Abstract
Dyes are widely used as additives in the food industry. As some dyes may pose health risks when being in contact with food, their removal in industrial processes is an important issue. Since the surfactant sodium dodecyl sulphate (SDS) covers a wide range of variety of task to remove oily materials, and it is often used in detergency processes, the association of some relevant dyes to SDS micellar aggregates has been investigated. In particular, the binding constants of the dyes, bromophenol blue, malachite green, crystal violet, congo red, calcon, methyl orange, and methyl red, to the SDS micelles have been measured using a UV-Vis spectrophotometric method. In all cases, a strong association of the different substrates has been probed.
Los colorantes son ampliamente usados como aditivos en la industria alimentaria. Debido a que algunos de estos colorantes, por estar en contacto con alimentos, presentan riesgos para la salud, su eliminación en procesos industriales es un tema importante. Puesto que el dodecil sulfato sódico (SDS) cubre un amplio rango en la eliminación de materiales aceitosos y es frecuentemente usado en procesos de detergencia, se ha investigado la asociación de diversos colorantes a agregados micelares de SDS. En particular, se han medido por un método espectrofotométrico UV-Vis las constantes de asociación de los colorantes azul de bromofenol, verde malaquita, violeta cristal, rojo congo, calcon, naranja de metilo y rojo de metilo a micelas de SDS. En todos los casos se observó una fuerte asociación de los diferentes sustratos, lo que demuestra su aplicabilidad en procesos de detergencia.
Keywords:
Palabras clave:
Introduction
A large portion of real-life chemical and biochemical processes take place in heterogeneous media. Due to the complexity of such media, there are not many contributions on these systems in chemistry and food science, and food technology courses (Rodríguez, Ríos, Mosquera, Ríos, & Mejuto, Citation1995). In the last few years, the intensive research about these media has increased so that any chemist is nowadays familiarized with heterogeneous media (Astray et al., Citation2010; De la Rosa, Mercado-Mercado, Rodrigo-García, González-Aguilar, & Álvarez-Parrilla, Citation2010; Deb, Shannigrahi, & Bagchi, Citation2008; García Río, Leis, Mejuto, & Pérez-Lorenzo, Citation2007; García Río, Mejuto, & Pérez-Lorenzo, Citation2004; Ghosh & Guchhait, Citation2009). Microheterogenous media are, as their name implies, heterogeneous microscopically. Micellar systems are one of the simplest but most useful classes of microheterogenous media, which are exemplified by many industrial and commercial products such as domestic detergents (Fogarty, Dempsey, & Regan, Citation2003; Ghaemi, Khosravi-Fard, & Neshati, Citation2005; Savarino, Montomeri, Musso, & Boffa, 2010). Micellar systems have also been used as simple models of biological membranes and to control chemical reactions (Beck, Li-Blatter, Seelig, & Seelig, Citation2010; Bombelli et al., Citation2010; Dutta et al., Citation2010; Fendler, Citation1982; Fendler & Fendler, Citation1975; Smith, Vinchurkar, Gronbech-Jensen, & Parikh, Citation2010).
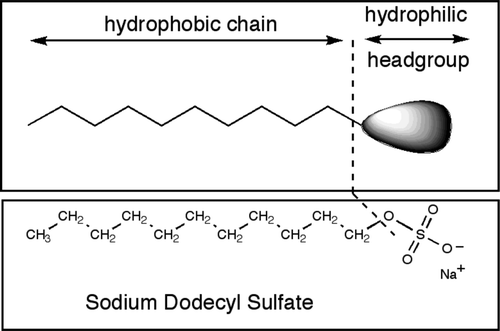
Micelles are aggregates of molecules known as surfactants (that correspond with the acronym surface-active agents). Surfactant molecules are composed of a hydrophilic (water-loving) part and a hydrophobic (water-hating) part. In the surfactant used in the experiment described in the present article, sodium dodecyl sulphate (SDS), the hydrophobic tail is the apolar hydrocarbon chain ‒(CH2)11‒CH3, and the hydrophilic head is the sulphate group ‒OSO3 – (). When a surfactant is added to water at a concentration exceeding a threshold called the critical micelle concentration (CMC), the hydrophobic tails of the excess surfactant molecules associate so that the surfactant forms more or less spherical aggregates (micelles) with the hydrophilic heads pointing out into the bulk water and the hydrophobic tails pointing in toward the centre. The surfactant molecules of the micelles are in constant motion and are continually exchanged with the surfactant dissolved in the bulk medium (Luisi, Citation2001). SDS is a highly effective surfactant. Industrial uses of SDS cover a wide range of variety of task to remove oily materials and residues. It is an important component in detergency processes such as engine degreasers, floor cleaners, car wash soaps, toothpastes, biomolecular techniques, shampoos, shaving foams, etc. (Davis, Morton III, Counce, Depaoli, & Hu, 2006; Duarte, Coelho, & Leite, Citation2002; Kabir-Ud-Din, Kumar, & Parveen, 2008; Queiroga-Neto, Bora, Diniz, Cavalheiro, & Souza, Citation2009; Torres-Arreola, Pacheco-Aguilar, Sotelo-Mundo, Rouzaud-Sández, & Ezquerra-Brauer, 2008; Wu, Hettiarachchy, & Rhee, Citation1998).
Dyes are colored substances that have affinity to the substrate to which it is being applied. Because of this, dyes are used in foods and drinks to color them. Another color-generating compound used in foods is the indicators. Food packaging technologists are continuously seeking new insights into the preservation of foods in order to indicate the quality of fresh, refrigerated, and processed foods. As a customer convenience type of active packaging, time temperature indicators and microwave susceptors are typically separate components of the package (Kerry, O'Grady, & Hogan, Citation2006; Labuza & Breene, Citation1989; Rooney, Citation1995). The use of color indicator has been developed to investigate both the food freshness in packaging (Smolander, Citation2003; Smolander, Hurme, & Ahvenainen, Citation1997) and the fermentation of vegetable products, and to evaluate on its applicability to packaging, storage, and distribution (Hong & Park, Citation2000).
Many dyes and indicators in contact with food may pose health risks. It has been demonstrated that some organic azo dyes affect adversely and alter biochemical markers in vital organs, e.g. liver and kidney in rats, not only at higher doses but also at lower doses (Amin, Hameid, & Elsttar, 2010; Mekkawy, Ali, & El-Zawahry, Citation1998). In particular, azo dyes and their degradation products have been probed to gain mutagenic and carcinogenic effects (Chung, Citation1983; Chung, Fulk, & Andrews, Citation1981). Dyes such as malachite green and crystal violet belong to the group of triphenylmethane dyes, which are on the Food and Drug Administration's (FDA's) priority list for fish drugs. In contrast, some natural food dyes have been found to be less toxic, non-carcinogenic, good antibiotics and antioxidants, and consequently, less health hazardous (Siva, Citation2007; Siva et al., Citation2011).
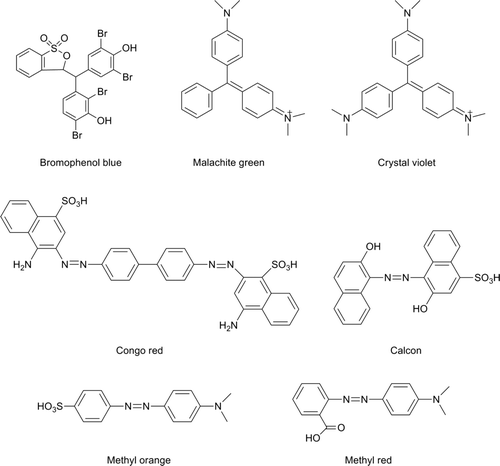
Since (i) dyes and indicators are believed to be toxic and carcinogenic or to be prepared from known carcinogens (Kariminiaae-Hamedaani, Sakurai, & Sakakibara, Citation2007; Novotny et al., Citation2006) and (ii) due to their detergency properties, surfactants have been used to remove a wide variety of substances in food industry (Jurado Alameda, Bravo Rodríguez, Altmajer Vaz, & de Cassia Siqueira Curto Valle, Citation2011), in this study, we have investigated the removal of different dyes in presence of micellar aggregates. In particular, the association of the dyes (bromophenol blue, malachite green, crystal violet, congo red, calcon, methyl orange, and methyl red) (), which cover a wide range in terms of lipophilic properties and are used in food industry as sensors (Morris, Citation2006), to self-assembly colloids (SDS micelles) was investigated.
Material and methods
The surfactant SDS and the dyes 4-[(4-dimethylaminophenyl)-phenyl-methyl]-N,N-dimethyl-aniline (crystal violet), 4,4′-(1,1-dioxido-3H-2,1-benzoxathiole-3,3-diyl)bis(2,6-dibromophenol) (bromophenol blue), 2-[(4-dimethyl-aminophenyl)diazenyl]benzoic acid (methyl red), sodium 4-[(2-hydroxynaphthalen-1-yl)hydrazinylidene]- 3-oxonaphthalene-1-sulfonate (calcon), 4-[(4-dimethylamino-phenyl)phenyl-methyl]-N,N-dimethylaniline (malachite green), 4-dimethylaminoazo-benzene-4′-sulfonic acid sodium salt (methyl orange), and sodium 3,3′-(1E,1′E)-biphenyl-4,4′-diylbis(diazene-2,1-diyl)bis(4-aminonaphthalene-1-sulfonate) (congo red) were supplied by Aldrich and used as received. To obtain the data shown in , the absorbance or a fixed quantity of dye in the presence of different concentrations of surfactant SDS, [SDS] is measured. The wavelength of measurement was chosen by comparison of UV-Vis spectra in the absence of surfactant and at large amount of surfactant (0.3 M of tensioactive), where we can assume that a large amount of dye will be bound to the micellar aggregate. A Kontron spectrophotometer (model Uvicon 923) was used. Measurements were carried out at 25°C. Temperature was kept constant using a Polyscience thermostat-cryostat temperature controller with an error of ±0.1°C.
CMC values of SDS have been obtained using surface tension measurements. The surface tension, σ/m Nm−1, was measured using a Kruss tensiometer (model K9) using the procedure of the Wilhelmy plate (Astray et al., Citation2011a,b). All of the measurements of surface tension had been carried out at 25.0 ± 0.1°C using a Polyscience thermostat-cryostat temperature controller. Nonlinear regression was carried out using Grafit 5.0 supplied by Erithacus Software Ltd.
Partition coefficient values (LogP) were calculated with the Chem3D Ultra® Molecular Modelling and Analysis software, version 12.0. The CLogP Driver was used.
Results and discussion
The association constants of dyes to micellar aggregates can be spectrophotometrically determined using reported procedures in the literature (García Río, Hervés, Mejuto, Parajó, & Pérez-Juste, Citation1998). This determination is based on the fact that changes in the medium will modify the UV-Vis spectrum (associations of the crystal violet dye with β-cyclodextrin and hexadecyltrimethylammonium chloride mixed systems have been previously investigated using UV-Vis NIR spectroscopy (García Río & Godoy, Citation2007)). The association of dyes to the micelles will imply changes in the position and in the intensity of their UV-Vis peaks. We can conclude that the absorbance measured (A) in the presence of micelles corresponds with the sum of dye absorbance in water (A w) and at the micellar aggregate (A m) (Equation (1)).
Taking into account Beer–Lambert law, Equation (1) can be rewritten as a function of total dye concentration [dye]t, Equation (2).
where, [dye]w, ϵw, [dye]m, and ϵm are the dye concentrations and molar absorption coefficients in the continuous medium and in the micellar aggregate, respectively. l is the light pathway (in a standard cell, it is equal to 1 cm).
The three concentrations of Equation (2) are related to the binding constant of a dye to the micellar aggregate, K dye, defined as:
where, [D n] is the micellized surfactant (total concentration of surfactant minus the CMC).
Using Equation (3) and the corresponding matter balance ([dye]t = [dye]m + [dye]w), Equations (4) and (5) can be easily deduced:
Equations (4) and (5) imply:
Because ϵappl[dye]t is equal to the total absorbance (A), ϵwl[dye]t is the absorbance in the absence of micellar aggregate (A w), and ϵml[dye]t corresponds with the absorbance when all the substrate is included in the micellar media (A m), Equation (6) can be written as:
If we measure the absorbance at various surfactant concentrations at a fixed concentration of dye, the binding constant can be calculated from the Equation (7).
shows the values of A against the corresponding [D n], with a leveling-off behavior fitted in accordance with Equation (7). Since the CMC of the surfactant used is 8.1·103 mol/dm3, [D n] is obtained as [SDS] – CMC. The K dye values obtained are in agreement with other published values (Armstrong, Menges, & Han, Citation1988) and are shown in .
An alternative treatment of experimental data can be done using a linearized equation:
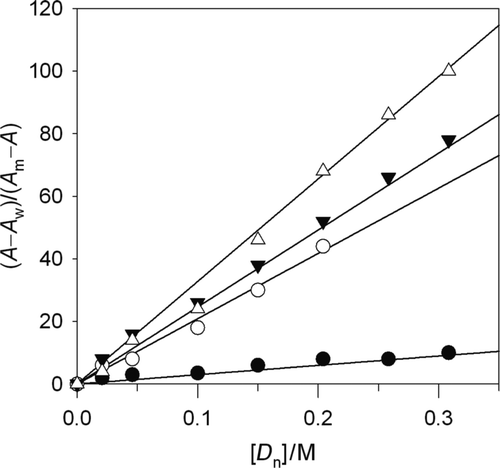
In this case, A w corresponds with the measurement in the absence of surfactant and A m can be substituted by absorbance value at high concentrations of [D n] or from the direct extrapolation of the plateau. The fits of the experimental data to Equation (8) are shown in . The values of K dye are compatible with the values obtained from the non-linear regression using Equation (7).
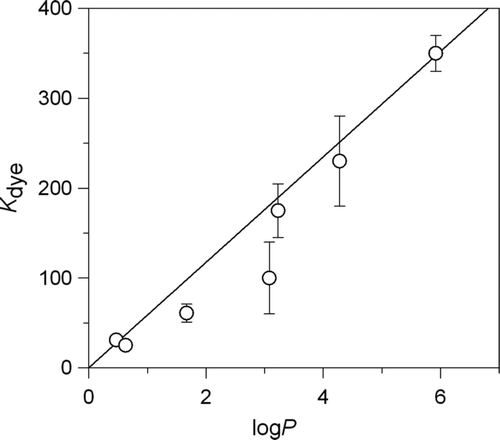
To measure the lipophilicity of these compounds, the logarithm of 1-n-octanol/water partition coefficients (logP), Equation (9), of each dye was calculated.
It is well known that logP has been extensively used as hydrophobicity parameter and it is easily obtained both from experimental data (partition coefficient between water and 1-n-octanol) and from computational procedures. As can be observed in , a correlation between logP and the binding constants were found (R = 0.95). Because the core of the SDS self-assembly aggregates presents hydrophobic properties, the higher the lipophilicity of a dye, the higher the affinity of this dye to SDS micellar aggregates, which demonstrates that the main driving force for the inclusion of the substrates to micellar aggregates is the hydrophobicity of the substrate. More hydrophobicity of dye implies better capacity of SDS for detergency procedures in food industry.
Conclusions
The binding of a wide range of dyes with SDS self-assembly colloids has been studied by UV-Vis spectroscopic methods. In all cases, high values of binding constants have been observed. This implies a significant association between dyes and SDS micellar aggregates. Considering the toxicity of these substances and their applications in the food industry, a simple process of washing with commercial SDS-based detergents could effectively remove these substances. The correlation between the logP and the binding constants observed implies that the higher the hydrophobicity of a dye, the higher the inclusion in SDS micelles, and therefore, it will be removed easily.
tcyt_a_585718_sup_27125295.pdf
Download PDF (927.3 KB)Acknowledgments
We thank the Xunta de Galicia (10 PXIB 383 187 PR) and Excma. Diputación Provincial de Ourense (INOU) (K125 131H 64702) for financial support. A.C. & J.M. thank the University of Vigo for a research training grant. G.A. thanks the Govern of Spain for an FPU research training grant (P.P. 0000421S 14006). Thanks are also given for the valuable comments made by the referees.
References
- Amin , K. A. , Abdel-Hameid , H. and Abd-Elsttar , A. H. 2010 . Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats . Food and Chemical Toxicology , 48 : 2994 – 2999 .
- Armstrong , D. W. , Menges , R. A. and Han , S. M. 1988 . Evaluation of dye-micelle binding constants using diffusion sensitive band broadening effects . Journal of Colloid and Interface Science Colloid , 126 : 239 – 242 .
- Astray , G. , Cid , A. , García-Río , L. , Lodeiro , C. , Mejuto , J. C. , Moldes , O. and Morales , J. 2010 . Cyclodextrin-surfactant mixed systems as reaction media . Progress in Reaction Kinetics and Mechanism , 35 : 105 – 129 .
- Astray , G. , Cid , A. , Manso , J. A. , Mejuto , J. C. , Moldes , O. and Morales , J. 2011a . Basic degradation of 3-keto-carbofuran in the presence of non-ionic self-assembly colloids . Fresenius Environmental Bulletin , 20 : 354 – 357 .
- Astray , G. , Cid , A. , Manso , J. A. , Mejuto , J. C. , Moldes , O. and Morales , J. 2011b . Influence of anionic and non-ionic micelles upon hydrolysis of 3-hydroxy-carbofuran . International Journal of Chemical Kinetics , 43 : 402 – 408 .
- Beck , A. , Li-Blatter , X. , Seelig , A. and Seelig , J. 2010 . On the interaction of ionic detergents with lipid membranes. Thermodynamic comparison of n-alkyl- +N(CH3)3 and n-alkyl-SO4 – . The Journal of Physical Chemistry B , 114 : 15862 – 15871 .
- Bombelli , C. , Bernardini , C. , Elemento , G. , Mancini , G. , Sorrenti , A. and Villani , C. 2010 . Concentration as the switch for chiral recognition in biomembrane models . Journal of the American Chemical Society , 130 : 2732 – 2733 .
- Chung , K. -T. 1983 . The significance of azo-reduction in the mutagenesis and carcinogenesis of azo dyes . Mutation Research , 114 : 269 – 281 .
- Chung , K. -T. , Fulk , G. E. and Andrews , A. W. 1981 . Mutagenicity testing of some commonly used dyes . Applied and Environmental Microbiology , 42 : 641 – 648 .
- Davis , A. N. , Morton , S. A. III , Counce , R. M. , Depaoli , D. W. and Hu , M. Z.-C. 2006 . Effect of ionic strength on oil removal from stainless steel in the presence of ionic surfactant . Separation Science and Technology , 41 : 3313 – 3328 .
- De la Rosa , L. A. , Mercado-Mercado , G. , Rodrigo-García , J. , González-Aguilar , G. A. and Álvarez-Parrilla , E. 2010 . Peach polyphenol oxidase inhibition by β-cyclodextrin and 4-hexylresorcinol is substrate dependent . CyTA – Journal of Food , 8 : 87 – 93 .
- Deb , N. , Shannigrahi , M. and Bagchi , S. 2008 . Use of fluorescence probes for studying kamlet−taft solvatochromic parameters of micellar system formed by binary mixture of sodium dodecyl sulfate and triton-X 100 . The Journal of Physical Chemistry B , 112 : 2868 – 2873 .
- Duarte , A. C.P. , Coelho , M. A.Z. and Leite , S. G.F. 2002 . Identification of peroxidase and tyrosinase in green coconut water . CyTA – Journal of Food , 3 : 266 – 270 .
- Dutta , A. , Kim , T. -Y. , Moeller , M. , Wu , J. , Alexiev , U. and Klein-Seetharaman , J. 2010 . Characterization of membrane protein non-native states. 2. The SDS-unfolded states of rhodopsin . Biochemistry , 49 : 6329 – 6340 .
- Fendler , J. H. 1982 . Membrane mimetich chemistry: Characterizations and applications of micelles, microemulsions, monolayers, bilayers, vesicles, host-guest systems, and polyions , New York : John Wiley .
- Fendler , J. H. and Fendler , E. J. 1975 . Catalysis in micellar and macromolecular systems , New York : Academic .
- Fogarty , B. , Dempsey , E. and Regan , F. 2003 . Potential of microemulsion electrokinetic chromatography for the separation of priority endocrine disrupting compounds . Journal of Chromatography A , 1014 : 129 – 139 .
- Jurado Alameda , E. , Bravo Rodríguez , V. , Altmajer Vaz , D. and de Cassia Siqueira Curto Valle , R. 2011 . Effectiveness of starch removal in bath-substrate-flow (BSF) device using surfactants and α-amylase . Food Hydrocolloids , 25 : 647 – 653 .
- García Río , L. and Godoy , A. 2007 . Use of spectra resolution methodology to investigate surfactant/β-cyclodextrin mixed systems . Journal of Physical Chemistry B , 111 : 6400 – 6409 .
- García Río , L. , Hervés , P. , Mejuto , J. C. , Parajó , M. and Pérez-Juste , J. 1998 . Association constant of crystal violet in micellar aggregates: Determination by spectroscopic techniques . Journal of Chemical Research , Part S : 716 – 717 .
- García Río , L. , Leis , J. R. , Mejuto , J. C. and Pérez-Lorenzo , M. 2007 . Microemulsions as microreactors in physical organic chemistry . Pure and Applied Chemistry , 79 : 1111 – 1123 .
- García Río , L. , Mejuto , J. C. and Pérez-Lorenzo , M. 2004 . Modification of reactivity by changing microemulsion composition. Basic hydrolysis of nitrophenyl acetate in AOT/isooctane/water systems . New Journal of Chemistry , 28 : 988 – 995 .
- Ghaemi , M. , Khosravi-Fard , L. and Neshati , J. 2005 . Improved performance of rechargeable alkaline batteries via surfactant-mediated electrosynthesis of MnO2 . Journal of Power Sources , 141 : 340 – 350 .
- Ghosh , S. and Guchhait , N. 2009 . Chemically induced unfolding of bovine serum albumin by urea and sodium dodecyl sulfate: A spectral study with the polarity-sensitive charge-transfer fluorescent probe (E)- 3-(4-methylaminophenyl)acrylic acid methyl ester . A European Journal of Chemical Physics and Physical Chemistry , 10 : 1664 – 1671 .
- Hong , S. -I. and Park , W. -S. 2000 . Use of color indicators as an active packaging system for evaluating kimchi fermentation . Journal of Food Engineering , 46 : 67 – 72 .
- Kabir-Ud-Din , Kumar S. and Parveen , N. 2008 . The clouding phenomenon for anionic sodium dodecyl sulfate + quaternary bromides in polar nonaqueous-water-mixed solvents . Journal of Surfactants and Detergents , 11 : 335 – 341 .
- Kariminiaae-Hamedaani , H. R. , Sakurai , A. and Sakakibara , M. 2007 . Decolorization of synthetic dyes by a new manganese peroxidase producing white rot fungus . Dyes and Pigments , 72 : 157 – 162 .
- Kerry , J. P. , O'Grady , M. N. and Hogan , S. A. 2006 . Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review . Meat Science , 74 : 113 – 130 .
- Labuza , T. P. and Breene , W. M. 1989 . Applications of active packaging for improvement of shelf-life and nutritional quality of fresh and extended shelf-life foods . Journal of Food Processing and Preservation , 13 : 1 – 69 .
- Luisi , P. L. 2001 . Are micelles and vesicles chemical equilibrium systems? . Journal of Chemical Education , 78 : 380 – 384 .
- Mekkawy , H. A. , Ali , M. O. and El-Zawahry , A. M. 1998 . Toxic effect of synthetic and natural food dyes on renal and hepatic functions in rats . Toxicology Letters , 95 : 155
- Morris , R. J. 2006 . U.S. patent no. 2006/0121165 , Vero Beach , FL : US Patent and Trademark Office .
- Novotny , C. , Dias , N. , Kapanen , A. , Malachova , K. , Vandrovcova , M. , Itävaara , M. and Lima , N. 2006 . Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes . Chemosphere , 63 : 1436 – 1442 .
- Queiroga-Neto , V. , Bora , P. S. , Diniz , Z. N. , Cavalheiro , J. M.D.O. and Souza , P. A.S. 2009 . Partial evaluation of Dipteryx lacunifera seed kernel as a nutritional food . CyTA – Journal of Food , 7 : 23 – 29 .
- Rodríguez , M. F. , Ríos , M. C. , Mosquera , M. , Ríos , A. M. and Mejuto , J. C. 1995 . Fluorescence quenching in microheterogenous media: A laboratory experiment determining micelle aggregation number . Journal of Chemical Education , 72 : 662 – 663 .
- Rooney , M. L. 1995 . Active food packaging , 1 – 37 . London : Blackie .
- Savarino , P. , Montoneri , E. , Musso , G. and Boffa , V. 2010 . Biosurfactants from urban wastes for detergent formulation: Surface activity and washing performance . Journal of Surfactants and Detergents , 13 : 59 – 68 .
- Siva , R. 2007 . Status of natural dyes and dye-yielding plants in India . Current Science , 92 : 916 – 925 .
- Siva , R. , Palackan , M. G. , Maimoon , L. , Geetha , T. , Bhakta , D. , Balamurugan , P. and Rajanarayanan , S. 2011 . Evaluation of antibacterial, antifungal, and antioxidant properties of some food dyes . Food Science and Biotechnology , 20 : 7 – 13 .
- Smith , A. M. , Vinchurkar , M. , Gronbech-Jensen , N. and Parikh , A. N. 2010 . Order at the edge of the bilayer: Membrane remodeling at the edge of a planar supported bilayer is accompanied by a localized phase change . Journal of the American Chemical Society , 132 : 9320 – 9327 .
- Smolander , M. 2003 . “ The use of freshness indicators in packaging ” . In Novel food packaging techniques , Edited by: Ahvenainen , R. 128 – 143 . Cambridge , UK : Woodhead Publishing Ltd .
- Smolander , M. , Hurme , E. and Ahvenainen , R. 1997 . Leak indicators for modified-atmosphere packages . Trends in Food Science & Technology , 8 : 101 – 106 .
- Torres-Arreola , W. , Pachecho-Aguilar , R. , Sotelo-Mundo , R. R. , Rouzaud-Sández , O. and Ezquerra-Brauer , J. M. 2008 . Partial characterization of collagen from mantle, fin, and arms of jumbo squid (Dosidicus gigas) . CyTA – Journal of Food , 6 : 101 – 108 .
- Wu , W. , Hettiarachchy , N. S. and Rhee , K. C. 1998 . Enhancing storage stability of emulsions by binding detergents with soy protein isolate and its hydrolysates . Journal of Surfactants and Detergents , 3 : 387 – 392 .
Supplementary material
Supplementary Table 1. Binding constants of dyes obtained from Equation (7).
Tabla 1. Constantes de asociación de los colorantes obtenidas de la Ec. 7
Supplementary Figure 2. Chemical structures of the studied dyes.
Figura 2. Estructuras químicas de los colorantes estudiados.
Supplementary Figure 3. Influence of micellized surfactant upon absorbance. (⧫) Bromophenol blue, (ˆ) methyl red, and (•) congo red. Solid line corresponds with the fit of experimental results to Equation (7).
Figura 3. Influencia del surfactante micelizado en la absorbancia. (⧫) Azul de bromofenol, (ˆ) rojo de metilo y (•) rojo congo. La línea sólida corresponde al ajuste de los resultados experimentales a la Ec. 7.
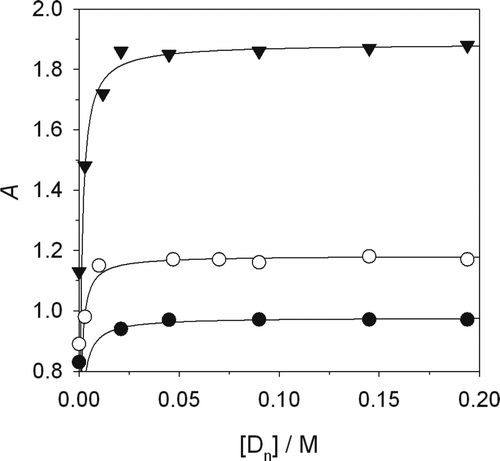
Supplementary Figure 4. Linearization of experimental data according to Equation (8). (Δ) Bromophenol blue, (▾) methyl red, (•) malachite green, and (ˆ) congo red.
Figura 4. Linealización de los datos experimentales de acuerdo con la Ec. 8. (Δ;) Azul de bromofenol, (▾) rojo de metilo, (•) verde malaquita y (ˆ) rojo congo.