Abstract
The sun, oven (50 °C and 70 °C) and microwave oven (210 and 700 W) drying behaviors of tomato slices were investigated. Effects of these drying methods in terms of mineral content, antioxidant activity and color parameters of tomato slices were also studied. “Page, Modified Page and Midilli and Küçük” models exhibited generally high coefficient of determination (R 2) values ranging between 0.990 and 0.999, while two-term model fit better for sun drying. The calculated effective diffusivity (D eff) values (m2/s) of tomato slices for the drying processes ranged between 0.24 and 111.13 × 10−9. The concentrations (mg/kg dry matter) of major minerals such as K, Mg, Na, and P in dried tomato slices varied between of 31567–38003, 1183–1419, 822–1927, and 3256–4999, respectively. Microwave oven-dried (700 W) samples revealed better a* and b* color values than the other dried samples. Fresh and oven-dried (50 °C) samples exhibited the lowest trolox equivalent antioxidant capacity (TEAC) and 2,2-diphenyl-1-picrylhydracyl (DPPH) radical scavenging activities.
Se investigó el comportamiento de secado al sol, horno (50 y 70 °C) y horno microondas (210 y 700 W) de rodajas de tomate. También se estudiaron los efectos de estos métodos de secado en términos de contenido mineral, actividad antioxidante y parámetros de color de las rodajas de tomate. Los modelos Page, Modificado de Page y Midilli y Küçük mostraron en general un mayor coeficiente de determinación (R 2), con valores entre 0.990–0.999, mientras el modelo de dos términos resultó mejor para secado al sol. Los valores de difusividad efectiva (D eff) calculados (m2/s) de rodajas de tomate para los procesos de secado se situaron entre 0.24–111.12 × 10−9. La concentración (mg/kg de materia seca) de minerales mayores, como K, Mg, Na y P, en rodajas de tomate secas oscilaron entre 31567–38003, 1183–1419, 822–1927, 3256–4999, respectivamente. Las muestras secadas en el horno microondas (770 W) mostraron mejores valores de color a* y b* que las otras muestras secas. Las muestras de fresco y secado al horno (50 °C) presentaron la menor Capacidad Antioxidante Equivalente a Trolox (TEAC) y las actividad de barrido del radical DPPH.
Palabras clave:
Nomenclature
| a, b, c | = |
empirical constants in drying models |
| D eff | = |
effective water diffusivity, (m2/s) |
| k, k 0, k 1 | = |
empirical constants in drying models |
| L 0 | = |
slice half-thickness (mm) |
| MR | = |
moisture ratio (dimensionless) |
| M | = |
moisture content at any time |
| M e | = |
equilibrium moisture content |
| M 0 | = |
initial moisture content |
| MRexp.i | = |
ith experimental moisture ratio |
| MRpre.i | = |
ith predicted moisture ratio |
| N | = |
number of observations |
| n | = |
number of constants |
| χ 2 | = |
Chi-square |
| R 2 | = |
correlation coefficient |
| T | = |
drying time (h) |
| Y | = |
empirical constant in drying models |
Introduction
Tomato is one of the most important fruits in the world. It can be easily and widely cultivated and is adapted to a wide range of soils and climates. Tomato is also recognized as the source of vitamins and minerals (Halevy, Koth, & Guggenheim, Citation1957). It can be processed and canned easily as a paste, juice, sauce, powder, or as a whole. In addition, tomato farming is also a good income-generating enterprise to many farmers around the world (Hamid, Citation1985).
The composition and the antioxidant components of the raw tomato fruit grown in different environments and locations in the world fall within a narrow range (Kerkhofs, Lister, & Savage, Citation2003). They are an excellent source of vitamin C, vitamin E, folic acid, potassium, and secondary metabolites such as b-carotene, lycopene, and phenolic compounds. The quantity and quality of phytochemicals detected in tomato fruits are known to depend greatly on genotype and environmental condition (Gahler, Otto, & Bohm, Citation2003; Giuntini, et al., Citation2005; Luthria, Mukhopadhyay, & Krizek, Citation2006).
Although epidemiological studies highlight the positive role of consuming fresh fruit and vegetables, it is clear that, as tomatoes have a relatively short shelf-life, a significant proportion of the crop is consumed cooked and processed. Typical home processing of tomatoes leads to loss of some antioxidant properties and change of color (Sahlin, Savage, & Lister, Citation2004). It has been reported that boiling and baking have a relatively small effect on ascorbic acid, total phenolics, lycopene, and total antioxidant activity, while frying significantly reduces these important nutrients (Sahlin et al., Citation2004). Considerable amounts of tomatoes are processed and dried and used as a component for pizzas and various vegetable dishes (Giovanelli, Zanoni, Lavelli, & Nanic Citation2002; Kerkhofs, Lister, & Savage, Citation2005).
However, tomato is highly perishable in the fresh state leading to wastage and losses during the peak harvesting period. The prevention of these losses and wastage is of major interest especially when there is subsequent imbalance in supply and demand at the harvesting off-season. Dehydration processes offer an alternative way of providing tomato to commerce. The dehydration of tomato has been practiced for many years as a means of preserving tomato. The most popular method of drying tomato in the tropics is hot air drying. This may result in physical and structural changes such as migration of soluble solids, shrinkage, and case hardening, loss of volatiles and aroma, and slower water absorption during rehydration. To minimize these changes, some drying methods are practiced such as freeze drying, air drying at low temperature, and vacuum drying (Akanbi & Oludemi, Citation2003; Akanbi, Adeyemi, & Ojo, Citation2006).
Dried tomato products (i.e. tomato halves, slices, quarters, and powders), being commonly dried at high temperatures in the presence of oxygen, show the highest sensitivity to oxidative damage (Giovanelli et al., Citation2002). Air drying of tomato caused a severe oxidative heat damage of product, shown by both a marked loss of ascorbic acid and an increase in the 5-hydroxymethyl-2-furfural (HMF) content, resulting in undesirable color and appearance changes of dried tomatoes (Zanoni, Peri, Nani, & Lavelli, Citation1999). Conversely, lycopene had a high stability during drying (Giovanelli et al., Citation2002).
The aim of the work was to establish the sun, oven, and microwave drying characteristics of tomato slices comparing traditional sun drying and conventional oven drying methods to the microwave drying method, and to determine the effects of these different drying techniques on some properties of tomato, such as the mineral content, antioxidant activity, and color values.
Materials and methods
Tomatoes
Fresh, mature, and ripe tomatoes (Lycopersicon esculentum Mill var. 8354 F1) were purchased from a local market in Konya, Turkey. The tomatoes were cut into slices of approximately 10.0 ± 0.1 mm thickness with a sharp stainless steel knife in the direction perpendicular to the vertical axis. Three measurements were made on each slice for its thickness using a calipier (Mitutoyo Corp. Model no SC-6, Japan) and their average values were considered. Moisture content of the tomato was immediately measured on arrival. The chemicals used in the assays of minerals and antioxidant activity assays were all of analytical grade (Merck, Germany). After drying, samples were packed in dark colored glass jars to prevent light damage and stored at +4 °C in a domestic refrigerator for a maximum of 7 days until all the analyses were carried out.
Drying of the tomato slices
Oven drying
100 g of tomato slices were distributed uniformly as a thin layer onto the stainless steel trays of size 0.3 × 0.2 m and dried in an oven (Nüve FN055 Ankara, Turkey, 55 L volume) at 50 °C and 70 °C (Balladin & Headley, Citation1999).
Sun drying
100 g of tomato slices were distributed uniformly as a thin layer onto the stainless steel trays of size 0.3 × 0.2 m and dried under direct sunlight at temperatures between 20 °C and 30 °C in August in Konya, Turkey (Balladin & Headley, Citation1999). The average value of wind velocity was 2.22 m/s and the proportional moisture was 56% during the days in which the materials were dried.
Microwave oven drying
A programmable domestic microwave oven (Arçelik ARMD 580, Turkey) with maximum output of 700 W and 2450 MHz was used for drying experiments. The dimensions of the microwave cavity were 345 mm × 340 mm × 225 mm. One dish containing 100 g of sample was placed on the centre of a turntable fitted inside the microwave cavity and processed until the slices were completely dried. The microwave oven was operated by a control terminal which could control microwave power level and emission time (1 s to 100 h).
The mass of the samples were measured every 1 h during oven and sun drying (Günhan, Demir, Hancioglu, & Hepbasli, Citation2005; Maskan, Kaya, & Maskan, Citation2002) and every 90 s during microwave oven drying (Fathima, Begum, & Rajalakshmi, Citation2001; Soysal, Öztekin, & Eren, Citation2006) using a digital balance, measuring to an accuracy of 0.001 g (Sartorius LE623S, Germany) (Gikuru & Olwal, Citation2005). A tray with the sample was taken out of the drying chamber, weighed on the digital balance, and placed back into the chamber. The digital balance was kept very close to the drying unit and the weight measurement process took about 10 s (Sharma, Verma, & Pathare, Citation2005). Experiments were repeated three times and mean values were used.
The initial moisture content of the slices was measured by drying in an oven at 105 °C for 24 h and expressed as kg water/kg dry solids which varied between 13.62 and 14.04 kg water/kg dry solids (AOAC, Citation1990). The equilibrium moisture content was assumed zero for microwave drying (Maskan, Citation2001).
Empirical modeling of drying curves
For mathematical modeling, the equations in Supplementary were tested to select the best model for describing the drying curve equation of tomato slices during drying. The moisture ratio of tomato slices during drying was calculated using the equation MR = (M − M e)/(M 0 − M e), where MR is the moisture ratio, M is the moisture content at a specific time (g water per g dry solids), M0 is the initial moisture content (g water per g dry solids), and M e is the equilibrium moisture content (g water per g dry solids) (Yaldız, Ertekin, & Uzun, 2001).
The regression was performed in Statistica computer program (Statistica for Windows 5.0). The correlation coefficient (R 2) was used to select the best equation to account for the variation in the drying curves of the dehydrated sample. In addition to R 2, Chi-square (χ 2), the mean square of the deviations between the experimental and estimated values for the models was used to determine the goodness of fit. The lower the values of the Chi-square, and the higher the values of R 2 values indicate the high fit of the model (Sharma et al., Citation2005). Chi-square can be calculated as:
Calculation of effective diffusivity
It has been accepted that the drying characteristics of biological products in the falling rate period can be described by using Fick's diffusion equation. Although the diffusivity equation is not the best equation to fit experimental data, it provides an approximate method to present a common quantitative comparison between different products in the aspect of moisture transfer because it can provide a description for average diffusion coefficient in the entire drying process. The solution to this equation developed by Crank (Citation1975) can be used for various regularly shaped bodies, such as rectangular, cylindrical, and spherical products. For a long drying period, this solution can be written in a logarithmic form as follows (Sun et al., Citation2007; Tutuncu & Labuza, Citation1996):
Determining the mineral composition
Approximately 0.5 g dried tomato was finely ground in a mortar and pestle to 40-mesh screened through a sieve to obtain uniform particle size sample was put into a burning cup, and 15 mL of pure HNO3 was added. The sample was incinerated in a MARS 5 Microwave Oven (CEM Corp., USA, 3100 Smith Farm Road, Matthews, NC) at 200 °C temperature and diluted to a certain volume (50 mL) with distilled water. Mineral concentrations were determined by inductively coupled plasma atomic emission spectrometer (ICP-AES) (Skujins, Citation1998). Process conditions of ICP-AES (Varian-Vista) were as follow; RF power: 0.7–1.5 kw (1.2–1.3 kw for Axial), plasma gas flow rate: (Ar) 10.5–15 L/min (radial), 15 L/min (axial); auxiliary gas flow rate (Ar):1.5; viewing height: 5–12 mm; copy and reading time: 1–5 s (max. 60 s), copy time: 3 s (max. 100 s).
Color measurement
Color of tomato slices was measured by a Minolta Chroma meter CR 400 color meter (Minolta Co., Osaka, Japan) before and after drying. The color meter was calibrated against a standard calibration plate of a white surface and set to CIE Standard Illuminant C. The L*, a*, b* values were the average of 10 readings. The color brightness coordinate L* measures the whiteness value of a color and ranges from black at 0 to white at 100. The chromaticity coordinate a* measures red when positive and green when negative, and chromaticity coordinate b* measures yellow when positive and blue when negative (Doymaz, Tugrul, & Pala, Citation2006).
Extraction of antioxidant compounds
Methanolic extracts were prepared as reported previously by Luthria et al. (Citation2006). Approximately 400 ± 1 mg of ground, dried tomato sample was placed in a 15 mL centrifuge tube with 5 mL of the solvent mixture MeOH:H2O (80:20, %v/v). The vials were then placed in a sonicator bath at ambient temperature for 30 min. The mixture was centrifuged and the supernatant was transferred into a 10-mL volumetric flask. The residue was resuspended in 5 mL of MeOH:H2O (80:20, %v/v), gently mixed manually, and sonicated for an additional 30 min followed by centrifugation. The supernatant was combined with the initial extract and the volume of combined supernatant was made up to 10 mL with the extraction solvent and appropriate aliquots of extracts were filtered over Whatman No. 1 filter paper and assayed for antioxidant activity. For each sample, triplicate extractions and analyses were carried out.
DPPH radical scavenging activity
Antioxidant activity was evaluated by measuring the radical scavenging effect of dried tomatoes methanolic extracts towards the 2,2-diphenyl-1-picrylhydracyl (DPPH•) as reported previously by Bamdad, Kadivar, and Keramat (Citation2006) and Singh, Murthy, and Jayaprakasha (Citation2002). Five millilitres of a 0.1 mM methanol solution of DPPH (Fluka) were added to 0.1 mL of several concentrations of methanol extracts of fresh and dried tomato samples. The tubes were allowed to stand at 27 °C for 20 min. The decrease in absorbance at 517 nm was recorded in a spectrophotometer (Shimadzu UV-vis mini spectrophotometer 1240). Radical scavenging activity was expressed as inhibition percentage and was calculated using the following formula:
Total antioxidant activity assay
Total antioxidant activity was determined using the ABTS method adapted from Miller and Rice-Evans (Citation1997). Decolorisation of the ABTS•+ radical cation by sample extract was measured spectrophotometrically at 734 nm (Shimadzu UV-vis mini spectrophotometer 1240 (Osaka, Japan)) in relation to a Trolox® (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, Sigma-Aldrich) standard. In the present study, metmyoglobin was used to generate the ABTS•+ radical cation in potassium phosphate-buffered saline (PBS), pH 7.4. Metmyoglobin (final concentration 400 μM) and ABTS (final concentration 5.0 mM) in PBS (5.0 mM) were mixed and the reaction was initiated by the addition of hydrogen peroxide (final concentration 0.1 mM). The ABTS•+ radical cation solution thus obtained was diluted with PBS (v/v) to give an absorbance of about 0.8 at 734 nm. For each experiment, a solvent blank was run. The decreases in absorbance for all the samples were measured at the moment which a sharp decrease in absorbance was observed for the 2.5 mM Trolox solution. These absorbances reflected the ABTS•+ radical cation scavenging capacity and was plotted against the concentration of the antioxidant. The trolox equivalent antioxidant capacity (TEAC) value represents the ratio between the slope of this linear plot for scavenging of ABTS•+ radical cation by the samples compared to the slope of this plot for ABTS•+ radical cation scavenging by Trolox. Results were expressed as Trolox® equivalent antioxidant capacity (μmol TEAC/kg dry matter).
Statistical analysis
The data are presented as the mean of three determinations ± standard deviation. Data were analysed by ANOVA and Duncan's multiple-range test by using SPSS (Version 13.0) (SPSS Inc., Chicago, IL). P values <0.05 were regarded as significant. The statistical analysis of drying experiments for model fitting was performed with a statistical software package (STATISTICA for Windows, 5.5, 1999; Statsoft, Inc., Tulsa, OK, USA).
Results and discussion
Drying characteristics of tomato slices
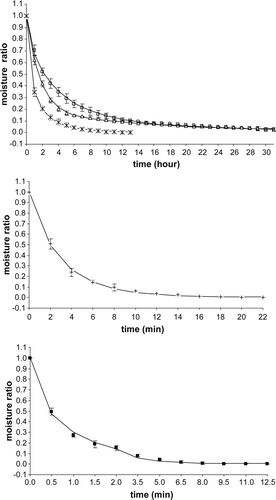
The drying curves were prepared based on the variation of the product water content as a function of time. Plots of the moisture ratio versus time curves are shown in Supplementary Figure 1, which represents the experimental curve of drying characteristics of tomato slices. By using oven-drying method (50 °C and 70 °C), the drying time up to the moisture content of approximately 85 g/kg wet weight basis (WWB) could be shortened by 2.08% and 78.12%, respectively when compared to sun drying method. For microwave oven drying at 210 W and 700 W, the drying times to reach the 85 g/kg WWB moisture content were 17 min and 7 min, respectively. The moisture content of the material was very high during the initial phase of the drying, which resulted in high drying rates due to the higher moisture diffusion. The entire drying process for the samples occurred in the range of falling rate period.
The time taken to reach a moisture content of 136.9 g/kg (WWB) for the oven drying at 50 °C was 42 h, a moisture content of 160 g/kg (WWB) for the oven drying at 70 °C was 9 h, a moisture content of 131 g/kg (WWB) for the sun drying methods was 42 h and at the 8 th min of microwave drying, a moisture content of 55.11 g/kg (WWB) and 55.11 g/kg (WWB) was obtained at 700 W and 210 W, respectively. The reductions in moisture contents of the samples were very slow and/or there were no significant reductions after the times mentioned above. The time was longer for sun drying due to the fluctuating temperature during the drying period. Water accounts for the bulk of the dielectric component of most food systems, especially the high-moisture fruits and vegetables. Hence, these products are very responsive to microwave application and will absorb the microwave energy quickly and efficiently as long as there is residual moisture. The microwave applications for drying therefore offer a distinct advantage, i.e. energy absorption proportional to the residual moisture content (Feng, Tang, & Cavalieri, Citation2002). Even though it was taking much less time for drying, one of the disadvantages of this drying method is the lack of temperature control (Walde, Velu, Jyothirmayi, & Math, Citation2006).
The drying rates were higher in the beginning of the drying processes and they gradually decreased through the end of the drying process. As previously reported by Sharma et al. (Citation2005), this can be attributed to the absorption of more energy by the water at the product surface initially, resulting in faster drying and with the product surface drying out subsequently, heat penetration through the dried layer decreased thus retarding the drying rates.
Evaluation of the models
Eight different MR models were used to predict the moisture content as a function of drying time (Supplementary ). The statistical values and R 2 and χ 2 values, which are obtained under specific drying conditions for these models are shown in Supplementary . Page, modified Page, and Midilli and Küçük models exhibited high coefficient of determination (R 2) values for all the drying methods used in the assay, except sun drying which two-term model fit better than the other models; and the values of the statistical parameter (χ 2) ranged between 0.19 × 10−4 and 206.18 × 10−4. The models generally fit well with closer R 2 and χ 2 values.
Effective diffusivity
The calculated D eff values (m2 s−1) of tomato slices for the sun, oven 50 °C and 70 °C, microwave 210 W and 700 W drying process were 0.26 × 10−9, 0.24 × 10−9, 1.36 × 10−9, 51.56 × 10−9 and 111.13 × 10−9, respectively. Microwave drying at high power level (700 W) yields the highest D eff values which were approximately 81 fold higher than that of oven (70 °C) drying. D eff value of microwave drying at 700 W was 2.15 fold higher than microwave drying at 210 W. As expected, the values of D eff increased with the increase in temperature in the case of oven drying. The value of D eff for the oven drying at 70 °C was 5.66 fold higher than that for the oven drying at 50 °C. The values of D eff lay in general within the range of 10−11 to 10−9 m2 s−1 for food materials dried using the conventional oven and solar drying methods (Madamba, Driscoll, & Buckle, Citation1996). Akanbi et al. (Citation2006) reported the D eff values of tomato samples (slice thickness 15 mm) oven dried at 45 °C, 60 °C, and 75 °C between 3.72 and 12.27 m2 s−1 × 10−9. The D eff values for tomato halves (slice thickness 10–20 mm) which were cabinet air dried at 60 °C, 80 °C, and 110 °C were reported in the range 2.3 × 109 to 9.1 × 10−9 m2 s−1 by Giovanelli et al. (Citation2002). The values obtained for oven dryings at 50 °C and 70 °C in the present study were lower than the D eff values reported by Akanbi et al. (Citation2006) and Giovanelli et al. (Citation2002), and even the slice thickness were closer or lower than those used in these two studies. This might be probably due to air circulation applied in their experiments.
Mineral contents
The mineral contents of fresh, oven-dried, sun-dried and microwave oven-dried tomato slices are given in Supplementary . The mineral compositions of dried tomato slices were higher because of increasing dry matter content. Fresh and dried tomato slices had high amounts of K, Mg, Na, and P minerals.
The differences between Al, B, Cr, Fe, Na, V, and Zn contents of fresh and dried tomato slices were not statistically significant. The remaining minerals were higher in sun-dried samples than the fresh samples. The highest Mg and Mn values were determined in sun-dried samples. This might be attributed to incidence of more chemical reactions (formation of stabile compounds such as stabile aluminium or ferric oxides) in the samples dried by the hot air convective oven and microwave oven. Therewith, microwave oven drying (700 W) led to lowest values of Mg, Mn, Na, and Ni in the samples. Se, Cu, and K contents of oven-dried samples were lower than the sun- and microwave oven-dried samples. The convective style of energy and wave strength of oven drying method might cause damage on these minerals.
Guil-Guerrero and Rebolloso-Fuentes (Citation2009) reported some minerals of eight tomato varieties collected from greenhouse in Spain as (mg/100g in wet weight) Na 4.0–17.4; K 249–319; Mg 10.8–22.4; P 7.8–27.3; Mn 66–306; Fe 488–3513; Cu 45–392; Zn 155–5479; and Se 0.09–1.45. When the unit was converted to mg/kg and the values were considered in dry matter, Mn, Fe, Cu, and Zn values determined in the present study were lower, P values were higher, and Na, K, and Mg values were closer to the values of Guil-Guerrero and Rebolloso-Fuentes (Citation2009) where they determined the minerals in eight different tomato varieties as Cherry, Cherry Pera, Daniela Larga Vida, Lido, Pera, Racimo, Raf, and Rambo.
Hernández Suárez, Rodriguez Rodriguez, and Diaz Romero (Citation2007) reported the minerals and trace element concentrations of various tomato cultivars as (mg/kg wet weight) Na 79–115; K 2429–2834; Mg 110–128; P 222–271; Mn 0.54–0.66; Fe 1.80–2.19; Cu 0.24–0.32; and Zn 0.69–0.86. Our results were higher than the values reported by Hernández Suárez et al. (Citation2007). Hernández Suárez et al. (Citation2007) also claimed that besides the many factors influencing the mineral and trace element concentrations, such as cultivar, cultivation method, production region or sampling period, the high Na concentration in soil is due mainly to the influence of the marine aerosol (Larcher, Citation2003). Also, the high salinity of the water used in the irrigation (Vargas Chavez & Rodriguez Rodriguez, 2000) could explain the relatively high concentration of Na in the tomatoes.
Discrepancies between data in the present study and the other studies could be the result of a wide variety of factors such as plant, age, cultivar, environmental conditions, nutrient availability, and storage conditions of the harvested plant (Thompson et al., Citation2005).
Color assessment
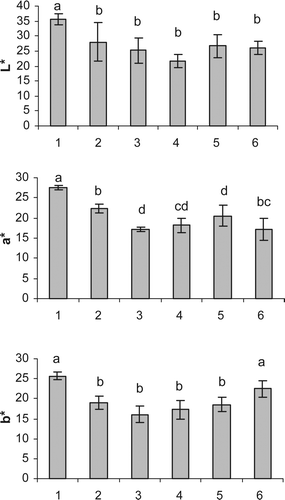
Drying methods and temperatures exert a significant effect on the color changes of tomato slices. Product color is the other quality parameter that needs to be maintained during tomato drying. Supplementary Figure 2 shows the color data in terms of L*, a*, and b* values of fresh and dried tomato slices. L* values of tomato slices decreased with drying which is an expected change as dried samples would normally be darker than the fresh samples. There were no significant differences between L* values of dried samples with different methods.
a* values of fresh samples were higher than the dried samples. Oven- (50 °C and 70 °C) and microwave oven-dried (210 W) samples had lower a* values than the sun- and microwave oven-dried (700 W) samples. The longer drying time required during microwave drying at low output and convective heat transfer style and high temperatures involved in oven drying might lead to reductions in the redness of the samples. Shi, Le Maguer, Kakuda, Liptay, and Kiekamp (Citation1999) reported that color degradation of tomato was less severe when the drying temperature was lowered from 90 to 55 °C.
Krokida and Maroulis (Citation1999) showed that microwave drying prevented color damages during drying. But in the present study, this was not true for the microwave drying at low output. Pott, Neidhart, Mühlbauer, and Carle (Citation2005) reported that high temperatures and excessive drying resulted in a noticeable increase in redness in mango slices. The change of color could be attributed to the browning reactions (Maillard) that occur during drying (Adam, Mühlbauer, Esper, Wolf, & Spiess, Citation2000).
b* values of dried samples were lower than the fresh samples except microwave dried (700 W) samples which did not show a significant decrease in yellowness. Similarly to a* values, b* values of microwave-dried samples at high output did not adversely affected during drying process. Soysal (Citation2004) also reported that a* and b* values of microwave-dried parsley leaves were not significantly different from the values of fresh leaves and indicated that the change in color values was not dependent on the microwave output power which is not in agreement with our results. In another study, air-dried carrot slices were reported to be darker with less yellow and red hues as compared to microwave-dried ones (Sumnu, Turabi, & Öztop, Citation2005). Maskan (Citation2001) reported that for kiwi fruit an L* value of about 40 was reached after 5 min for microwave drying and about 325 min for hot air drying and therefore, microwave would give a destruction rate 65 times faster than hot air (60 °C). A similar trend was found by Maskan (Citation2001) for kiwifruit microwave drying, increase in a* value and decrease in b* value. These color changes are due to a combination of non-enzymatic browning (Maillard reaction) and lycopene degradation (Zanoni et al., Citation1999). Kerkhofs et al. (Citation2005) reported the L*, a*, and b* values of fresh and air-dried tomato fruits from three commercially grown cultivars (Aranka, Encore, and Flavourine) between 30.2–47.2, 20.5–39.4, 17.9–28.7 and 32.8–34.6, 16.1–21.5, 18.4–22.8. The color values determined in the present study fall between the ranges reported by Kerkhofs et al. (Citation2005).
Antioxidant activity
Supplementary shows the average antioxidant activity and radical scavenging capacity of fresh and dried tomato slices. The antioxidant activity of tomato samples were significantly affected by the drying conditions. The TEAC values for dried tomatoes varied between 33775.0 and 57774.6 μmol TEAC/kg dry matter (DM) and DPPH radical scavenging capacities of the dried tomatoes were in the range of 53.08–78.06%. The sun- and microwave oven-dried (700 W) tomatoes gave the highest TEAC and DPPH radical scavenging activity while microwave oven-dried tomatoes at low output energy (210 W) showed high activity in terms of DPPH radical scavenging activity. Meanwhile, fresh and oven-dried (50 °C) samples exhibited the lowest TEAC and DPPH radical scavenging activities among them. The temperature did not negatively affect the antioxidant activity of tomato during oven drying. In fact, compared to sun drying less air circulation and longer drying time were required when drying at 70 °C; the oven drying at 50 °C did not reveal the desired conditions. In previous studies, it was reported that bound antioxidants are released by processing of tomatoes (Stahl & Sies, Citation1992; Tonucci et al., Citation1995) and other, labile antioxidant compounds are being destroyed (Abushita, Daood, & Biacs, Citation2000).
It was previously reported that long dehydration times together with high temperatures (Perez-Galvez, Hornero-Mendez, & Minguez-Mosquera, Citation2005) lead to poor quality products due to caramelization, Maillard reactions, enzymatic reactions, pigment degradation, and l-ascorbic acid oxidation (Horner, Citation1993). Kim, Lee, Park, Lee, and Hwang (Citation2006) reported that modified drying, which is short time and low temperature drying of cut red pepper pods, was certainly more effective than conventional drying in reducing the destruction of the antioxidant activity, ascorbic acid, and color.
Conclusions
For microwave oven drying at 210 W and 700 W, the drying times to reach the 8.5 g/100g (WWB) moisture content were 17 min and 7 min, respectively. By using the oven-drying method (50 °C and 70 °C), the drying time up to the moisture content of approximately 8.5 g/100g WWB could be shortened by 2.08% and 78.12%, when compared to sun drying, respectively. Page, modified Page, and Midilli and Küçük models exhibited high coefficient of determination (R 2) values for all the drying methods used in the assay, except sun drying which two-term model fit better than the other models. D eff value of microwave drying at 700 W was 2.15 fold higher than microwave drying at 210 W. The value of D eff for the oven drying at 70 °C was 5.66 fold higher than that for the oven drying at 50 °C. Probably due to incidence of more chemical reactions (formation of stabile compounds such as stabile aluminium or ferric oxides) in the samples dried by the hot air convective oven and microwave oven, microwave oven drying (700 W) led to lowest values of Mg, Mn, and Na and oven-dried samples had lower values of Ni and Se, Cu, and K contents. The longer drying time required during microwave drying at low energy output (210 W) and convective heat transfer style and high temperatures involved in oven drying (50 °C and 70 °C) led to reductions in the redness of the samples. Nevertheless, for the oven drying, the longer drying time decreased the antioxidant activity of tomato. Fresh and oven-dried (50 °C) samples exhibited lower TEAC and DPPH radical scavenging activities than the sun-, microwave- and oven-dried (70 °C) samples. It may therefore be concluded that microwave oven drying at 700 W offer advantages due to lower drying time required, higher effective moisture diffusivity, and lower destruction of the antioxidant activity and color.
Supplementary material
The supplementary material for this article is available online at http://dx.doi.org/10.1080/19476337.2010.522734
tcyt_a_522734_sup_21461527.pdf
Download PDF (188.8 KB)Acknowledgments
This study was supported by Selcuk University Office of Scientific Research Projects (S.Ü.-BAP, Konya-Turkey).
References
- Abushita , A. A. , Daood , H. G. and Biacs , P. A. 2000 . Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors . Journal of Agricultural and Food Chemistry , 48 : 2075 – 2081 .
- Adam , E. , Mühlbauer , W. , Esper , A. , Wolf , W. and Spiess , W. 2000 . Quality changes of onion (Allium cepa L.) as affected by the drying process . Nahrung , 44 : 32 – 37 .
- Akanbi , C. T. , Adeyemi , R. S. and Ojo , A. 2006 . Drying characteristics and sorption isotherm of tomato slices . Journal of Food Engineering , 73 : 157 – 163 .
- Akanbi , C. T. and Oludemi , F. O. 2003 . Effect of processing and packaging on the lycopene content of tomato products . International Journal of Food Properties , 7 ( 1 ) : 139 – 152 .
- AOAC. 1990 . Official methods of analysis , Washington, DC : Association of Official Analytical Chemists .
- Ayensu , A. 1997 . Dehydration of food crops using a solar dryer with convective heat flow . Solar Energy , 59 : 121 – 126 .
- Balladin , D. A. and Headley , O. 1999 . Evaluation of solar dried thyme (Thymus vulgaris Linne.) herbs . Renewable Energy , 17 : 523 – 531 .
- Bamdad , F. , Kadivar , M. and Keramat , J. 2006 . Evaluation of phenolic content and antioxidant activity of Iranian caraway in comparison with clove and BHT using model systems and vegetable oil . International Journal of Food Science and Technology , 41 ( Suppl. 1 ) : 20 – 27 .
- Crank , J. 1975 . The mathematics of diffusion , Oxford, , England : Clarendon Press .
- Diamante , L. M. and Munro , P. A. 1993 . Mathematical modelling of the thin layer solar drying of sweet potato slices . Solar Energy , 51 : 271 – 276 .
- Doymaz , İ. , Tugrul , N. and Pala , M. 2006 . Drying characteristics of dill and parsley leaves . Journal of Food Engineering , 77 : 559 – 565 .
- Fathima , A. , Begum , K. and Rajalakshmi , D. 2001 . Microwave drying of selected greens and their sensory characteristics . Plant Foods for Human Nutrition , 56 : 303 – 311 .
- Feng , H. , Tang , J. and Cavalieri , R. P. 2002 . Dielectric properties of dehydrated apples as affected by moisture and temperature . Transaction of the American Society of Agricultural Engineers , 45 : 129 – 135 .
- Gahler , S. , Otto , K. and Bohm , V. 2003 . Alterations of vitamin C, total phenolics, and antioxidant capacity as affected by processing tomatoes to different products . Journal of Agriculture and Food Chemistry , 51 : 7962 – 7968 .
- Gikuru , M. and Olwal , J. O. 2005 . The drying kinetics of kale (Brassica oleracea) in a convective hot air dryer . Journal of Food Engineering , 71 : 373 – 378 .
- Giovanelli , G. , Zanoni , B. , Lavelli , V. and Nanic , R. 2002 . Water sorption, drying and antioxidant properties of dried tomato products . Journal of Food Engineering , 52 : 135 – 141 .
- Giuntini , D. , Graziani , G. , Lercari , B. , Fogliano , V. , Soldatini , G. F. and Ranieri , A. 2005 . Changes in carotenoid and ascorbic acid contents in fruits of different tomato genotypes related to the depletion of UV-B radiation . Journal of Agriculture and Food Chemistry , 53 : 3174 – 3181 .
- Guil-Guerrero , J. L. and Rebolloso-Fuentes , M. M. 2009 . Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties . Journal of Food Composition and Analysis , 22 : 123 – 129 .
- Günhan , T. , Demir , V. , Hancioglu , E. and Hepbasli , A. 2005 . Mathematical modelling of drying of bay leaves . Energy Conversion and Management , 46 : 1667 – 1679 .
- Halevy , S. , Koth , H. and Guggenheim , K. 1957 . The vitamin and mineral content of fruits and vegetables grown in Israel . British Journal of Nutrition , 11 : 409 – 413 .
- Hamid , M. D. 1985 . Effect of plant density on tomato yield. Training report of the 3rd Regional Training Course in Vegetable Production and Research , Kamphaeng Saen, Nakhon Pathom, Thailand : TOP-AVRDC, Kasetsart University .
- Henderson , S. M. and Pabis , S. 1961 . Grain drying theory I: Temperature effect on drying coefficient . Journal of Agricultural Research Engineering , 6 : 169 – 174 .
- Hernández Suárez , M. , Rodriguez Rodriguez , E. M. and Diaz Romero , C. 2007 . Mineral and trace element concentrations in cultivars of tomatoes . Food Chemistry , 104 : 489 – 499 .
- Horner , W. F.A. 1993 . “ Drying: chemical changes ” . In Encyclopaedia of food science, food technology and nutrition , Edited by: Marcrae , R. , Robinson , R. K. and Sadler , M. J. 1485 – 1489 . London : Academic Press .
- Kerkhofs , N. S. , Lister , C. E. and Savage , G. P. 2003 . Antioxidant compounds in fresh tomatoes . Proceeding Nutrition Society of New Zealand , 28 : 96 – 104 .
- Kerkhofs , N. S. , Lister , C. E. and Savage , G. P. 2005 . Change in colour and antioxidant content of tomato cultivars following forced-air drying . Plant Foods for Human Nutrition , 60 : 117 – 121 .
- Kim , S. , Lee , K. W. , Park , J. , Lee , H. J. and Hwang , I. K. 2006 . Effect of drying in antioxidant activity and changes of ascorbic acidmand colour by different drying and storage in Korean red pepper (Capsicum annuum, L.) . International Journal of Food Science and Technology , 41 ( Suppl. 1 ) : 90 – 95 .
- Krokida , M. K. and Maroulis , Z. B. 1999 . Effect of microwave drying on some quality properties of dehydrated products . Drying Technology , 17 : 449 – 466 .
- Larcher , W. 2003 . Physiological plant ecology. Ecophysiology and stress physiology of functional groups , 4th ed. , Berlin : Springer .
- Luthria , D. L. , Mukhopadhyay , S. and Krizek , D. T. 2006 . Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation . Journal of Food Composition and Analysis , 19 : 771 – 777 .
- Madamba , P. S. , Driscoll , R. H. and Buckle , K. A. 1996 . Thin-layer drying characteristics of garlic slices . Journal of Food Engineering , 29 : 75 – 97 .
- Maskan , M. 2001 . Kinetics of colour change of kiwifruits during hot air and microwave drying . Journal of Food Engineering , 48 : 169 – 175 .
- Maskan , A. , Kaya , S. and Maskan , M. 2002 . Hot air and sun drying of grape leather (pestil) . Journal of Food Engineering , 54 : 81 – 88 .
- Midilli , A. and Küçük , H. 2003 . Mathematical modelling of thin layer drying of pistachio by using solar energy . Energy Conversion and Management , 46 : 1667 – 1679 .
- Miller , N. and Rice-Evans , C. 1997 . Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay . Free Radical Research , 26 : 195 – 199 .
- Özdemir , M. and Devres , Y. O. 1999 . The thin layer drying characteristics of hazelnuts during roasting . Journal of Food Engineering , 42 : 225 – 233 .
- Perez-Galvez , A. , Hornero-Mendez , D. and Minguez-Mosquera , M. I. 2005 . Dependence of carotenoid content and temperature-time regimes during the traditional slow drying of red pepper for paprika production at La Vera county . European Food Research and Technology , 221 : 645 – 652 .
- Pott , I. , Neidhart , S. , Mühlbauer , W. and Carle , R. 2005 . Quality improvement of non-sulphited mango slices by drying at high temperatures . Innovative Food Science and Emerging Technologies , 6 : 412 – 419 .
- Sahlin , E. , Savage , G. P. and Lister , C. E. 2004 . Investigation of the antioxidant properties of tomatoes after processing . Journal of Food Composition and Analysis , 17 : 635 – 647 .
- Shi , J. , Le Maguer , M. , Kakuda , Y. , Liptay , A. and Kiekamp , F. 1999 . Lycopene degradation and isomerisation in tomato dehydration . Food Research International , 32 : 15 – 21 .
- Sharma , G. P. , Verma , R. C. and Pathare , P. 2005 . Mathematical modeling of infrared radiation thin layer drying of onion slices . Journal of Food Engineering , 71 : 282 – 286 .
- Singh , R. P. , Murthy , K. N.C. and Jayaprakasha , G. K. 2002 . Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in-vitro models . Journal of Agricultural and Food Chemistry , 50 : 81 – 86 .
- Skujins , S. 1998 . Handbook for ICP-AES (Varian-Vista). A short guide to Vista series , Switzerland : Varian Int . ICP-AES Operation. AG, Zug, Version 1.0
- Soysal , Y. 2004 . Microwave drying characteristics of parsley . Biosystems Engineering , 89 : 167 – 173 .
- Soysal , Y. , Öztekin , S. and Eren , Ö. 2006 . Microwave drying of parsley: Modelling, kinetics, and energy aspects . Biosystems Engineering , 93 : 403 – 413 .
- Stahl , W. and Sies , H. 1992 . Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans . Journal of Nutrition , 122 : 2161 – 2166 .
- Sumnu , G. , Turabi , E. and Öztop , M. 2005 . Drying of carrots in microwave and halogen lamp-microwave combination ovens . Lebensmittel Wissenschaft und Technologie , 38 : 549 – 553 .
- Sun , J. , Hu , X. , Zhao , G. , Wu , J. , Wang , Z. , Chen , F. and Liao , X. 2007 . Characteristics of thin-layer infrared drying of apple pomace with and without hot air pre-drying . Food Science and Technology International , 13 : 91 – 97 .
- Thompson , L. , Morris , J. , Peffley , E. , Gren , C. , Pare , P. , Tissue , D. and Kane , C. 2005 . Flavonol content and composition of spring onions grown hydroponically or in potting soil . Journal of Food Composition and Analysis , 18 : 635 – 645 .
- Togrul , I. T. and Pehlivan , D. 2002 . Mathematical modelling of solar drying of apricots in thin layers . Journal of Food Engineering , 55 : 209 – 216 .
- Tonucci , L. H. , Holden , J. M. , Beecher , G. R. , Khachik , F. , Davies , C. S. and Mulokozi , G. 1995 . Carotenoid content of thermally processed tomato-based food products . Journal of Agricultural and Food Chemistry , 43 : 579 – 586 .
- Tutuncu , M. A. and Labuza , T. P. 1996 . Effect of geometry on the effective moisture transfer diffusion coefficient . Journal of Food Engineering , 30 : 433 – 447 .
- Vargas , Chavez, G.E. and Rodri´guez , Rodriguez, A. 2000 . Influencia de las aguas de riego en los procesos de salinizacio´n y sodificacio´n de suelos en cultivos de pla´tanos y tomates (I. Canarias) . Edafologi´a , 7 : 129 – 136 .
- Walde , S. G. , Velu , V. , Jyothirmayi , T. and Math , R. G. 2006 . Effects of pretreatments and drying methods on dehydration of mushroom . Journal of Food Engineering , 74 : 108 – 115 .
- Yaldız , O. , Ertekin , C. and Uzun , H. I. 2001 . Mathematical modeling of thin layer solar drying of sultana grapes . Energy , 26 : 457 – 465 .
- Zanoni , B. , Peri , C. , Nani , R. and Lavelli , V. 1999 . Oxidative heat damage of tomato halves as affected by drying . Food Research International , 31 : 395 – 394 .
Supplementary material
Supplementary Figure 1. Variations of moisture ratio as a function of time for sun, oven and microwave drying of tomato slices. Δ, sun; □, oven 50 °C; *, oven 70 °C; +, microwave (210 W), ▪, microwave (700 W). The lines show the predicted values which fit well for the corresponding drying methods (Two term model for sun drying, Midilli and Kucuk (2003) model for oven (50 °C), microwave (210 and 700W), modified Page model for oven (70 °C) drying. Data given are the mean values of three replications.
Figura adicional 1. Variaciones del nivel de humedad como función de tiempo para rodajas de tomate secadas al sol, horno y microondas.
Supplementary Figure 2. Effects of different drying methods on L*, a*, and b* values of tomato slices. (1) fresh, (2) sun dried, (3) oven dried 50 °C, (4) oven dried 70 °C, (5) microwave (210 W), (6) microwave (700 W). Bars with different letters are significantly different (P < 0.05).
Figura adicional 2. Efectos de diferentes métodos de secado en valores L*, a* y b* de rodajas de tomate.