Abstract
The relationship between the time-to-detection (TTD) and the generation time (GT) of Escherichia coli was studied under different pH values (4.5, 5.5, 6.5, and 7.4) and temperatures (30°C, 35°C, 37°C or 42°C). An adequate linear relationship was found between both parameters (R 2 = 0.899). The regression of the ratio ln(TTD/GT) only introduced the pH in the model. The validation of the model was carried out with a new dataset. Differences were observed at 30°C, and they increased with pH 5.5. The relative rate to detection (RRT) and the relative GT (RGT) were calculated. The results suggest that both are equivalent measures, so the GT could be obtained from the TTD and the RRD under optimal pH values. This conclusion would reinforce the initial statement, i.e. the relationship between the TTD and the GT.
Se ha estudiado la relación entre el tiempo de detección (TTD) y el tiempo de generación (GT) de Escherichia coli bajo distintas condiciones de pH (4,5, 5,5, 6,7, y 7,4) y de temperatura (30, 35, 37 y 42°C). Se encontró una relación lineal entre ambos parámetros (R2 = 0,899). La regresión de la relación ln(TTD/GT) sólo introdujo el pH en el modelo. La validación se realizó con un nuevo grupo de datos. Se observaron diferencias a 30°C, que aumentaron con un pH 5,5. Se calcularon la tasa de detección relativa y el GT relativo. Los resultados sugieren que ambos son equivalentes, por lo que el GT podría obtenerse a partir del TTD y de la tasa de detección relativa bajo valores de pH óptimos. Esta conclusión refuerza la idea inicial: la relación entre el TTD y el GT.
Introduction
The increased need for accurate data is difficult to meet with the classical viable count methods (Francois et al., Citation2005; Lindqvist, Citation2006). Automated optical density (or absorbance) measurement methods are useful to estimate microbial growth parameters because they are rapid and non-destructive (Dalgaard & Koutsoumanis, Citation2001). The ability of turbidimetric methods to produce vast amounts of accurate data on growth is of great interest to the industrial laboratories for rapid predictive microbiology development (Membré & Lambert, Citation2008). Several approaches exist to estimate growth parameters from the turbidity data (Lindqvist, Citation2006). They are based on fitting primary growth equations (i) to curves of absorbance values (Zhao, Montville, & Schaffner, Citation2000), (ii) to curves of log-transformed absorbance values (Begot, Desnier, Daudin, Labadie, & Lebert, Citation1996), (iii) to curves of transmittance values (McMeekin, Olley, & Ross, Citation1993), or (iv) to time-to-detection (TTD) values obtained from serially diluted cultures (Baranyi & Pin, Citation1999; Dalgaard & Koutsoumanis, Citation2001; Firstenberg-Eden & Eden, Citation1984).
In predictive microbiology, a bacterial growth curve is characterized by two kinetic parameters: the lag time (LT) and the generation time (GT). Many microbiologists assume a proportional (constant) relationship between the LT and the GT for bacterial cultures having identical physiological states at the moment of inoculation in a broth and being cultivated under different conditions (Baranyi & Roberts, Citation1994; Cooper, Citation1963; Delignette-Muller, Citation1998; Zhao et al., Citation2000). Several authors also noticed the relationship between the LT and the specific growth rate μ (Baranyi & Roberts, Citation1994; Dalgaard & Koutsoumanis, Citation2001; Mellefont, McMeekin, & Ross, Citation2003, Citation2005; Mellefont & Ross, Citation2003; Pin, García de Fernando, Ordoñez, & Baranyi, Citation2002; Robinson, Ocio, Kaloti, & Mackey, Citation1998; Swinnen, Bernaerts, & Van Impe, Citation2006).
Baranyi and Pin (Citation1999) and Robinson et al. (Citation1998) proposed the use of the TTD (the time needed by a bacterial population to reach a detectable level of turbidity) as an alternative to the LT. The TTD measurements obtained from absorbance techniques are as valid as the traditional plate techniques used for growth curves, although TTD focuses on a part of the growth curve near the end of the exponential growth (Membré & Lambert, Citation2008). The measurement of the TTD provides a simple means of establishing reasonable estimates of growth rate under different conditions (Cuppers & Smelt, Citation1993). Several authors used the TTD for growth parameters estimation (Francois et al., Citation2005; Lindqvist, Citation2006; McKellar & Knight, Citation2000) or for predicting the interactions between antimicrobial factors (Bidlas & Lambert, Citation2008; Lambert & Bidlas, Citation2007). The following assumptions are taken into account: (i) the TTD is the sum of the LT plus the t TTD (time needed to reach the TTD from the end of the LT phase) (Pin & Baranyi, Citation2008); (ii) the LT is constant for bacterial cultures with the same physiological state at the moment of inoculation in a broth (Baranyi & Pin, Citation1999); and (iii) the t TTD depends on the GT (the more the GT values decrease, the less the t TTD values are).
The aim of the present study was to analyze the relationship between the TTD and the GT under different pH values and non-limiting temperatures. Three different approaches to estimate the TTD and the GT from Escherichia coli absorbance curves were used. The absorbance values were not previously transformed to CFU/ml values because it is beyond the scope of this article to take this transformation into account. Finally, a simple linear method to estimate the GT of E. coli from the TTD is used.
Materials and methods
Strain and cultures
A non-pathogenic E. coli strain provided by the Spanish Type Culture Collection (University of Valencia, Spain) was used: E. coli CECT 516. The strain was reconstituted in Brain Heart Infusion (BHI, Difco Laboratories, Detroit, Mich., USA) and incubated at 37°C for 24 h. Four bottles with 250 ml of BHI were prepared. The pH value was adjusted in each bottle to 4.5, 5.5, 6.5, and 7.4 with HCl and NaOH. Nine ml from each bottle were transferred to tubes and sterilized by autoclaving at 121°C for 15 min. These tubes were used to dilute the inoculum previously cultivated in BHI at 37°C for 24 h (ca. 4.8 × 109 CFU/ml).
Absorbance-CFU linear range and threshold of detection
The linear range is the absorbance (A) range where the Beer–Lambert's law is verified (Koch, Citation1981). It was determined by plotting A vs. CFU/ml. The cultures of E. coli were diluted at 1/2, 1/4, 1/5, 1/8, 1/10, 1/16, 1/20, 1/50, 1/100, 1/500, and 1/1000 with BHI. They were enumerated in PCA (plate count agar; Difco) at 37°C for 24 h. Two hundred μl from each dilution was placed into six wells, and 200 μl from non-inoculated BHI were placed into 12 wells. Absorbance was measured at 595 nm. The TTD corresponds to the bacterial concentration that involves a significant change of the A values. The TTD was selected as the lower limit of the linear range by plotting A vs. CFU/ml (McMeekin et al., Citation1993). This value was determined as 0.102 (ca. 9.6 × 106 CFU/ml). Below that value no linear relationship exists between A and bacterial population; and above it the changes of the bacterial population can be detected with A measurements. As TTD = LT + t TTD (Pin & Baranyi, Citation2008), minimizing the t TTD approximates the TTD to the LT. An initial concentration of ca. 5 × 106 CFU/ml was used to inoculate the microtiter plate wells, so the growth would start just below the TTD and the t TTD would be minimized. The A of the non-inoculated medium was experimentally obtained as 0.06.
Absorbance measurements
Two hundred μl of BHI bacterial cultures (ca. 5 × 106 CFU/ml) were distributed into 96-well micro-titer plates and immediately incubated at 30°C, 35°C, 37°C or 42°C in a microplate reader (SLT 340 ATTC, SLT Labinstruments, Austria) during 13–15 h. Two hundred μl of non-inoculated BHI was also distributed into the microplate wells and incubated at the same conditions (control test). The A was measured at a wavelength of 595 nm. Twenty-four replications of each combination of temperature and pH were done. The experiment was replicated twice.
TTD and GT estimation from log-transformed absorbance curves
The A data were transformed for each measurement at time t as follows (Begot et al., Citation1996): A i, the mean of the A of 24 replicates; A ni, the mean of the non-inoculated medium (0.06); A min, the lowest A value above the threshold of detection (0.103); ΔA t = A i – A ni; and, log10(ΔA t/A min). The growth curves were fitted (with Microsoft Excel loaded with the Solver add-in tool) using the modified Gompertz equation (Zwietering, Jongenburger, Rombouts, & van´t Riet, Citation1990):
where, N is the bacterial population at time t, N 0 is the bacterial population at the moment of inoculation, P is the logarithmic increase of the bacterial population, TTD is the time-to-detection (h), μ is the specific growth rate (h−1), and t is time (h). The GT was derived from μ:
TTD and GT estimation from transmittance curves
The A is related with the percentage of transmittance (%T) by the equation:
The change in the %T increases linearly as the population density increases logarithmically. Plotting log10(A) vs. %T reveals that there is a linear correspondence between these measures over a narrow, but useful range: 20–60%T. A doubling of the population within this range is represented by a 24.5% decrease in the %T (McMeekin et al., Citation1993). The A was transformed to %T by the Equation (3), i.e. %T = 10(2–A). The decrease in the percentage of transmission (%Ti–%Tt) was plotted against the time (where the %Ti is the initial percentage of transmittance, and the %Tt is the percentage of transmittance at time t). By plotting the change in %T vs. time, a sigmoid curve was obtained. The three-parameter Gompertz function (Gibson, Bratchell, & Roberts, Citation1987) was applied:
where, A595 is the absorbance at 595 nm at the time t, A595(0) is the absorbance at 595 nm at the time zero, C is the change of A595 between the inoculation and the stationary phase, B is the specific growth rate (h−1), M is the time to reach B, and t is the time (h). It was fitted with Microsoft Excel loaded with the Solver add-in tool. The parameters from Equation (4) were used to calculate the TTD (h). The GT was calculated as reported by Neumeyer, Ross, and McMeekin, (Citation1997):
where, the term 24.5 is the percentage of decrease in %T when a doubling of the population is represented (McMeekin et al., Citation1993), e is the Euler's number (2.71828), and 1.13 is a correction factor developed by Ross (Neumeyer et al., Citation1997; Ross, Citation1993).
TTD and GT estimation from absorbance curves
According to Zhao et al. (Citation2000), the three-parameter Gompertz function (Gibson et al., Citation1987) was used (Equation (4)) and fitted with Microsoft Excel loaded with the Solver add-in tool. The TTD was calculated with Equation (5). The GT (h) was derived from the maximum growth rate μ((B × C)/e; A595/h) as follows:
Linear relationship between the TTD and the GT
The natural logarithmic transformation of the variables TTD and GT were used to stabilize their variances. The linear relationship between the dependent variable (y = ln(GT)) and the independent variable (x = ln(TTD)) from the sample follows the model yi
= α0 + α1
xi
+ ϵ
i
, i = 1,2, … ,n. The linear relationship between the ln(TTD) and the ln(GT) was fitted with JMP Statistical Software (SAS Institute, Madrid, Spain). The goodness-of-fit was measured with the coefficient of determination R
2, that is, the proportion of the variation in the response that can be attributed to terms in the model rather than to the random error.
ln(TTD/GT) model fitting
A backward stepwise regression was carried out using JMP. The temperature and the pH were considered as independent variables. The ln(TTD/GT) ratio was used as the dependent variable. The probability selected for each parameter to enter or to leave the model was 0.250 or 0.100, respectively. A level of significance greater than 0.05 is recommended to reduce the risk of omitting important variables (Quinn & Keough, Citation2002). The root mean square error (RMSE) was used to evaluate the models (Ratkowsky, Citation2004). The sum of squares (SSE) and the mean square error (MSE) were also obtained. The estimation of the GT could be derived from the regression equations as follows:
where, y is the TTD, z is the GT, x 1 is the pH, x 2 is the temperature T, and the coefficients a, b, and c are those estimated by the regressions. Since ln(y/z) = ln(y)−ln(z),
Validation
In order to evaluate the model (9), a validation dataset (GTv) was obtained from new experiments. The new dataset was carried out at 30°C, 34°C, 38°C, and 42°C, with 4.5, 5.5, 6.5, and 7.4 pH values, and 2.5% of NaCl. Graphical comparisons between the GT values from this study (GTo) and the GTv were done.
Results and discussion
The standard deviation of each measurement point was calculated (0.001–0.099). Absorbance results from the conditions pH 4.5 at 42°C were not considered, since growth was not observed along the study. The linear range between the A and the CFU/ml was found between A = 0.102 and 1.148 (A = 2(−10) × CFU + 0.146; R 2 = 0.975). The initial population of E. coli (ca. 5 × 106 CFU/ml) was selected as close as possible to the lower limit of the range (A = 0.102; ca. 9.6 × 106 CFU/ml).
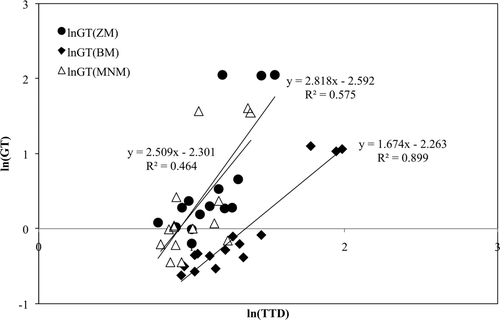
The parameters TTD and GT were calculated using the three methods proposed (). The natural logarithmic transformation was applied. It was previously used to stabilize the variance of the GT values (Alber & Schaffner, Citation1992; Delignette-Muller, Citation1998; Ratkowsky, Ross, Macario, Dommett, & Kamperman, Citation1996), the LT values (Alber & Schaffner, Citation1992; Delignette-Muller, Citation1998; Ratkowsky, Ross, McMeekin, & Olley, Citation1991; Zwietering, Cuppers, de Wit, & van't Riet, Citation1994), or the ratio LT/GT (Delignette-Muller, Citation1998). Bivariate scattergrams were plotted to display the linear relationship between the ln(TTD) and the ln(GT) for each dataset (). For a strictly constant ratio between the TTD and the GT (i.e. TTD/GT), the slope of the regression equations should theoretically be equal to 1 (Delignette-Muller, Citation1998). All the factors of the independent variable x (ln(TTD)) were positive (slopes 2.82, 1.67, and 2.51), that is, the relationship showed an increasing linear trend between ln(TTD) and ln(GT). Delignette-Muller (Citation1998) studied the linear relationship between the ln(LT) and the ln(GT). Data from E. coli O157:H7 was used, obtaining a linear relationship with R 2 = 0.59. This author considered that value as a reinforcement of the assumption of a proportional relationship between both parameters. In the present study, the best linear relationship between the ln(TTD) and the ln(GT) was found using the method proposed by Begot et al. (Citation1996) (BM; R 2 = 0.899) with the slope closer to one (1.67). Some differences among the three methods were found. Those differences were more evident under non-favorable pH values (4.5). These methods have different approaches for modeling the A growth curves: the BM applies a decimal logarithmic transformation to the A curves, and the methods proposed by Zhao et al. (Citation2000) (ZM) and McMeekin et al. (Citation1993) (MNM) do not apply any transformation to the A or %T curves, respectively. That logarithmic transformation would increase the linearity between the TTD and the GT inside the linear range.
A forward stepwise regression to the three datasets was carried out (). Only the pH was introduced by the regression in the three models. If the dependent variables, ln(GT) and ln(TTD), are considered independently, the effect temperature T appears included in both regressions (data not shown): i.e. ln(TTDBM) = 3.866 – 0.0308 × T – 0.2412 × pH, and ln(GTBM) = 3.813 – 0.0341 × T – 0.4411 × pH. As ln(TTD/GT) = ln(TTD) – ln(GT), the subtraction of these polynomials cancels the effect T: the result of (−0.0308 × T) – (−0.0341 × T) is very low (0.0033). So, the T does not appear in the regression of ln(TTD/GT). The statistics of the regression are also shown in the . The highest R 2 (0.758) was observed for the ln(TTD/GTMNM) regression. The lowest SSE, MSE, and RMSE were obtained for the ln(TTD/GTBM) regression (0.616, 0.047, and 0.217, respectively). The regression process showed the best fit of the ln(TTD/GT) using the BM, with the lower RMSE value (0.217). R 2 is probably the most frequently used measure of goodness-of-fit. However, the simplest and the most informative measure of goodness-of-fit for regression models, both linear and nonlinear, is the RMSE (Ratkowsky, Citation2004). This measure may be considered as the average discrepancy between the observed data and their predicted values. Its magnitude is useful in assessing whether a given model truly fits the data well. Delignette-Muller (Citation1998) studied the influence of environmental factors on the ratio LT/GT. The stepwise regression applied to the E. coli data included the environmental factors T, pH, and NaCl, with an adjusted-R 2 = 0.842 (the RMSE was not published). This author concluded that there is a proportional relationship between the LT and the GT. The adjusted-R 2 adjusts the R 2 to make it more comparable to models with different numbers of parameters by using the degrees of freedom in its computation. It cannot be used in our study because the three models have the same number of parameters. The results obtained in the present work reinforce the idea of a proportional relationship between the TTD and the GT.
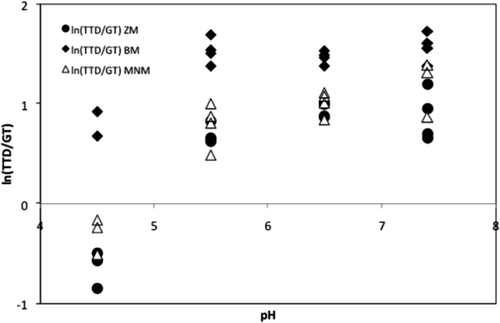
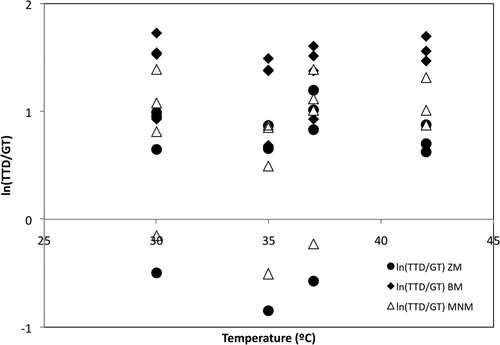
The effect of the pH on the ln(TTD/GT) ratio for each method dataset is shown in . The BM maintained the influence of the pH over the ln(TTD/GT) inside a narrow range of positive values (between 0 and 2). The ZM and the MNM showed a similar trend between them. Both needed a wider range, including negative values. The effect of the temperature on the ln(TTD/GT) ratio is shown in . The values of the ratio followed a linear trend between 1 and 2 using the BM. The ZM and the MNM gave a wider range of values, including negative values. The regression of the ratio ln(TTD/GT) only introduced the term pH in the model. The range of selected temperatures could be the reason of the absence of the term T in the final models. That assumption could be reinforced by the conclusions reported by several authors. Pin et al. (Citation2002) published that a change in the growth temperature from 1°C to 11°C did not affect the amount of work needed to adapt the cells to the new environmental conditions, but, under increased CO2 and/or O2 in the atmosphere surrounding the cells, there was an extra amount of work required to adapt to these new environmental conditions. Mellefont and Ross (Citation2003) and Swinnen et al. (Citation2006) demonstrated that the proportionality between the LT and the GT does not keep over the full range of temperature. The range of temperatures selected in this article only include those favorable to the growth of the microorganism, that is, 35–37°C ± 5 (30°C, as a high room temperature; and 42°C, used in the enumeration procedure for fecal coliforms in water and food, 44.5°C).
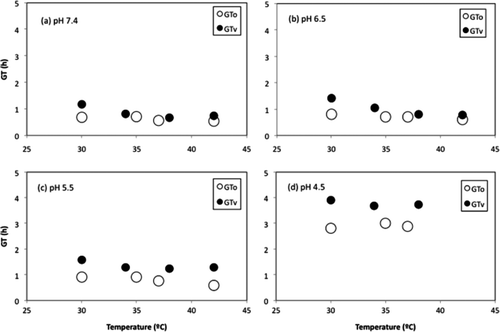
shows the graphical comparison between the observed GT (GTo) and the validated GT (GTv) values, and the effect of the T and the pH. The results were similar for pH 7.4 (a)) and 6.5 (b)) at 34–35°C, 37–38°C, and 42°C between the GTo and the GTv. At 30°C, the GTv was greater than the GTo for both pH values. The difference could be due to the combination of a less optimal temperature and the presence of NaCl. This could reinforce the increased differences founded between the GT values under pH 5.5 ((c)), because these three environmental parameters (30°C, 2.5% NaCl, and pH 5.5) would affect the growth of the microorganism. The combined effect of the pH 4.5 and the 2.5% NaCl increased the GT to values between 2.79 and 3.91 h ((d)). The goal of the use of NaCl as an additional environmental condition in the dataset for validation was to introduce a new differential factor in the system. This factor would introduce new insights in the results for the interpretation of the results.
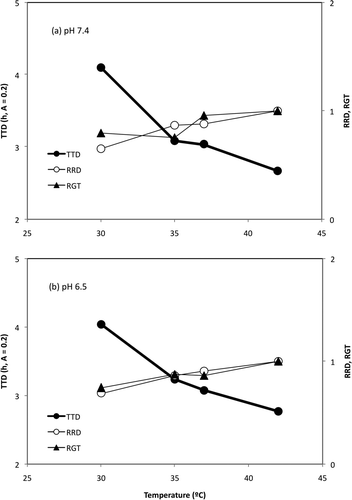
One particular problem with using the TTD is the limited number of models available (Membré & Lambert, Citation2008). Lambert and Bidlas (Citation2007) and Bidlas and Lambert (Citation2008) have used the TTD to quickly obtain the areas of growth/no-growth. Lambert and Bidlas (Citation2007) published that the relative specific growth rate and the relative rate to detection (RRD = TTDref/TTDobs; TTDref = the reference TTD or the shortest TTD; TTDobs = the observed TTD for a given set of environmental conditions) are equivalent measures for a constant A (i.e. at any point where the number of microorganisms is equal). These authors suggested that the specific growth rate μ could be obtained using the TTD and the RRD at any point of a temperature series with the same number of microorganisms (i.e. the same A value). In the present work, all microorganisms had the same initial state. Baranyi and Roberts (Citation1994) and Baranyi and Pin (Citation1999) described the product LT × μ as the initial physiological state of the microorganisms or h0. As LT and μ are closely related with TTD and GT, we considered the product TTD × GT when a constant A value is considered. An A value of 0.5 gave a bacterial population of ca. 1.2 × 109 CFU/ml, so an A = 0.2 was considered as the constant value (ca. 3.0 × 108 CFU/ml) (Lambert & Bidlas, Citation2007). This A value is almost the middle point between the inoculation level and the maximal population density. The RRD and the relative GT (RGT = GTref/GTobs) were calculated from our BM data. shows the effect of the T on the TTD, the RRD, and the RGT with pH 7.4 ((a)) and 6.5 ((b)). The results suggest that the RGT and the RRD are equivalent measures, so the GT could be obtained from the TTD and the RRD under optimal pH values, as Lambert and Bidlas (Citation2007) previously stated. This conclusion would reinforce the initial statement, i.e. the relationship between the TTD and the GT.
Some authors showed the ratio of the LT to the GT. Baranyi and Roberts (Citation1994) defined the term h0 as a relationship between the LT and the GT. This term was called W or “the work to be done” by Robinson et al. (Citation1998). Pin et al. (Citation2002) studied the lag-growth rate relationship of Yersinia enterocolitica through the parameters h0 and W. These authors, and also Mellefont and Ross (Citation2003), assumed proportionality between both parameters, and they demonstrated that this assumption does not keep over the full range of temperatures. Mellefont et al. (Citation2003, Citation2005) quantified the effect of the temperature upshifts and downshifts on the “relative lag” (ratio LT – GT) of E. coli. The “relative lag” increased significantly when the difference between the previous and the current temperature was higher than 20°C. Similar results were reported by Swinnen et al. (Citation2006).
As stated by Baranyi, Ross, McMeekin, and Roberts (Citation1996) and Delignette-Muller, Rosso, and Flandrois (Citation1995), parsimonious models should be preferred in predictive microbiology, due to their better robustness. A model with unnecessary parameters may become specific for the dataset to which it is fitted, so a non-parsimonious model may have worse abilities in prediction than in adjustment. Assuming a constant ratio of the TTD to the GT contributes to the minimization of the number of parameters (Delignette-Muller, Citation1998). This author found that focusing on the relation linking LT and GT will often lead to models with less unnecessary parameters.
Because the TTD is a parameter obtained from several automated techniques (turbidimetry, colorimetry or impedanciometry), its relationship with the GT could be useful. The TTD/GT ratio would permit to estimate the GT from the TTD, and this could be an interesting tool in prediction for the food industry. The TTD using optical density techniques is valid for rapid predictive microbiology and its application to industry (Membré & Lambert, Citation2008). The results showed the importance of the method selected for the primary modeling. More research is needed to improve the knowledge about the TTD/GT ratio. The influence of several stress conditions will be studied in the near future.
tcyt_a_596286_sup_27125771.pdf
Download PDF (850.7 KB)Acknowledgements
A.J.S. Eduardo received a scholarship of the AECI (Spanish Agency of International Cooperation), Spain.
Additional information
Notes on contributors
E. J. Quinto
†Present address: Institute of Nursing, University Agostinho Neto, Morro Bento, Luanda, Angola‡Present address: Area of Food Science and Nutrition, School of Medicine, University of Valladolid, 47005, Valladolid, SpainReferences
- Alber , S. A. and Schaffner , D. W. 1992 . Evaluation of data transformations used with the square root and Schoolfield models for predicting bacterial growth rate . Applied and Environmental Microbiology , 58 : 3337 – 3342 .
- Baranyi , J. and Pin , C. 1999 . Estimating bacterial growth parameters by means of detection times . Applied and Environmental Microbiology , 65 : 732 – 736 .
- Baranyi , J. and Roberts , T. A. 1994 . A dynamic approach to predicting bacterial growth in food . International Journal of Food Microbiology , 23 : 277 – 294 .
- Baranyi , J. , Ross , T. , McMeekin , T. A. and Roberts , T. A. 1996 . Effects of parameterization on the performance of empirical models used in predictive microbiology . Food Microbiology , 13 : 83 – 91 .
- Begot , C. , Desnier , I. , Daudin , J. D. , Labadie , J. C. and Lebert , A. 1996 . Recommendations for calculating growth parameters by optical density measurements . Journal of Microbiological Methods , 25 : 225 – 232 .
- Bidlas , E. and Lambert , R. J.W. 2008 . Quantification of hurdles: Predicting the combination of effects – Interaction vs non-interaction . International Journal of Food Microbiology , 128 : 78 – 88 .
- Cooper , K. E. 1963 . “ The theory of antibiotic inhibition zones ” . In Analytical microbiology , Edited by: Kavanagh , F. 1 – 86 . New York : Academic Press .
- Cuppers , H. G.A.M. and Smelt , J. P.P.M. 1993 . Time to turbidity measurement as a tool for modeling spoilage by Lactobacillus . Journal of Industrial Microbiology , 12 : 168 – 171 .
- Dalgaard , P. and Koutsoumanis , K. 2001 . Comparison of maximum specific growth rates and lag times estimated from absorbance and viable count data by different mathematical models . Journal of Microbiological Methods , 43 : 183 – 196 .
- Delignette-Muller , M. L. 1998 . Relation between the generation time and the lag time of bacterial growth kinetics . International Journal of Food Microbiology , 43 : 97 – 104 .
- Delignette-Muller , M. L. , Rosso , L. and Flandrois , J. P. 1995 . Accuracy of microbial growth predictions with square root and polynomial models . International Journal of Food Microbiology , 27 : 139 – 146 .
- Firstenberg-Eden , R. and Eden , G. Impedance Microbiology, Letchworth, Hertfordshire . pp. 41 – 72 . UK : Research Studies Press Limited .
- Francois , K. , Devlieghere , F. , Standaert , A. R. , Geeraerd , A. H. , Cools , I. , Van Impe , J. F. and Debevere , J. 2005 . Environmental factors influencing the relationship between optical density and cell count for Listeria monocytogenes . Journal of Applied Microbiology , 99 : 1503 – 1515 .
- Gibson , A. M. , Bratchell , N. and Roberts , T. A. 1987 . The effect of sodium chloride and temperature on the rate and extent of growth of Clostridium botulinum type A in pasteurized pork slurry . Journal of Applied Bacteriology , 62 : 479 – 490 .
- Koch , A. L. 1981 . “ Growth measurement ” . In Manual of methods for general bacteriology , Edited by: Gerhardt , P. , Murray , R. G.E. , Costilow , R. N. , Nester , E. W. , Wood , W. A. , Krieg , N. R. and Phillips , G. B. 179 – 207 . Washington : ASM Press .
- Lambert , R. J.W. and Bidlas , E. 2007 . A study of the Gamma hypothesis: Predictive modelling of the growth and inhibition of Enterobacter sakazakii . International Journal of Food Microbiology , 115 : 204 – 213 .
- Lindqvist , R. 2006 . Estimation of Staphylococcus aureus growth parameters from turbidity data: Characterization of strain variation and comparison of methods . Applied and Environmental Microbiology , 72 : 4862 – 4870 .
- McKellar , R. C. and Knight , K. 2000 . A combined discrete-continuous model describing the lag phase of Listeria monocytogenes . International Journal of Food Microbiology , 54 : 171 – 180 .
- McMeekin , T. A. , Olley , J. N. and Ross , T. 1993 . Predictive microbiology: Theory and application , Taunton , UK : Research Studies Press .
- Mellefont , L. A. , McMeekin , T. A. and Ross , T. 2003 . The effect of abrupt osmotic shifts on the lag phase duration of foodborne bacteria . International Journal of Food Microbiology , 83 : 281 – 293 .
- Mellefont , L. A. , McMeekin , T. A. and Ross , T. 2005 . Viable count estimates of lag time responses for Salmonella typhimurium M48 subjected to abrupt osmotic shifts . International Journal of Food Microbiology , 105 : 399 – 410 .
- Mellefont , L. A. and Ross , T. 2003 . The effect of abrupt shifts in temperature on the lag phase duration of Escherichia coli and Klebsiella oxytoca . International Journal of Food Microbiology , 83 : 295 – 305 .
- Membré , J. M. and Lambert , R. J.W. 2008 . Application of predictive modelling techniques in industry: From food design up to risk assessment . International Journal of Food Microbiology , 128 : 10 – 15 .
- Neumeyer , K. , Ross , T. and McMeekin , T. A. 1997 . Development of a predictive model to describe the effects of temperature and water activity on the growth of spoilage Pseudomonas . International Journal of Food Microbiology , 38 : 45 – 54 .
- Pin , C. and Baranyi , J. 2008 . Single-cell and population lag times as a function of cell age . Applied and Environmental Microbiology , 74 : 2534 – 2536 .
- Pin , C. , García de Fernando , G. D. , Ordoñez , J. A. and Baranyi , J. 2002 . Analysing the lag-growth rate relationship of Yersinia enterocolitica . International Journal of Food Microbiology , 73 : 197 – 201 .
- Quinn , G. P. and Keough , M. J. 2002 . “ Fitting the best regression model ” . In Experimental design and data analysis for biologists , Edited by: Quinn , G. P. and Keough , M. J. 139 – 140 . Cambridge , UK : Cambridge University Press .
- Ratkowsky , D. A. 2004 . “ Model fitting and uncertainty ” . In Modeling microbial responses in food , Edited by: McKellar , R. C. and Lu , X. 151 – 196 . Boca Raton , Florida : CRC Press .
- Ratkowsky , D. A. , Ross , T. , Macario , N. , Dommett , T. W. and Kamperman , L. 1996 . Choosing probability distributions for modeling generation time variability . Journal of Applied Bacteriology , 80 : 131 – 137 .
- Ratkowsky , D. A. , Ross , T. , McMeekin , T. A. and Olley , J. 1991 . Comparison of Arrhenius-type and Belehradek-type models for prediction of bacterial growth in foods . Journal of Applied Bacteriology , 71 : 452 – 459 .
- Robinson , T. P. , Ocio , M. J. , Kaloti , A. and Mackey , B. M. 1998 . The effect of the growth environment on the lag phase of Listeria monocytogenes . International Journal of Food Microbiology , 44 : 83 – 92 .
- Ross , T. 1993 . A philosophy for the development of kinetic models in predictive microbiology (Ph.D. Thesis) , Hobart , Australia : University of Tasmania .
- Swinnen , I. A. , Bernaerts , K. and Van Impe , J. F. 2006 . Modelling the work to be done by Escherichia coli to adapt to sudden temperature upshifts . Letters in Applied Microbiology , 42 : 507 – 513 .
- Zhao , L. , Montville , T. J. and Schaffner , D. W. 2000 . Inoculum size of Clostridium botulinum 56A spores influences time-to-detection and percent growth-positive samples . Journal of Food Science , 65 : 1369 – 1375 .
- Zwietering , M. H. , Cuppers , H. G.A.M. , de Wit , J. C. and van't Riet , K. 1994 . Evaluation of data transformations and validation of a model for the effect of temperature on bacterial growth . Applied and Environmental Microbiology , 60 : 195 – 203 .
- Zwietering , M. H. , Jongenburger , I. , Rombouts , F. M. and van´t Riet , K. 1990 . Modeling of the bacterial growth curve . Applied and Environmental Microbiology , 56 : 1875 – 1881 .
Supplementary material
Supplementary Table 1. Time-to-detection (TTD; h) and generation time (GT; h) values. The methods suggested by Begot et al. (Citation1996), McMeekin et al. (Citation1993), and Zhao et al. (Citation2000) were applied: BM, MNM, and ZM, respectively. ND: bacterial growth was not detected.
Tabla 1. Tiempos de detección (TTD; h) y tiempos de generación (GT; h). Se aplicaron los métodos propuestos por Begot et al. (1996), Zhao et al. (2000) y McMeekin et al. (1993): BM, ZM, y MNM, respectivamente. ND: no detectado.
Supplementary Table 2. Forward stepwise regression and statistics of ln(TTD/GT) for each method used (see ). F: statistic to test the significance of a term. Prob > F: the significance level associated with the F-statistic.
Tabla 2. Regresión y estadísticas de la relación ln(TTD/GT) para cada método utilizado (ver Tabla 1). F: estadística para comprobar la significación del término. Prob > F: nivel de significación asociado a la estadística F.
Supplementary Figure 1. Bivariate scattergram displaying the linear relationship between ln(TTD) and ln(GT) of E. coli for each method used. The three points with higher ln(GT) values from each dataset represent the combinations of 30°C, 35°C, and 37°C with pH 4.5. Growth was not detected at 42°C with pH 4.5.
Figura 1. Relación lineal entre ln(TTD) yln(GT) de E. coli para cada uno de los métodos utilizados. Los tres puntos con un valor de ln(GT) más alto para cada uno de los grupos de datos representan las combinaciones de 30, 35, y 37°C con pH 4.5. No se detectó crecimiento a 42°C con pH 4.5.
Supplementary Figure 2. Effect of the pH on the ln(TTD/GT) ratio for each method used.
Figura 2. Efecto del pH sobre el ln(TTD/GT) para cada uno de los métodos utilizados.
Supplementary Figure 3. Effect of the T on the ln(TTD/GT) ratio for each method used.
Figura 3. Efecto de T sobre el ln(TTD/GT) para cada uno de los métodos utilizados.
Supplementary Figure 4. Effect of the pH on both the observed GT values (GTo) and the predicted values (GTv) obtained after the application of the Equation (9) to the validation dataset.
Figura 4. Efecto del pH sobre los valores de GT observados (GTo) y predichos (GTv) obtenidos tras la aplicación de la ecuación (9) al grupo de datos destinados a la validación.
Supplementary Figure 5. Effect of temperature on the time-to-detection (TTD), the relative TTD (RRD), and the relative generation time (RGT) of E. coli under 7.4 and 6.5 pH values. The TTD and the GT data were obtained with the BM (see ).
Figura 5. Efecto de la temperatura sobre el tiempo de detección (TTD), el TTD relativo (RRD), y el tiempo de generación relativo (RGT) de E. coli con pH 7.4 y 6.5. Los valores del TTD y del GT fueron obtenidos mediante el BM (ver Tabla 1).