Abstract
Among three animal fats evaluated, chicken fat (CF) has close similarity in fatty acid profiles with cod liver oil (CLO), compared with mutton fat and beef fat; therefore, CF can be one of the potential adulterants in CLO. Fourier transform infrared (FTIR) spectra of CLO, CF, and their mixtures were measured on direct contact with horizontal attenuated total reflectance (HATR) in mid infrared region (4000–650 cm−1) at 32 scanning and 4 cm−1 resolution. The chemometrics of partial least square (PLS) and discriminant analysis (DA) were chosen for the quantification and classification of oil adulterant in CLO. The results showed that FTIR spectroscopy coupled with PLS calibration can predict the level of CF in CLO with coefficient of determination (R2) for the relationship between actual and FTIR predicted value of CF in CLO is 0.996. The root means square errors of calibration (RMSEC) and prediction (RMSEP) obtained using seven principal components (PCs) are 0.346 and 0.513, respectively. DA using the Coomanss plot can classify pure CLO and that adulterated with CF accurately. Besides, DA using 10 PCs can be successfully exploited for the classification of CLO and CLO adulterated with the mixture of animal fats.
Entre tres grasas animales evaluadas, la grasa de pollo (CF) mostró una gran semejanza en perfiles de ácidos grasos con aceite de hígado de bacalao (CLO), comparado con grasa de cordero y grasa de buey; la grasa de pollo puede ser uno de los posibles adulterantes de aceite de hígado de bacalao. Espectros FTIR de aceite de hígado de bacalao, grasa de pollo y sus mezclas se midieron en contacto directo con la reflectancia total atenuada horizontal en la región media de infrarrojos (4000 – 650 cm−1) a 32 barridos y resolución de 4 cm−1. Las quimometrías de cuadrados mínimos parciales (PLS) y análisis discriminante (DA) se eligieron para la cuantificación y clasificación de aceite adulterante en aceite de hígado de bacalao. Los resultados mostraron que la espectroscopia FTIR asociada con la calibración de cuadrados mínimos parciales puede predecir el nivel de grasa de pollo en aceite de hígado de bacalao con coeficiente de determinación (R2) para la relación entre valores de reales y FTIR predichos de grasa de pollo en aceite de hígado de bacalao es 0,996. La raíz media cuadrada de errores estándar de calibración (RMSEC) y predicción (RMSEP) obtenidos usando 7 componentes principales (PCs) son 0,346 y 0,513, respectivamente. El análisis discriminante usando el gráfico de Coomans puede clasificar aceite de hígado de bacalao puro y aquél adulterado con CF de una forma precisa. Además, el análisis discriminante usando 10 componentes principales puede ser aprovechado con éxito para la clasificación de aceite de hígado de bacalao y aceite de hígado de bacalao adulterado con la mezcla de grasas animales.
Introduction
Currently, the authentication of high value edible fats and oils such as cod liver oil (CLO) is an attractive issue for food scientist due to the legal compliance, economic, food quality, and religious reasons (halal and kosher) (Kamm, Dionisi, Hischenhuber, & Engel, Citation2001). The adulteration usually involved the replacement of high value oils with low grade ones (Lizhi, Toyoda, & Ihara, Citation2010). In the last few decades, researchers have taken into account the importance of a proper consumption of dietary fatty acids (FAs) from fish oils such as CLO (Mondello, Tranchid, Dugo, & Dugo, Citation2006).
CLO is one of the high valuable oils and its production is about 10,000 tones per year. It has long been marketed as a source of vitamins A and D. Besides, CLO was also as a source of the long-chain of ω-3 fatty acids, especially docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (Gunston, Citation2004; Raeder, Steen, Vollset, & Bjelland, Citation2007). Several factors contributed to the nutritional and price values of CLO, namely the raw material quality, species used, and processing history from extraction until storage. According to new EC regulations (Commission Regulation 1662/2006) as cited from Standal, Prae, McEvoy, Axelson, & Aursand (Citation2008) “raw material used for the preparation of fish oil for human consumption must be derived from the fishery products suitable for human consumption, be prepared in an approved establishment, and transported and stored in a hygienic condition.” This regulation has a consequence that traceability and authenticity of fish oils, including CLO must be verified.
Retrospective studies reported that daily use of CLO was associated with reduced risk of death in patients with solid tumors and lung cancer (Skeie, Braaten, Hjartaker, Brustad, & Lund, Citation2009). CLO was also reported to be correlated with the lower risk of diabetes mellitus insulin-dependent (Type I) in children in the offspring during pregnancy or by the baby in the first year of life caused by vitamin D or the ω-3 fatty acids of EPA and DHA present in CLO (Stene, Ulriksen, Magnus, & Joner, Citation2000). Terkelsen, Eskild-Jensen, Kjeldsen, Barker, & Hjortdal (Citation2000) reported that CLO with dose 25% (wt/wt) in ointment preparation can significantly accelerate both the epithelial and the vascular component of healing compared with saline. This effect was contributed by the high vitamin A in CLO. For the reason of beneficial effects of CLO, it is important to ensure the authenticity of CLO from other fat adulterants using fast and reliable method. One of the instrumental techniques offering such a method is Fourier transform infrared (FTIR) spectroscopy.
Combined with powerful chemometrics technique, FTIR spectroscopy method has been highlighted for the authentication purposes of high value fats and oils such as olive oil (Alam & Hamid, Citation2007; Rohman & Che Man, Citation2010) and virgin coconut oil (Manaf et al., Citation2007; Rohman & Che Man, Citation2009a). In this study, Fourier transform infrared using handling sample technique of horizontal attenuated total reflectance (FTIR–HATR) spectroscopy combined with chemometrics was used as a rapid, non destructive, and free from chemical preparations for detection and quantification of chicken fat (CF) using partial least square (PLS). Further analysis was carried out to distinguish CLO from three animal fats and on the basis of their FTIR spectra using discriminant analysis (DA) method.
Materials and methods
Sample preparation
CLO was purchased from the local market in Jogjakarta, Indonesia. The animal fats of mutton (MF), chicken (CF), and beef (BF) were prepared by rendering adipose tissues of corresponding animals according to Rohman & Che Man (Citation2009b). Standards of fatty acid methyl esters (FAMEs) were obtained from Sigma (Sigma Chemicals, St. Louis, MO). All reagents and solvents used were of proanalytical grade.
Calibration and validation
For calibration, a number of standard or training sets consisting of CF in CLO at concentration ranges of 1%–50% v/v were prepared. For validation, a series of independent sample was built. The spectra of pure CLO and CF as well as their blends were analyzed using FTIR spectrometer. The spectral frequency regions where the variations were observed were chosen for developing PLS model in order to quantify CF in CLO.
Discriminant analysis (DA)
In the first study of DA, a series of training sets of pure CLO in concentration range of 1%–90% v/v in chloroform was prepared and assigned as “pure CLO.” Subsequently, CLO was mixed with animal fats in certain concentration (1%–50% v/v in CLO) and marked as “adulterated.” In the second study, CLO adulterated with the mixture of three animal fats in chloroform was compared and classified using DA.
FTIR instrumental analysis
FTIR analysis was performed according to our previous paper (Rohman, Che Man, Ismail, & Hashim, 2011). The spectra of all oil samples were scanned using FTIR spectrometer Nicolet 6700 (Thermo Nicolet Corp., Madison, WI) equipped with a detector of deuterated triglycine sulphate (DTGS) and connected to software of OMNIC operating system (Version 7.0 Thermo Nicolet). FTIR spectra were collected over 32 scans with resolution of 4 cm−1 at regions of 4000–650 cm−1. These spectra were subtracted against background air spectrum. After every sample scanning, a background of reference air spectrum was taken. The ATR plate was carefully cleaned in situ with hexane twice followed by acetone.
Statistical and chemometrics analysis
The mean and standard deviation (SD) of FA concentration were performed using Microsoft Excel 2007. All FA compositions were performed in three replications and subjected to analysis of variance (ANOVA) and followed with Duncan multiple comparison using SPSS version 17.0 software (SPSS Inc., Chicago, USA). The significance value (p) less than 0.05 was statistically different. Chemometrics analysis of PLS and DA was done using the software TQ Analyst™ (Thermo electron Corporation). The leave-one-out cross-validation procedure was used to verify the calibration model. The calibration performance of PLS was assessed using the values of root mean square error of calibration (RMSEC) and coefficient of determination (R2). In addition, R2 and root mean square error of prediction (RMSEP) were used for the evaluation of prediction capability of PLS.
Yi and are the actual and predicted concentration of CF in CLO, respectively. M and N are the number of samples in calibration and validation datasets, respectively. The lower the RMSEC and RMSEP values for the selected dataset, the better the performance of the model (Gurdeniz & Ozen, Citation2009).
Fatty acid analysis
The profiles of FA of CLO and three animal fats (BF, CF, and MF) were quantitatively analyzed as FAME derivate according to method used by Nor Hayati, Che Man, Tan, & Nor Aini (Citation2009) with slight modification. Fifty micrograms of CLO and animal fats were accurately weighed, dissolved in 0.8 mL hexane, and esterified by the addition of 0.2 mL sodium methoxide 2M. The mixture was mixed for 1 min using a vortex mixer, and subsequently 1 μL of the clear supernatant was taken and injected into gas chromatograph (Shimadzu GC-2010, Shimadzu Corp., Tokyo, Japan). The column used was RTX-5 capillary column (0.25 mm internal diameter, 30 m length, and 0.2 μm film thickness; Restex Corp., Bellefonte PA). The oven temperature was programmed as follows: 50 °C (hold for 1 min), ramped to 180 °C (8 °C/min), then 180–200 °C (8 °C/min), and finally held at 240 °C for 5 min. The flow rate of carrier gas (N2) was 6.8 mL/min, meanwhile the temperature of flame ionization detector and injector was set at 240 °C. The split ratio of injection was 1: 20. Standard FAMEs were used to calculate the percentage of FAs based on its peak area normalization.
Results and discussion
FTIR spectral analysis and fatty acid composition
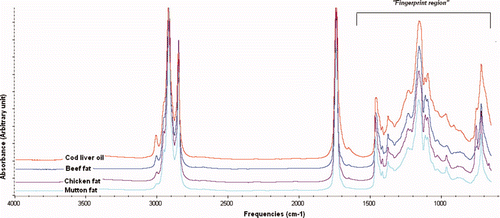
Edible fats and oils are essentially consisted of one molecule of glycerol esterified with three FAs with different substitution patterns and degrees of saturation of the chains and of other minor components such as tocopherols and sterols. Due to its ability as fingerprint analytical technique, FTIR spectrometer can be exploited as powerful tool to distinguish among edible fats and oils. shows FTIR spectra of CLO and animal fats (BF, CF, and MF) which reveal the characteristic bands of fats and oils spectra as described in our previous paper (Rohman & Che Man, Citation2009b).
FTIR spectra of the evaluated fats and oils appear very similar, however, they reveal slight differences in terms of peak absorbances and the exact frequencies at which the maximum absorbance are generated in each fats and oils, due to the different nature and composition of FAs in CLO and animal fats, especially at 3007 cm−1 and at fingerprint regions. FA compositions of CLO and three animal fats are shown in . The FA profiles of CLO are closely similar with those of CF; therefore, the presence of CF in CLO is difficult to detect using gas chromatography. For this reason, FTIR spectroscopy is exploited for detection and quantification of CF in CLO.
Taking into account the difference between CLO and CF spectra, it is obvious that peak intensities at 3007 cm−1 and at 1500–900 cm−1 are different. Therefore, these frequencies were selected to be optimized for analysis of CF in CLO because FTIR spectra variation was observed between CF and CLO. The interpretation of functional groups responsible for IR absorption in CLO and animal fat samples can be found in Pavia, Lampman, Kriz-jr, & Vyvyan (Citation2009) and Lerma-Garcia, Ramis-Ramos, Herrero-Martinez, & Simo-Alfonso (Citation2010).
Quantification of CF in cod liver oil
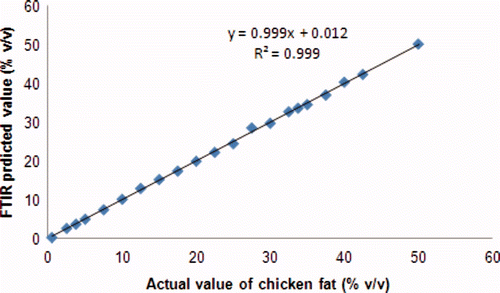
For calibration model, absorbencies of CF with concentration ranging from 0%–50% in CLO was recorded. PLS calibration was employed to make a relationship between actual and FTIR predicted value of CF (% v/v) in CLO. The frequency regions for determination of CF in CLO were optimized at 3020–3000, 1500–900, and at combined frequencies of 3020–3000, 1500–900 cm−1. The selection of frequency regions was based on their ability to provide the highest value of R2 and the lowest value of RMSEC. Finally, using these criteria, the frequencies at 1500–900 cm−1 was selected for such determination. The RMSEC value obtained was relatively low, i.e. 0.346, meaning that mean calibration error is low. The R2 value obtained for the relationship between actual value (x-axis) and FTIR predicted value (y-axis) of CF in PLS caibration model is 0.999 ().
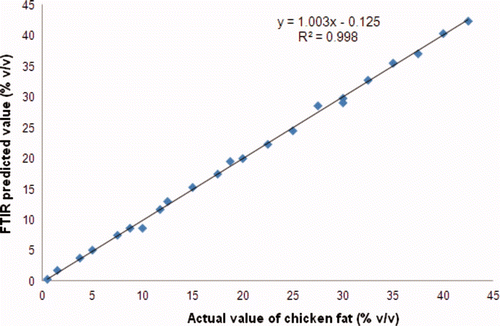
In order to validate PLS model, the cross validation was carried out using “leave-one-out” technique and the accuracy of the model was assessed using RMSEP and R2 values. The low RMSEP value indicated that mean prediction error in validation samples is low. In validation model, RMSEP and R2 values obtained for correlation between actual and FTIR predicted values of CF in CLO were 0.513 and 0.996, respectively. The scatter plot along with equation obtained for such relationship was shown in .
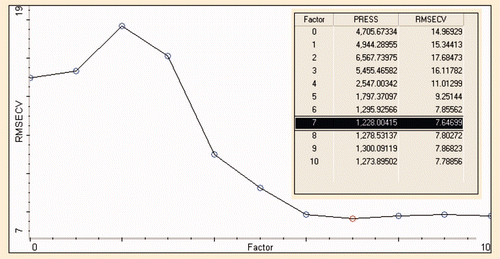
The predicted residual errors sum of square (PRESS) is subsequently calculated from the errors in the predictions obtained from cross validation and plotted as a function of number of factors or principle components (PCs) employed in PLS calibration. Eigen analysis and PRESS values revealed that the optimal number of PCs is seven as shown in . The optimum number of PCs corresponds to the point at which the PRESS value was minimum, or the PRESS plot begins to level off (Rohman & Che Man, Citation2010). It can be understood that root mean square error of cross validation (RMSECV) reaches a stable point, minimum after seven factors.
Discriminant analysis
DA is one of the supervised pattern recognition techniques which can be used to determine the class of CLO with animal fats by calculating the distance from each class center in Mahalanobis distance units (Miller & Miller, Citation2005). First study involved the classification of pure CLO and CLO adulterated with individual animal fats at concentrations of 1.0%–50.0% (v/v). Both classes of CLO and that adulterated with animal fats (BF, CF, and MF) were subjected to DA. The frequency regions together with number of PCs used for DA are shown in . The selection of frequency regions was performed in such a way that they give no or the least misclassification between the two groups.
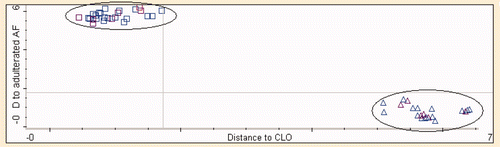
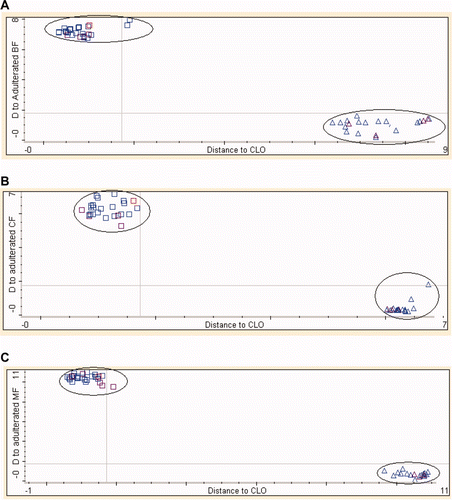
exhibits the Coomans plot for the classification of pure CLO and that spiked with 1%–50% v/v of animal fats using the optimized number of PCs and frequency regions as shown in . The x-axis shows Mahalanobis distance to CLO, while the y-axis shows the distance to the adulterated CLO class with animal fats. The Coomans plot clearly exhibits the separated group of pure CLO and adulterated CLO with animal fats. In this study, DA model classified 100% of all samples accurately according to its group.
In the second study, the pure CLO samples were compared with the mixture of animal fats (BF, CF, and MF). All animal fats were categorized into one group and compared with CLO samples. Furthermore, DA was used to categorize the samples into CLO and that adulterated with animal fats. exhibits the Coomans plot of CLO and that adulterated with animal fats using 10 PCs. The model successfully classified CLO samples and those adulterated. There is no misclassification reported for DA in this developed model.
Conclusion
It can be concluded that adulteration of CLO with animal fats can be monitored using FTIR spectroscopy using ATR sampling handle technique. PLS model can be successfully used for the quantification of chicken fat in CLO. In addition, DA makes the possibility to classify CLO and that adulterated with animal fats using 9 and 10 PCs.
tcyt_a_510211_sup_20735831.pdf
Download PDF (615.4 KB)Acknowledgments
Abdul Rohman thanks The Directorate for Higher Education (Dikti), The Ministry of National Education, Republic of Indonesia, for the scholarship during his PhD program in Halal Products Research Institute, UPM, Malaysia.
References
- Alam , M. A. and Hamid , S. F. 2007 . Application of FTIR spectroscopy in the assessment of olive oil adulteration . Journal of Applied Science Research , 73 : 102 – 108 .
- Gunston , F. D. 2004 . The chemistry of oils and fats: Sources, composition, properties and uses , UK : Blackwell Publishing Ltd .
- Gurdeniz , G. and Ozen , B. 2009 . Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data . Food Chemistry , 116 : 519 – 525 .
- Kamm , W. , Dionisi , F. , Hischenhuber , C. and Engel , K-H. 2001 . Authenticity assessment of fats and oils . Food Reviews International , 17 : 249 – 290 .
- Lerma-Garcia , M. J. , Ramis-Ramos , G. , Herrero-Martinez , J. M. and Simo-Alfonso , E. F. 2010 . Authentication of extra virgin olive oils by Fourier transform infrared spectroscopy . Food Chemistry , 118 : 78 – 83 .
- Lizhi , H. , Toyoda , K. and Ihara , I. 2010 . Discrimination of olive oil adulterated with vegetable oils using dielectric spectroscopy . Journal of Food Engineering , 96 : 167 – 171 .
- Manaf , M. A. , Che Man , Y. B. , Hamid , N. S.A. , Ismail , A. and Syahariza , Z. A. 2007 . Analysis of adulteration of virgin coconut oil by palm kernel olein using Fourier transform Infrared spectroscopy . Journal of Food Lipids , 14 : 111 – 121 .
- Miller , J. N. and Miller , J. C. 2005 . Statistics and chemometrics for analytical chemistry (5th ed.) , 213 – 239 . Edinburgh Gate Harlow, England : Pearson Education Limited .
- Mondello , L. , Tranchid , P. Q. , Dugo , P. and Dugo , G. 2006 . Rapid, micro-scale preparation and very fast gas chromatographic separation of cod liver oil fatty acid methyl esters . Journal of Pharmaceutical and Biomedical Analysis , 41 : 1566 – 1570 .
- Nor Hayati , I. , Che Man , Y. B. , Tan , C. P. and Nor Aini , I. 2009 . Physicochemical characteristics of soybean oil, palm kernel olein, and their binary blends . International Journal Food Science and Technology , 44 : 152 – 161 .
- Pavia , D. L. , Lampman , G. M. , Kriz-jr , G. S. and Vyvyan , J. R. 2009 . Introduction to Spectroscopy (4th ed.) , 15 – 88 . Australia : Cencage Learning Inc. pp. 15–88 .
- Raeder , M. B. , Steen , V. M. , Vollset , S. E. and Bjelland , I. 2007 . Associations between cod liver oil use and symptoms of depression: The Hordaland health study . Journal of Affective Disorders , 101 : 245 – 249 .
- Rohman , A. and Che Man , Y. B. 2009a . Monitoring of virgin coconut oil (VCO) adulteration with palm oil using Fourier transform infrared (FTIR) spectroscopy . Journal of Food Lipids , 16 : 618 – 628 .
- Rohman , A. and Che Man , Y. B. 2009b . Analysis of cod-liver oil adulteration using Fourier Transform Infrared (FTIR) spectroscopy . Journal of the American Oil Chemists' Society , 86 : 1149 – 1153 .
- Rohman , A. and Che Man , Y. B. 2010 . Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil . Food Research International , 43 : 886 – 892 .
- Rohman , A. , Che Man , Y. B. , Ismail , A. and Hashim , P. 2001 . FTIR spectroscopy combined with chemometrics for analysis of lard adulteration in some vegetable oils . CYTA-Journal of Food , 9 : 96 – 101 .
- Skeie , G. , Braaten , T. , Hjartaker , A. , Brustad , M. and Lund , E. 2009 . Cod liver oil, other dietary supplements and survival among cancer patients with solid tumors . International Journal of Cancer , 125 : 1155 – 1160 .
- Standal , I. B. , Prae , A. , McEvoy , L. , Axelson , D. E. and Aursand , M. 2008 . Discrimination of cod liver oil according to wild/farmed and geographical origins by GC and 13C NMR . Journal of the American Oil Chemists' Society , 85 : 105 – 112 .
- Stene , C. , Ulriksen , J. , Magnus , P. and Joner , G. 2000 . Use of cod liver oil during pregnancy associated with lower risk of Type I diabetes in the offspring . Diabetologia , 43 : 1093 – 1098 .
- Terkelsen , L. H. , Eskild-Jensen , A. , Kjeldsen , H. , Barker , J. H. and Hjortdal , V. E. 2000 . Topical application of cod liver oil ointment accelerates wound healing: an experimental study in wounds in the ears of hairless mice . Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery , 34 : 15 – 20 .
Supplementary material
Supplementary Figure 1. FTIR spectra of CLO and BF, CF and MF at mid infrared regions of 4000–650 cm−1.
Figura adicional 1. Espectro FTIR de aceite de hígado de bacalao y grasas de buey, pollo y cordero en regiones infrarrojas medias de 4000–650 cm−1.
Supplementary Figure 2. The linear regression for the relationship between actual (x-axis) and FTIR predicted values (y-axis) of CF in CLO in PLS calibration model.
Figura adicional 2. Regresión lineal para la relación entre valores real (eje x) y FTIR predicho (eje y) de grasa de pollo en aceite de hígado de bacalao en el modelo de calibración de cuadrados mínimos parciales.
Supplementary Figure 3. The scatter plot for the relationship between actual and FTIR predicted values of CF in CLO in validation model.
Figura adicional 3. Diagrama de dispersión de la relación entre los valores real y FTIR predicho de grasa de pollo en aceite de hígado de bacalao en el modelo de validación.
Supplementary Figure 4. The root mean square error of cross validation (RMSECV) versus principal components (PCs) for analysis of CF in CLO at frequency regions of 1500–900 cm−1.
Figura adicional 4. La raíz media cuadrada de errores de validación cruzada (RMSECV) en contraposición a los componentes principales (PCs) para el análisis de grasa de pollo en aceite de hígado de bacalao en regiones de frecuencia de 1,500–900 cm−1.
Supplementary Figure 5. The Coomans plot of CLO and CLO adulterated with animal fats: (□) CLO; (▵) CLO samples adulterated with animal fats of BF (A), CF (B), and MF (MF).
Figura adicional 5. Gráfico de Coomans de aceite de hígado de bacalao y aceite de hígado de bacalao adulterado con grasas animales (□) aceite de hígado de bacalao; (▵) muestras de aceite de hígado de bacalao adulteradas con grasas animales de buey (A), pollo (B) y cordero (MF).
Supplementary Figure 6. The Coomans plot of CLO and that adulterated with animal fats: (□) CLO; (▵) adulterated CLO samples with the mixture of three animal fats.
Figura adicional 6. Gráfico de Coomans de aceite de hígado de bacalao adulterado con grasas animales: (□) aceite de hígado de bacalao; (▵) muestras de aceite de hígado de bacalao adulterado con la mezcla de las tres grasas animales.