolic, and ethanol extracts of D. glomerata, were found as 64.36, 65.86, 86.27, 88.42, respectively. The metal scavenging effect of all extracts of D. glomerata and standard decreased in the order of ethanol extract > hydroethanolic extract > aqueous extract > catechin.
Table 6. Ferrous ion-chelating activity (%) of different amount of three extracts from D. glomerata.
Tabla 6. Actividad quelante del ión ferroso (%) de diferentes cantidades de tres extractos de D. glomerata.
Hydroxyl radical is the most reactive of the ROS and can be formed from superoxide anion and hydrogen peroxide, in the presence of metal ions, such as copper or iron. It attacks almost every molecule in the body. Hydroxyl radicals react with lipid, polypeptides, proteins, and DNA (deoxyribonucleic acid), especially thiamine and guanosine. It initiates the peroxidation of cell membrane lipids yielding malondialdehyde, which is mutagenic and carcinogenic. Hydroxyl radicals are formed in vivo from water by high-energy irradiation or from H2O2 in a metal-catalyzed process (Halliwell, Citation1991). Indeed, the deoxyribose assay in the presence of Fe3+-EDTA, H2O2 and a reducing agent has been proposed as a simple “test-tube” method for determining rate constants for the reaction of substrates with OH• (Omafuvbe & Kolawole, 2004). If deoxyribose is incubated with H2O2 and an Fe2+-EDTA complex (or an Fe3+-EDTA complex in the presence of a reducing agent such as ascorbate or superoxide, O2−) the resulting deoxyribose degradation is inhibited by any added scavenger of OH• to an extent that depends only on the concentration of scavenger relative to deoxyribose, and on the scavenger's second order rate constant for reaction with OH•. In vitro, D. glomerata extracts and catechin were able to scavenge in a concentration-dependent manner (0.25–1 mg/mL), the hydroxyl radical (), thus possibly capable of preventing mutagenesis and carcinogenesis. Like for the superoxide radical, the ethanol extract and catechin were significantly (p < 0.05) more effective than the hydroethanolic and aqueous extracts, respectively. Generally, all extracts possess some antioxidant activity, with the ethanol extract being more effective than catechin and hydroethanolic in scavenging free radicals and ROS. This property of D. glomerata could possibly be related to its higher polyphenol content.
Table 7. Percentage inhibition of hydroxyl radicals in the presence of different concentration of the extracts of D. glomerata.
Tabla 7. Porcentaje de inhibición de radicales hidroxilo en presencia de diferentes concentraciones de extractos de D. glomerata.
Sodium nitroprusside in aqueous solution at physiological pH spontaneously generates NO, which interacts with oxygen to produce nitrite and nitrate ions that can be measured using Griess reagent. Scavengers of NO compete with oxygen, leading to reduced production of nitrite ions. Thus, the scavenging activity of extract was based on their ability to prevent the formation of nitrite ions. The NO scavenging effects of extracts are presented in . Overall, the ethanol and the hydroethanolic extracts of D. glomerata showed the highest NO scavenging ability when compared with the aqueous extract and catechin. All three extracts and standard had a significant (p < 0.05) dose-related effect on the scavenging of NO. At the highest concentration (300 μg/mL of dry extract), the ethanol extract inhibited almost 96% of NO compared to 92, 54, and 60%, respectively for the hydroethanolic and aqueous extracts and catechin. Therefore, D. glomerata may have the property to counteract the effect of NO formation and in turn may be of considerable interest in preventing the ill effects of excessive NO generation in vivo.
Table 8. Percentage inhibition of nitric oxide in the presence of different concentration of the extracts of D. glomerata.
Tabla 8. Porcentaje de inhibición de óxido nítrico en presencia de diferentes concentraciones de extractos de D. glomerata.
The antioxidant quality of the plant extracts was also determined by the ability to inhibit LDL oxidation. The lipid peroxidation products in unoxidized LDL, oxidized LDL with Cu2+ in the presence or absence of extracts was assayed as TBARS and lipid hydroperoxides. The amount of lipid hydroperoxides (lipid-OOH) and TBARS formation in unoxidized LDL and Oxidized LDL in the presence or absence of extracts are presented in and . Even in the absence of metal ions, aerobic oxidation of LDL caused significant formation of TBARS and lipid-OOH, which were greatly increased by 11.4 and 14.2 in the presence of Cu2+. Addition of plant extracts and catechin in oxidation mixture containing LDL and Cu2+ inhibited the generation of lipid peroxidation products in a concentration dependent manner. Hydroethanolic extract gave the highest percentage of inhibition ( and ) followed by ethanol extract, catechin, aqueous extract, and ascorbic acid at the concentration of 1 μM. The LDL particle contains large amounts of polyunsaturated fatty acids that make this lipoprotein more prone to the oxidative degradation even in the absence of proxidants. Decomposition of the peroxidized fatty acid leads to the formation of lipid peroxidation products such as lipid-OOH and TBARS. Indeed, in vitro oxidation of LDL by metal ions (e.g. Cu2+ and Fe2+) occurs in three phases: an initial lag phase (consumption of endogenous antioxidant) a propagation phase (rapid oxidation of unsaturated fatty acids to lipid hydroperoxides), and a decomposition phase (hydroperoxides are converted to reactive aldehydes like malondialdehyde, 4-hydroxynonenal). These aldehydes react with lysine residues in apoB-100, resulting in oxidized LDL (Mertens & Holvoet, 2001). The modulation by the spice D. glomerata of LDL resistance to oxidative modification was tested using the classical copper-catalyzed oxidation systems. The Cu2+-catalyzed LDL oxidation depends on the reduction of the metal ion probably through the reaction with endogenous lipid hydroperoxides, with lipid hydroperoxyl radicals' production. The reduced copper (Cu+) in its turn decomposes preexisting peroxides, producing alkoxyl radicals. Therefore, the inhibition of the Cu2+-catalyzed oxidation represents the association of both chelation of metal ions and scavenging of different free radicals. Prevention of peroxidative changes in LDL lipid by extracts in the present work suggests that this spice may play a role in scavenging the free radicals from fatty hydroperoxides so as to inhibit the chain of peroxidation as well as in chelating metal ion.
Table 9. Inhibition of lipid hydroperoxide generation in oxidized LDL by Cu+2 in the presence or absence of different concentrations of extracts.
Tabla 9. Inhibición de la generación de hidroperóxido lípido en LDL oxidado por Cu+2 en presencia o ausencia de diferentes concentraciones de extractos.
Table 10. Inhibition of TBARS (MDA) generation in oxidized LDL by Cu+2 in the presence or absence of different concentrations of extracts.
Tabla 10. Inhibición de la generación de TBARS (MDA) en LDL oxidado por Cu+2en presencia o ausencia de diferentes concentraciones de extractos.
Table 11. Percentage inhibition of lipid hydroperoxide generation in oxidized LDL by Cu+2 in the presence or absence of different concentrations of extracts.
Tabla 11. Porcentaje de inhibición en la generación de hidroperóxido lípido en LDL oxidado por Cu+2 en presencia o ausencia de diferentes concentraciones de extractos.
Table 12. Percentage inhibition of TBARS (MDA) generation in oxidized LDL by Cu+2 in the presence or absence of different concentrations of extracts.
Tabla 12. Porcentaje de inhibición en la generación de TBARS (MDA) en LDL oxidado por Cu+2 en presencia o ausencia de diferentes concentraciones de extractos.
This study showed that among the ethanol, hydroethanolic and aqueous extracts of D. glomerata, the ethanol extract possesses greater significant antioxidant activity and their potency is in the order of ethanol > hydroethanolic > aqueous for most methods. Overall, the ethanol extract is the most potent in scavenging the DPPH and ABTS radicals, superoxide anion and NO as well as in reducing ferric ions whereas the hydroethanolic extract was the best modulator of LDL oxidation. In addition, the antioxidant activity of the ethanol and hydroethanolic extracts was greater or comparable to catechin and ascorbic acid, standard compounds that have been reported to contain potent antioxidant activity. The presence of high levels of phenolic compounds in extracts may have contributed to the observed antioxidant activity. The results of this study show that the extract of D. glomerata pods can be used as easily accessible source of natural antioxidants and as a possible food supplement or in pharmaceutical industry. However, in vivo studies are beneficial to further understand the mechanism of action of this plant as an antioxidant.
Acknowledgements
The University of Yaoundé I and the Gateway Health Alliances, CA, USA, through the Laboratory of Nutrition and Nutritional Biochemistry, provided all reagents and equipment used for this study.
References
- Agbor , A. G. , Oben , E. J. , Ngogang , Y. J. , Xinxing , C. and Vinson , J. A. 2007 . In vitro antioxidant activity of three piper species . Journal of Herbal Pharmacotherapy , 7 ( 2 ) : 49 – 64 .
- Agbor , A. G. , Oben , E. O. , Ngogang , Y. J. , Xinxing , C. and Vinson , J. A. 2005 . Antioxidant capacity of some herbs/spices from Cameroon: A comparative study of two methods . Journal of Agricultural and Food Chemistry , 53 : 6819 – 6824 .
- Benzie , I. F.F. and Strain , J. J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Brand-Williams , W. , Cuvelier , M. E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . Lebens Wissen Tech , 28 : 25 – 30 .
- Dinis , T. C.P. , Madeira , V. M.C. and Almeida , L. M. 1994 . Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers . Archive of Biochemistry Biophysics , 315 : 161 – 169 .
- Garcia-Parra , M. , Mejiade , Gardia , M. and Weigandt , H. 1977 . “ A new method for lipoprotein isolation by precipitation ” . In Protides of biological fluids , Edited by: Peeters , H. 411 – 415 . New York : Pergamon Press . Proceedings of 25th Colloquium Brugge
- Halliwell , B. 1991 . Reactive oxygen species in living systems: source, biochemistry and role in human disease . American Journal of Medicine , 91 : 14 – 22 .
- Halliwell , B. and Gutteridge , J. M. 1984 . Oxygen toxicology, oxygen radicals, transition metals and disease . Biochemical Journal , 219 : 1 – 4 .
- Halliwell , B. , Gutteridge , J. M. and Aruoma , O. I. 1987 . The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals . Analytical Biochemistry , 165 : 215 – 219 .
- Harma , M. , Harma , M. and Erel , O. 2003 . Increased oxidative stress in patients with hydatidiform mole . Swiss Medical Weekly , 133 : 563 – 566 .
- Hollman , P. C.H. and Katan , M. B. 1999 . Dietary flavonoids: Intake health effect and bioavailability . Food and Chemical Toxicology , 37 : 937 – 942 .
- Jiménez-Escrig , A. , Jiménez-Jiménez , I. , Pulido , R. and Saura-Calixto , F. 2001 . Antioxidant activity of fresh and processed edible seaweeds . Journal of the Science of Food and Agriculture , 81 : 530 – 534 .
- Kumaran , A. and Karunakaran , R. J. 2007 . Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus . Food Chemistry , 100 ( 1 ) : 356 – 361 .
- Lakenbrink , C. , Lapczynski , S. , Maiwald , B. and Engelhardt , U. H. 2000 . Flavonoids and other polyphenols in consumer brews of tea and other caffeinated beverages . Journal of Agricultural and Food Chemistry , 48 : 2848 – 2852 .
- Lee , J. , Koo , N. and Min , D. B. 2004 . Reactive oxygen species, aging, and antioxidative nutraceuticals . Comprehensive Reviews in Food Science and Food Safety , 3 : 21 – 33 .
- Lowry , O. H. , Rosebrough , N. J. , Farr , A. L. and Randall , R. I. 1951 . Protein determination using Folin–Ciocalteu reagent . Journal of Biological Chemistry , 193 : 438 – 448 .
- Mbuya , L. P. 1994 . Useful trees and shrubs for Tanzania: Identification, propagation and management for agricultural and pastoral communities Regional Soil Conservation Unit (RSCU), Swedish International Development Authority (SIDA)
- Mertens , A. and Hobvoet , P. 2001 . Oxidized LDL and HDL: Antagonists in atherothrombosis . Journal of the Federation of American Societies for Experimental Biology , 15 : 2073 – 2084 .
- Mitsuda , H. , Yuasumoto , K. and Iwami , K. 1996 . Antioxidation action of indole compounds during the autoxidation of linoleic acid . Eiyo to Shokuryo , 19 : 210 – 214 .
- Miyazawa , T. 1989 . Determination of phospholipid hydroperoxides in human blood plasma by a chemiluminescence-HPLC assay . Free Radical Biology and Medicine , 7 : 209 – 217 .
- Mondon , P. , Leclercq , L. and Lintner , K. 1999 . Evaluation of helianthus annuus free-radical scavenger effects of extracts using new ex vivo stripping methods . Cosmetics, Aerosols and Toiletries in Australia , 12 ( 4 ) : 87 – 98 .
- Moskovitz , J. , Yim , M. B. and Chock , P. B. 2002 . Free radicals and disease . Archives of Biochemistry and Biophysics , 397 : 354 – 359 .
- Nerurkar , S. V. and Taskar , S. P. 1985 . Lipoprotein fractionation by precipitation (a comparison of two methods) . Journal of Postgraduate Medicine , 31 : 89 – 94 .
- Omafuvbe , B. O. and Kolawole , D. O. 2004 . Quality assurance of stored pepper (Pipper guineense) using controlled processing methods . Pakistan Journal of Nutrition , 3 : 244 – 249 .
- Pérez-Jiménez , J. , Arranz , S. , Tabernero , M. , Díaz-Rubio , M. E. , Serrano , J. , Goñi , I. and Saura-Calixto , F. 2008 . Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results . Food Research International , 41 : 274 – 285 .
- Pérez-Jiménez , J. and Saura-Calixto , F. 2008 . Antioxidant capacity of dietary polyphenols determined by ABTS assay: A kinetic expression of the results . International Journal of Food Science Technology , 43 : 185 – 191 .
- Re , R. , Pellegrini , N. , Preoteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Rice-Evans , C. , Miller , N. and Paganga , G. 1996 . Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .
- Rubin , B. A. , Merzlyak , M. N. and Juferova , S. G. 1976 . Oxidation of lipid components in isolated chloroplasts under lighting. The substrates and products of lipid peroxidation . Fiziol Rast , 23 : 254 – 261 . (in Russian)
- Saint-Cricq de Gaulejacn , N. , Glories , Y. and Vivas , N. 1999 . Free radical scavenging effect of anthocyanins in red wines . Food Research International , 32 : 327 – 333 .
- Sánchez-Moreno , C. , Larrauri , J. A. and Saura-Calixto , F. 1998 . A procedure to measure the antiradical efficiency of polyphenols . Journal of the Science of Food and Agriculture , 76 : 270 – 276 .
- Sánchez-Moreno , C. , Larraurin , J. A. and Saura-Calixto , F. 1999 . Free radical scavenging capacity of selected red, rose and white wines . Journal of the Science of Food and Agriculture , 79 ( 10 ) : 1301 – 1304 .
- Shahidi , F. 2008 . Antioxidants. extraction, identification application and efficacy measurement . Electronic Journal of Environmental, Agricultural and Food Chemistry , 7 ( 8 ) : 3325 – 3330 .
- Siddhuraju , P. and Becker , K. 2007 . The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts . Food Chemistry , 101 : 10 – 19 .
- Siddhuraju , P. , Mohan , P. S. and Becker , K. 2002 . Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp . Food Chemistry , 79 : 61 – 67 .
- Silva , E. M. , Souza , J. N.S. , Rogez , H. , Rees , J. F. and Larondelle , Y. 2007 . Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region . Food Chemistry , 101 ( 3 ) : 1012 – 1018 .
- Singleton , V. L. and Rossi , J. A. 1965 . Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Sreejayan , N. and Rao , M. N.A. 1997 . Nitric oxide scavenging by curcuminoids . Journal of Pharmacy and Pharmacology , 49 : 105 – 107 .
- Srinivasan , K. 2005 . Spices as influencers of body metabolism: An overview of three decades of research . Food Research International , 38 : 77 – 86 .
- Tchiégang , C. and Mbougueng , P. D. 2005 . Chemical composition of spices used in the cooking of Nah poh and Nkuiof western cameroon . Tropicultura , 23 ( 4 ) : 193 – 200 . (in French)
- Vellosa , J. C.R. , Khalil , N. M. , Formenton , V. A.F. , Ximenes , V. F. , Fonseca , L. M. and Furlan , M. 2006 . Antioxidant activity of Maytenus ilicifora root bark . Fitoterapia , 77 : 243 – 244 .
- Vergara-Valencia , N. , Granados-Pérez , A. , Agama-Acevedo , E. , Touvar , J. , Ruales , J. and Bello-65-Pérez , A. 2007 . Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient . Food Science and Technology , 40 ( 4 ) : 722 – 729 .
- Wong , S. P. , Leong , L. P. and Koh , J. H.W. 2006 . Antioxidant activities of aqueous extracts of selected plants . Food Chemistry , 99 : 775 – 783 .
- Yeum , K. J. , Aldini , G. , Chung , H. Y. , Krinsky , N. I. and Russell , R. M. 2003 . The activities of antioxidant nutrients in human plasma depend on the localization of attacking radical species . Journal of Nutrition , 133 : 2688 – 2691 .