Abstract
The aim of this study was to evaluate the behavior of biogenic amines (BAs), especially histamine (HIS), and its correlation with pH, water activity (Aw), sodium chloride (NaCl), and microbiological parameters in innovative bluefin tuna (Thunnus thynnus) – raw, salted, seasoned and cooked products. The sensory analysis showed that this seafood typology, especially the pressed and cooked products, could be accepted willingly by consumer. The results showed amine profile trends that are similar to those observed during ripening and seasoning of pig meat products; HIS levels were found to be lower than the limits set by EU legislation in all products and during the whole storage period. To ensure the safety of the analyzed products, despite using raw materials of excellent quality, it is important to select process parameters that reduce the BA production.
El objetivo de este estudio fue evaluar el comportamiento de aminas biógenas (BAs), particularmente histamina (HIS), y su correlación con el pH, actividad acuosa (Aw), cloruro de sodio (NaCl) y parámetros microbiológicos en productos innovadores crudos salados y cocinados de atún rojo (Thunus thynnus). Análisis sensoriales muestran que la tipología de estos productos, en especial los prensados y cocinados, podría ser aceptada por el consumidor. Los resultados mostraron tendencias del perfil de amina similares a los observados durante la maduración y sazonado de productos de carne de cerdo. Los niveles de HIS fueron más bajos que los límites establecidos por la legislación de la UE en todos los productos y durante todo el periodo de almacenamiento. Para garantizar la inocuidad de los productos analizados a pesar del uso de materiales crudos de una calidad excelente, es importante seleccionar parámetros de procesos que reduzcan la producción de BA.
Palabras clave:
Introduction
The interest on food quality has contributed to guide consumers’ choice toward fishery products which are considered to be of high nutritional value and capable of influencing human health in a positive way. Fish cannot be easily absorbed by the market as whole fish only. The diversification of the commercial offers and the production and promotion of value-added fresh and processed fish products, which could fulfill consumers’ demands, may represent a solution to this problem. Since the presence of bluefin tuna (Thunnus thynnus) is limited to some fishing areas and the species undergoes a high fishing pressure, the commercial value of this species, especially in the oriental market, is very high. Recently, market demand has prompted research aiming at fully exploiting the potential of this species through the production of innovative tuna-based products.
Despite the value of tuna and tuna products, one of the most important safety problems is related to the presence of biogenic amines (BAs); BAs are aliphatic, alicyclic, or heterocyclic organic bases of low molecular weight which result from the metabolic processes of animals, plants, and microorganisms (Vale & Gloria, Citation1997). The formation of the amines such as histamine (HIS), putrescine (PUT), cadaverine (CAD), spermidine (SPD), and spermine (SPR) is primarily due to the enzymatic decarboxylation of free amino acids; their production depends on the availability of free amino acids, the presence of microbial decarboxylase, and the conditions that promote enzymatic processes and bacterial growth, such as temperature, pH, and saline and oxygen concentrations (Halasz, Barath, Simon-SarKadi, & Holzapel, Citation1994; Kerr, Lawicki, Aguirre, & Rayner, Citation2002; Mercogliano, De Felice, Chirollo, & Cortesi, Citation2010).
Biogenic amines are normally present at very low levels in fresh fish. Bacterial growth results in gradual accumulation of amines, and the presence of high concentrations is indicative of microbial spoilage (Kerr et al., Citation2002; Veciana-Nogues, Marine-Font, & Vidal-Carou, Citation1997). Among BAs, in fish belonging to Scombridae, HIS plays an important role; it is a biologically active amine produced from free histidine by decarboxylasing bacteria (Emborg & Dalgaard, Citation2006). The optimal temperatures for these bacteria are generally in the range of 20–30°C (Auerswald, Morren & Lopata, Citation2006; McMekkin, Olley, Ross, & Ratskowsky, Citation1993), but some HIS-producing bacteria, such as Vibrio species, are known to grow at below 10°C. Mishandling during processing and storage may cause the production of HIS toxic levels (Kerr et al., Citation2002; Lehane & Olley, Citation2000) which could cause HIS fish poisoning (HFP) or “Scombroid poisoning”. However, storage at refrigeration temperatures, together with NaCl concentrations >3.5% and low O2 levels, may limit HIS levels. EU legislation (COMMISSION REGULATION (EC) No 2073/2005) settled HIS as a food safety parameter and fixed a limit of 100–200 mg/kg in fresh fish and of 200–400 mg/kg in fishery products which have undergone enzyme maturation treatment in brine, belonging to the Scombridae, Clupeidae, Engraulidae, Corifenidae, Pomatomidae and Scombresosidae families. This rule and the attention which must be paid to HIS are confirmed by the Community Summary Report on Trends and Sources of Zoonoses and Zoonotic Agents and Food-Borne Outbreaks in the European Union, published each year and that confirmed many HIS outbreaks in recent years (Report EFSA Journal, Citation2010).
Since the prepared and processed tuna products are gaining increasing interest from consumers and producers, the aim of study was to evaluate the production of the most relevant BA during production process of innovative bluefin tuna-processed products and the relationship with some microbial and physicochemical parameters.
Materials and methods
Tuna sampling
The study was carried out on processed seafood produced from bluefin tuna dorsal and ventral muscles. Three types of smoked products were prepared:
| 1. | Two kinds of seasoned products traditionally smoked (hot smoking) with beech tree shavings; the first consisted of salted and seasoned whole muscle and the second of seasoned sausages named a1 and a2, respectively. | ||||
| 2. | One kind of pressed and cooked product, smoked with smoke flavorings (cold smoking) named b. | ||||
All typologies were produced from fresh tuna. Fish (no. 8) from Tyrrhenian Sea (South Italy) weighing 45–50 kg were cooled on board by immersing in slurry ice. Later on, the tunas were placed in polyurethane boxes with plenty of ice and delivered to the laboratory with a maximum postcatching time of 24 h. The fish were washed, decapitated, gutted, and placed in boxes with ice and divided into four lots. Lots 1 and 2 were used to produce a1 and a2 products; lots 3 and 4 to were used to produce the two typologies of b2 products.
Before processing, two samples of fish muscle from apical middle and caudal section of each tuna were taken to evaluate pH, Aw, and BA content.
The tuna products were processed as follows:
(a1) Raw pieces obtained with a sterile knife from whole muscle cuts of the tuna dorsal and ventral regions were dry salted (NaCl 60 g/kg, KNO3 0.15 g/kg, ground white pepper 1 g/kg). The products were seasoned for 14 days, traditionally smoked using beech tree shavings, divided according to their diameter into large (∼9.5 cm), medium (∼6.5 cm), and small (∼4.5 cm), vacuum-packaged, and stored at + 3°C.
(a2) Seasoned sausages (tuna “salami”) were produced from different tuna muscles, which were dry salted (NaCl 50 g/kg, KNO3 0.15 g/kg, ground white pepper 1 g/kg) and put into a collagen casing (Fibran SA, 55 mm). Sausages were pressed for 3 days at room temperature of 26–28°C, seasoned for 10 days at the same temperatures, smoked with beech tree shavings, vacuum-packaged, and stored at + 3°C.
(b) For salted, pressed, and cooked tuna products, the muscles of the first batch (b1) were cut into cubic pieces (∼3 cm), brine-salted with 200 g/kg NaCl, added with 1 g/kg of smoking flavoring (EF, Europrodotti S.p.A), and cooked at 71°C in the metal boxes used generally for cooking hams. The muscles of the second batch (b2) were cut into smaller pieces (<3 cm), salted with 250 g/kg NaCl brine, smoked with 1 g/kg smoking flavoring (EF, Europrodotti S.p.A), and cooked at 69°C in the same metal boxes. Polyphosphates (15 g/kg) were also added. The finished products were vacuum-packed and stored at + 3°C. All tuna samples were individually packaged in laminated film bags (HAFLIGER film type 145 bags; diffusion coefficient of 35 cm3/24 h m2 bar to O2 and 150 cm3/24 h m2 bar to CO2). Samples were taken at random and analyzed on days 11–18–25–32–39–46–67–81–95 from production; each determination was done in duplicate.
Sensory analysis
Samples were examined by seven selected and trained panelists for odor, juiciness, surface color, saltiness, and firmness. A continuous scale between 1 and 6 was used. A value of 1 corresponded to the lowest intensity for each parameter (fishy odor, dry, red pearly, unsalty, and nonfirm, respectively) and a value of 6 to the highest. Samples scoring less than 2 were rejected. The general acceptability was also evaluated based on a six-point hedonic scale, where 6 represented like extremely and 1 represented dislike extremely.
Physical and chemical analyses
All the solutions used were prepared from reagent-grade chemicals. Moisture and sodium chloride content (g of NaCl/100 g of product) were determined according to the methods recommended by the AOAC International (AOAC International, Citation2000). pH was measured with a pH meter (micropH 2001; Crison Instruments, SpA, Modena, Italy) after homogenizing 10 g of sample in 100 ml of distilled water. Water activity values (of the entire sample) were determined with a Novasina EEJA-3 instrument (Novasina, Pfaffikon, Switzerland).
Biogenic amines analysis
The concentration of PUT, CAD, SPD, SPR, and HIS was determined with a chromatographic direct method based on cation-exchange separation of the amines and detection with integrated pulsed amperometry according to Draisci et al. (Citation1998). Briefly, 200 g of sample was homogenized in an Ultra Turrax blender (IKA Werke GmbH and Co. KG, Staufen, Germany). A 10-g aliquot of homogenized sample was extracted with 0.375 M perchloric acid (Carlo Erba, Italy) and purified by liquid–liquid partition by using n-hexane. The aqueous phase was filtered through a 0.2-mm-pore-size filter and injected for analysis. Separations were performed on an IonPac CS10 cation-exchange column coupled to an IonPac CG10 guard column (Dionex Co., Sunnyvale, CA). Isocratic separation was performed with an eluent containing 1 M sodium perchlorate–0.375 M perchloric acid–water (81:5:14 [vol/vol/vol]) at a flow rate of 1 ml/min. Chromatographic separation was achieved with a model 45001 liquid chromatography (Dionex Co.), and an ED40 detector with an electrochemical cell equipped with an Au working electrode and a reference electrode in Na form (pH-Ag-AgCl; Dionex Co.). A reagent delivery module (Dionex Co.) was used for postcolumn sodium hydroxide addition. Putrescine, CAD, HIS, SPR, and SPD standards were obtained from Sigma-Aldrich (Milano, Italy).
Microbiological analysis
A 20-g portion (whole slice, including middle and periphery portions) was cut from each sample and diluted with sterile saline–peptone water (8 g/liter NaCl–1 g/liter bacteriological peptone; Oxoid, Ltd., Basingstoke, UK) at a 1:4 ratio and then homogenized in a stomacher. The homogenates were serially diluted and surface-plated on de Man Rogosa Sharpe agar (Difco, Becton Dickinson Sparks, MD, USA) plates to enumerate lactic acid bacteria (LAB). Bacterial colonies were counted after the plates were incubated at 30°C for 48 h. Bacterial numbers were expressed as log CFU per gram of sample.
Statistical analysis
The data were subjected to an analysis of variance and to a multiple comparison test by using the PC-based SYSTAT program (Systat, Software, Inc., Chicago, IL). Linear regression was calculated to determine the correlation of BA with LAB counts. The Tukey test was chosen for comparison of means (Systat, Software, Inc.). The results are expressed as the mean of three samples taken from the same group of products. The differences between the means are statistically significant at P < 0.05.
Results and discussion
Sensory analysis
The results obtained are shown in . It can be observed that the a1 products, after 81 days of storage, still received a very high score for parameter odor and firmness particularly. General acceptability was over three points of hedonic scale also during last sampling period. The tuna salted products showed a typical fluorescence on cutting surface and gradually lost their red pearly luster from 46th day, but they were still accepted by the tasters.
Table 1. Sensory scores of tuna products (see text) analyzed during storage.
Tabla 1. Resultados sensoriales de productos de atún analizados durante almacenado.
The a2 products showed good score for odor, color, and firmness of kneading until day 67; odor particularly was pleasant, spicy, and aromatic. The presence of greenish areas gives a low score in general acceptability.
For the cooked product (b), the score was lower for the samples of the first lot (b1) than those of the second lot (b2). These products showed graying of cutting surface from 46 days and the presence of liquid in bag coupled with an increased firmness from 67 days. On the other hand, the general acceptability of products of second lot was considered very good until the last time of sampling. Firmness particularly received the highest score.
Physical and chemical analyses
Aw and pH values in samples as a function of storage time were reported in and .
Figure 1. Water activity (Aw) behavior during ripening process and after vacuum packaging of tuna products.
Figura 1. Comportamiento de actividad acuosa (Aw) durante el proceso de maduración y tras envasado al vacío de productos de atún.
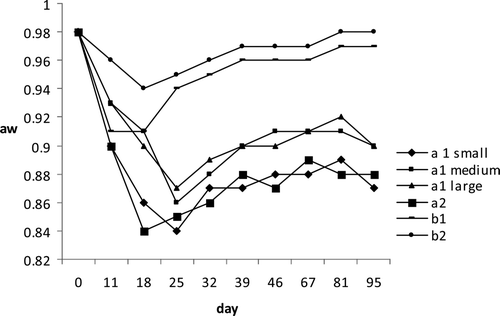
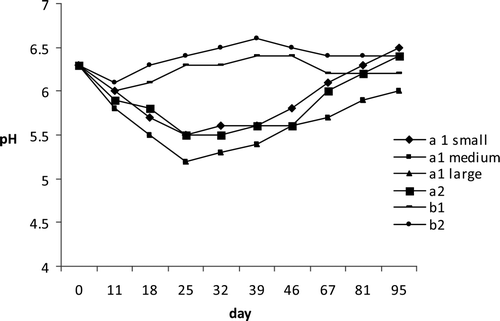
Figure 2. pH behavior during ripening process and after vacuum packaging of tuna products.
Figura 2. Comportamiento de pH durante el proceso de maduración y tras envasado al vacío de productos de atún.
Mean water activity values decreased from 0.98 in raw material to 0.84 and 0.87 for small and large diameter a1 samples and to 0.85 for a2 tuna products, respectively, before packaging. No significant differences between large and medium a1 products were observed. Progressive drying of tuna products led to a decrease in water activity up to values that, together with a fall of pH, can inhibit most of the contaminating microflora, including pathogens, potentially present in the raw material.
During storage under vacuum, Aw value increased until a final level of 0.87 and 0.90 for a1 and 0.88 for a2 samples, respectively. For cooked products, Aw (after vacuum packaging) increased from 0.96 and 0.91 up to levels of 0.98 and 0.97 in samples of the first and second batches, respectively (); this difference was probably due to different salt concentrations and the presence of polyphosphate in the second batch. The pH behavior in the raw seasoned seafood, in agreement with that previously reported by other authors for dry fermented sausages, showed an initial decrease from 6.3 (raw material) to 5.2 and 5.5 (at the beginning of vacuum packaging) for large a1 samples and small a2 products, respectively, strictly correlated with the lactic acid production (). Moreover, no significant differences between large and medium a1 products were observed for pH parameter. During storage in vacuum packaging, a rise until final value of 6.5 and 6.4 for a1 small and a2 products and of 6.0 for a1 large and medium caliber samples was observed, due probably to the reduced lactic acid production and the increased proteolytic activity of the LAB and of other bacteria. In b products, pH after an initial decrease, rose progressively showing mean values of 6.2 and 6.4 for the first and second lots, respectively; Suzzi and Gardini (Citation2003), in dry sausages, showed that the decrease of pH reduced enterococci and Enterobacteriaceae counts without affecting LAB growth; these effects on microbial population resulted in lower HIS and tyramine concentration (Maijala, Citation1993); a rapid reduction in pH in sausages is known to reduce the growth of the amine-positive microorganisms, particularly Enterobacteriaceae (Maijala, Citation1993; Bover-Cid, Hugas, Izquierdo-Pulido, & Vidal-Carou, Citation2001).
The mean salt concentrations were 4.0% and 3.8% for large medium and small a1 samples and 3.9% for a2 products. These values are in agreement with taste of products. In cooked products, the mean salt levels were 4.8 and 5.1 for first and second lots, respectively. Henry Chin and Koehler (Citation1986) in fermented sausages demonstrated that NaCl concentration ranging from 3.5% to 5.5% could inhibit HIS production. This influence can be mainly attributed to reduced cell yields obtained in the presence of high NaCl concentrations.
BA analysis
Biogenic amines evolution for different products during ripening was reported in –. In general, it can be observed that the examined tuna products showed amine profile trends similar to those observed during ripening and seasoning of pig meat products.
Figure 3. Biogenic amines profile during ripening in a1 small tuna products.
Figura 3. Perfil de aminas biógenas durante maduración en productos de atún pequeños a1.
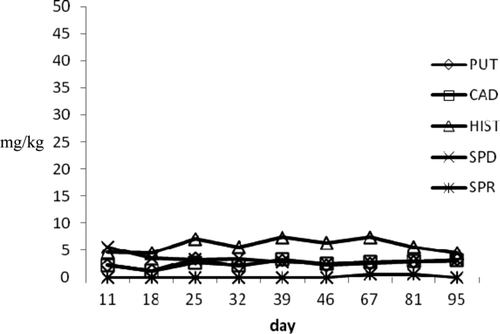
In a1 large diameter raw products, HIS profile showed a moderate increase from 3.82 to 5.24 mg/kg at the end of the experimental period. The concentrations of PUT and CAD, which are amines often considered to evaluate the shelf life of pork sausages and the seasoning process of dried salted meat products, increased reaching values of 6.28 mg/kg and 3.46 mg/kg, respectively, at 95th day. SPD and SPR, which are ubiquitous compounds present in different foods and did not retain a useful parameter to evaluate shelf life, showed decreasing values. In the medium diameter products, the HIS levels ranged from 1.1 to 8.53 mg/kg, the highest observed value in raw seafood. Putrescine and CAD reached the final value of 12.16 and 6.76 mg/kg. Differently from large diameter products, SPR gradually increased until final value of 11.61 mg/kg. The moderate BA increasing could be to highest values of Aw detected in these products that may influence the growth of microbial strain producing amines. On the other hand, small diameter products showed a reduction of amine concentration probably due to the low Aw values and high pH level (0.87 and 6.5, respectively) achieved during vacuum packaging. Particularly, HIS reached a final value of 3.92 mg/kg and SPR was not detected at the last control. Similar behavior was observed in tuna “salami” (a2 samples) where amine profile was characterized by a gradual HIS decrease, which dropped from initial values of 12.79–3.42 mg/kg on 165 d. Putrescine and CAD showed slight increase until levels of 6.33 and 3.99 mg/kg, respectively, at the end of storage period. In these products, Aw and pH values (0.88 and 6.4, respectively) during vacuum storage were superimposed to those detected in small a1 products.
The amine profile of the cooked products indicated differences between samples of the two batches. In the samples of the first batch, the highest HIS level (49.20 mg/kg) found on the 11th day gradually decreased to a final value of 12.89 mg/kg. Putrescine and CAD showed final values of 15.99 and 16.75 mg/kg, respectively. Spermidine concentration was constant during storage, and SPR was not detected at the end of analyses. In the samples of second batch, the levels detected were lower than products of first lot for all amines analyzed. The use of polyphosphates, the higher brine content, and the cutting of tuna muscles into smaller pieces allowed a rapid salt penetration, reduced Aw, and limited bacterial growth as well as BA production.
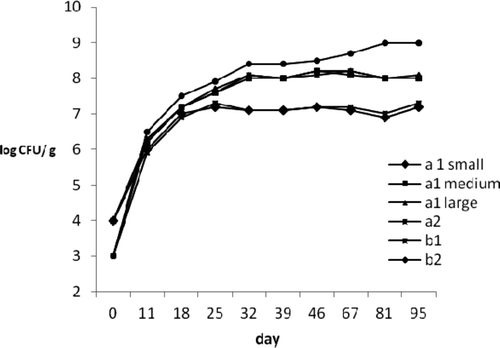
The results from LAB analysis were reported in . In a1 and a2 products, LAB, which had initial mean values of 4 and 5 log CFU/g at time zero, increased after vacuum packaging until final concentrations of 7 and 8 log CFU/g, respectively. In cooked samples, the behavior was similar for the two lots but with different LAB levels that ranged from 3 to 8 and from 4 to 9 in first and second lots, respectively. A comparative study between LAB counts and amine levels suggests a good correlation (r ∼0.70) between microbial behavior and amine evolution. A possible interpretation of this can be obtained by the pH observations. The pH level is an important factor influencing amino acid decarboxylase activity, which is stronger in an acidic environment with the optimum pH less than 5.5 (Teodorovic, Buncic, & Smiljanid, Citation1994). The increase in pH could lead to a reduction of the decarboxylase-positive lactic flora activity and to a lower BA production without inhibiting replication, as confirmed by respective LAB counts. This could be confirmed from lower BA concentrations detected in a1 small diameter and a2 products which showed a higher pH than other a1 samples.
Figure 4. Biogenic amines profile during ripening in a1 medium tuna products.
Figura 4. Perfil de aminas biógenas durante maduración en productos de atún medianos a1.
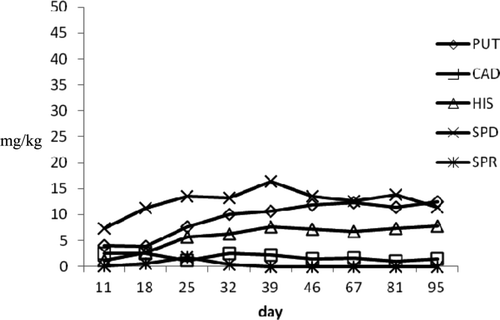
Figure 5. Biogenic amines profile during ripening in a1 large tuna products.
Figura 5. Perfil de aminas biógenas durante maduración en productos de atún grandes a1.
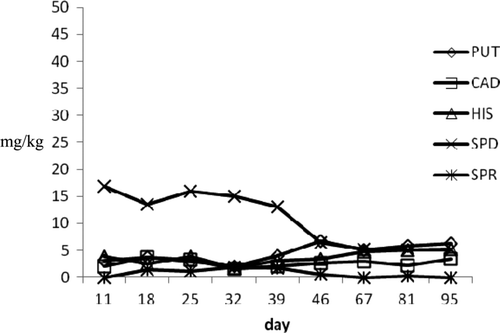
Figure 6. Biogenic amines profile during ripening in a2 tuna products.
Figura 6. Perfil de aminas biógenas durante maduración en productos de atún a2.
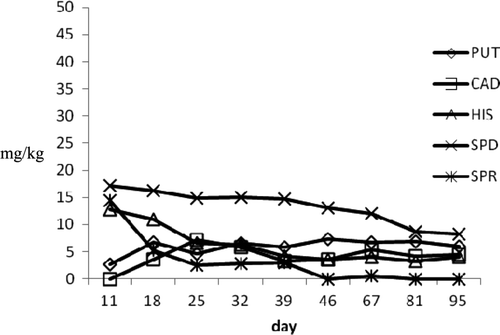
Figure 7. Biogenic amines profile during ripening in b1 tuna products.
Figura 7. Perfil de aminas biógenas durante maduración en productos de atún b1.
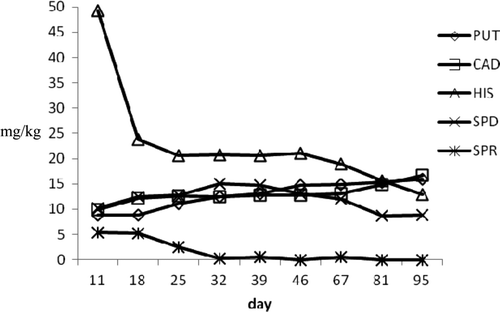
Figure 8. Biogenic amines profile during ripening in b2 tuna products.
Figura 8. Perfil de aminas biógenas durante maduración en productos de atún b2.
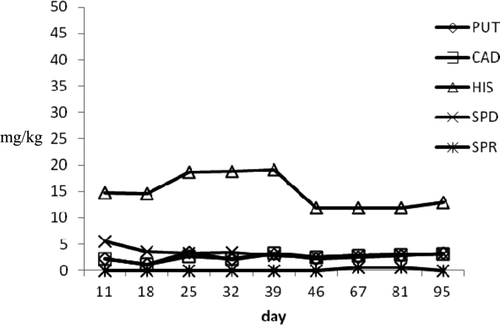
Figure 9. Changes in lactic acid bacteria (LAB) during ripening process and after vacuum packaging in tuna products.
Figura 9. Cambios en la bacteria ácido láctica (LAB) durante el proceso de maduración y tras envasado al vacío de productos de atún.
There are no data on BA formation and bacterial behavior concerning fish vacuum-packaged products similar to those of our study. However, recently, Ntzimani, Paleologos, Savvaidis, and Kontominas (Citation2008) showed that LAB were the dominant microbial population through the storage period of smoked turkey breast fillets vacuum packaged and HIS was one of the main BAs formed in term of concentration. Moreover, it has been reported (Morii, Cann, & Taylor, Citation1988) that, in mackerel, the family of Enterobacteriacae is not particularly active in the formation of BAs.
Moderate concentrations of HIS could be explained by the low percentage of this amine producer strains among decarboxylase-positive LAB, as demonstrated by others in different products (Brink, Damink, Joosten, & Huis in't Veld, 1990). Furthermore, the low levels of BAs could be attributed to the unfavorable conditions for the growth of Enterobacteriaceae, which can accumulate high amounts of HIS, as reported by Brink et al. (Citation1990) for dry fermented sausages. Unpublished data confirmed the low counts of this bacteria family. Recent studies in Denmark found that the psychrotolerant bacteria Morganella psychrotolerans and Photobacterium phosphoreum, which are known to be present in fresh tuna, are of particular concern because they form HIS at low temperatures (Emborg, Laursen, & Dalgaard, Citation2005). In a following article, Emborg and Dalgaard (Citation2008) who modeled the effect of temperature, carbon dioxide, Aw, and pH on the growth and HIS formation by Morganella psychrotolerans showed that the a minimum value of 0.963 for Aw was found, suggesting that Morganella psychrotolerans was slightly less NaCl-resistant than other psychrotolerant and Gram-negative bacteria from fresh and lightly preserved seafood. The same authors (2005) showed that in tuna vacuum-packaged, inoculated with both the psychrotolerant Morganella morganii-like isolates and Photobacterium phosphoreum, toxic concentrations of HIS at 2.1°C within 12–14 days were produced. The HIS levels in the uninoculated (control) vacuum-packaged tuna samples remained below 60 mg/kg, indicating that the HIS formation was due to the added bacteria. In the same study, dominating microbiota responsible for the formation of HIS in vacuum-packaged tuna that caused an outbreak of HFP were constituted by Morganella morganii-like isolates and Photobacterium phosphoreum; however, the temperature history of product was unknown. According to these data and considering that we analyzed raw material and products which had known temperature history, Aw, and NaCl detected in our study could further explain the low HIS values found.
Conclusion
Consumers’ demand for lightly preserved seafood is increasing. Salting, cooking and/or precooking, smoking, and curing are only some examples. These methods are receiving growing attention from food manufacturers interested in broadening and/or diversifying their productions. This interest regards, both at the experimental and commercial levels, not only the most common and more easily available species, but also those that have become the object of farming more recently. The results of our study show that, probably, these types of tuna products could be accepted by the consumer, although studies with a larger number of subjects should be carried out.
Biogenic amines behavior, especially HIS profile, of raw, salted, seasoned or cooked bluefin tuna products showed HIS levels lower than the EU limits; besides the correct application of cold chain, to ensure their safety, it is important to control the raw material quality, to process the raw material rapidly, to use good manufacturing and hygienic practices, and to employ technological procedures and process parameters that can reduce BA production, such as pH, salt concentration, and Aw values.
Acknowledgements
This work was supported by P.O.R. (Progetti Operativi Regionali) 2000/2006, Misura 4.23, Sottomisura 6 Regione Campania (Italy) project “Applicazione di tecnologie di affumicamento innovative per lo sviluppo di prodotti a base di Thunnus thynnus”.
References
- AOAC International . 2000 . Official methods of analysis of AOAC International , (17th ed.) , Gaithersburg, MD , , USA : Association of Analytical Communities .
- Auerswald , L. , Morren , C. and Lopata , A.L. 2006 . Histamine levels in seventeen species of fresh and processed South African seafood . Food Chemistry , 98 : 231 – 239 .
- Brink , B. , Damink , C. , Joosten , H.M. and Huis in't Veld , J.H. 1990 . Occurrence and formation of biologically active amines in foods . International Journal of Food Microbiology , 1 : 73 – 84 .
- Bover-Cid , S. , Hugas , M. , Izquierdo-Pulido , M. and Vidal-Carou , M.C. 2001 . Amino acid-decarboxylase activity of bacteria isolated from fermented pork sausages . International Journal of Food Microbiology , 66 : 185 – 189 .
- COMMISSION REGULATION (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs . Official Journal of the European Union, L 338/26 EN, 22.12.2005
- Draisci , R. , Riannetti , L. , Boria , P. , Lucentini , L. , Palleschi , L. and Cavalli , S. 1998 . Improved ion chromatography-integrated pulsed amperometric detection method for the evaluation of biogenic amines in food of vegetable or animal origin and in fermented foods . Journal of Chromatography A , 798 : 109 – 116 .
- Emborg , E. and Dalgaard , P. 2006 . Formation of histamine and biogenic amines in cold-smoked tuna: An investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning . Journal of Food Protection , 69 : 897 – 906 .
- Emborg , E. and Dalgaard , P. 2008 . Modelling the effect of temperature, carbon dioxide, water activity and pH on growth and histamine formation by Morganella psychrotolerans . International Journal of Food Microbiology , 128 : 226 – 233 .
- Emborg , J. , Laursen , B.G. and Dalgaard , P. 2005 . Significant histamine in tuna Thunnus albacares at 2°C – Effect of vacuum- and modified atmosphere packaging on psychrotolerant bacteria . International Journal of Food Microbiology , 101 : 263 – 274 .
- Halasz , A. , Barath , A. , Simon-SarKadi , L. and Holzapel , W. 1994 . Biogenic amine and their production by micro-organisms in food – Review . Trends in Food Science and Technology , 5 : 42 – 49 .
- Henry Chin , K.D. and Koehler , P.E. 1986 . Effects of salt concentration and incubation temperature on formation of histamine, phenethylamine, tryptamine and tyramine during miso fermentation . Journal of Food Protection , 49 : 423 – 427 .
- Kerr, M., Lawicki, P., Aguirre, S., & Rayner, C. (2002). Effect of storage conditions on histamine formation in fresh and canned tuna. Victoria, Australia: Public Health Division – Victoria Government of Human Services
- Lehane , L. and Olley , J. 2000 . Histamine fish poisoning revisited . International Journal of Microbiology , 58 : 1 – 37 .
- Maijala , R. 1993 . Formation of histamine and tyramine by some lactic acid bacteria in MRS-broth and modified decarboxylation agar . Letters in Applied Microbiology , 17 : 40 – 43 .
- McMekkin , T.A. , Olley , J. , Ross , T. and Ratskowsky , D.A. A thermodynamic approach to bacterial growth (Chapter 10) . Perspective microbiology: Theory and application . pp. 288 – 290 . Tauton, New York : Research Studies Press, Wiley .
- Mercogliano , R. , De Felice , A. , Chirollo , C. and Cortesi , M.L. 2010 . Production of vasoactive amines during the ripening of Pecorino Carmasciano cheese . Veterinary Research Communications , : 75 – 78 . Suppl 1
- Morii , H. , Cann , D.C. and Taylor , L.Y. 1988 . Histamine formation by luminous bacteria in mackerel stored at low temperatures . Nippon Suisan Gakkaishi , 54 : 299 – 305 .
- Ntzimani , A.G. , Paleologos , E.K. , Savvaidis , I.N. and Kontominas , M.G. 2008 . Formation of biogenic amines and relation to microbial flora and sensory changes in smoked turkey breast fillets stored under various packaging conditions at 4 degrees C . Food Microbiology , 25 : 509 – 517 .
- Report EFSA Journal . 2010 . The Community Summary Report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in the European Union in 2008 . EFSA Journal , 8 : 1496
- Suzzi , G. and Gardini , F. 2003 . Biogenic amines in dry fermented sausages: A review . International Journal of Food Microbiology , 88 : 41 – 54 .
- Teodorovic , V. , Buncic , S. and Smiljanid , D. 1994 . A study of factors influencing histamine production in meat . Fleischwirtschaft , 74 : 170 – 172 .
- Vale , S. and Gloria , M.B. 1997 . Biogenic amines in Brazilian cheeses . Food Chemistry , 63 : 343 – 348 .
- Veciana-Nogues , M.T. , Marine-Font , A. and Vidal-Carou , M.C. 1997 . Biogenic amines as hygienic quality indicators of tuna. Relationships with microbial counts, ATP-related compounds, volatile amines and organoleptic changes . Journal of Agriculture and Food Chemistry , 45 : 2036 – 2041 .