Abstract
Two enzymatic methods using amperometric and colorimetric detection based on two coupled activities (glyceroquinase and glycerol-3-phosphate oxidase) were compared for the glycerol content determination. The enzymatic conversion of glycerol consumes oxygen, which is measured amperometrically in a Clark-type electrode and correlated with the glycerol concentration. In addition to the enzymatic-colorimetric method, there is a third enzymatic reaction that can be used to form a coloured compound; this method involves the addition of peroxidase, 4-chlorophenol and 4-aminoantipyrine. The enzymatic methods studied showed good linearity in the range of 4 to 60 μmol L−1 glycerol with a detection limit of 1 μmol L−1. Studies of glycerol recovery in fortified sugar cane liquor showed a recovery of 98 ± 2% for the enzymatic-amperometric method and of 96 ± 1% for the enzymatic-colorimetric method. These two methods were applied to the determination of glycerol in beverages and good correlations were found between these methods.
Con el fin de determinar el contenido de glicerol y utilizando la detección amperométrica y colorimétrica, se compararon dos métodos enzimáticos basados en dos actividades acopladas (gliceroquinasa y glicerol-3-fosfato oxidasa). La conversión enzimática de glicerol consume oxígeno, proceso medido amperométricamente con un electrodo tipo Clark y correlacionado con la concentración de glicerol. Además del método enzimático-colorimétrico, a partir de una tercera reacción enzimática se puede formar un compuesto de color; este método implica agregar peroxidasa, 4-clorofenol y 4-aminoantipirina. Los estudios de los métodos enzimáticos demostraron buena linealidad en el rango de 4 a 60 μmol L−1 glicerol, con un límite de detección de 1 μmol L−1. Asimismo, los estudios de recuperación de glicerol en un licor fortificado de caña de azúcar demostraron una recuperación de 98% ± 2% para el método enzimático-amperométrico y de 96% ± 1% para el método enzimático-colorimétrico. La aplicación de ambos métodos para determinar glicerol en bebidas evidenció buenas correlaciones entre los mismos.
Introduction
Glycerol is a water-soluble product that is viscous, hygroscopic and odourless and has a sweet taste; it is a natural chemical constituent of many foodstuffs and is currently used in fruit juice, wine, vegetable oil, beer, tobacco and honey, among others. As a food additive, it belongs to the class of thickeners, stabilisers and emulsifiers. Glycerol contributes to the viscosity and sweetness of drinks such as wine, rum, sangria and grape juice with a favourable effect on the taste (Niculescu, Mieliauskiene, Laurinavicius, & Csöregi, Citation2003).
Glycerol is also a major fermentation product in wines after ethanol and carbon dioxide (Balli et al., Citation2003). It is naturally found in wines at a ratio of approximately 8 g of glycerol/100 g of ethanol. Glycerol concentrations in wines may vary between 1 and 10 g L−1 (Niculescu et al., Citation2003). Deviations from this value might indicate technological alterations during the process or deterioration of the harvested grape. High glycerol: ethanol ratios indicate either the addition of glycerol, the use of putrescent grapes or infection with Botrytis cinerea. Substantially low ratios can be due to the addition of ethanol or degradation of glycerol by undesirable microorganisms (Cañizares & Castro, Citation1995; Niculescu et al., Citation2003). Thus, glycerol is an important parameter for the industrial quality control of commercial beverages, requiring rapid, sensitive, simple, reliable and inexpensive methods for routine analysis. Furthermore, the glycerol content is also of interest for detecting the possible adulteration of wine and other beverages with added glycerol. This practice, for example, is not permitted by European Commission regulations and the European “Glycerol Project” was established to study possible methods to detect addition of synthetic or natural but exogenous glycerol in wine (Calderone, Naulet, Guillou, & Reniero, Citation2004).
There are several analytical methods that are employed in the literature for glycerol determination. The official methods for determining glycerol are those recommended by the AOAC involving gas (Method 968.09) and liquid chromatography (Method 972.10), which are time-consuming, tedious and expensive to use for routine analysis (Williams, 2010a, Citation2010b).
Enzymatic methods combining the selectivity of enzymes involved either in solution or immobilised with different detection systems have been extensively applied to develop prospective methods for measuring glycerol in clinical analysis (Merchie, Girard, Maisterrena, Michalon, & Couturier, Citation1992; Minakshi & Pundir, Citation2008), food products (Cañizares & Castro, Citation1995; Prodromidis, Stalikas, Tzouwara-Karayanni, & Karayannis, Citation1996; Niculescu et al., Citation2003; Gamella, Campuzano, Reviejo, & Pingarrón, Citation2008; Goriushkina et al., Citation2010; Monošík, Stred'anský, Tkac, & Šturdík, 2012), and biotechnological processes (Compagnone, Esti, Messia, Peluso, & Palleschi, Citation1998; Katrlik et al. Citation2006).
There is an enzymatic assay based on the methodology of substrate dosage by the final point method that uses glycerokinase (GK) and glycerol-3-phosphate oxidase (G3PO) for glycerol analysis in different matrices such as tobacco casing (Montoya et al., Citation1993), fermented products (Gamella et al., Citation2008), blood serum (Minakshi & Pundir, Citation2008) and biodiesel (Hwang, 2010; Macedo, Amado, Castro, & D'Elia, Citation2010; Luetkmeyer et al., Citation2010). In this assay, glycerol is converted into dihydroxyacetone phosphate by the sequential action of the GK and G3PO enzymes, consuming oxygen and producing hydrogen peroxide proportionally to the initial content of glycerol in the sample, as described in , where ATP and ADP are adenosine triphosphate and adenosine diphosphate, respectively.
Figure 1. Enzymatic reaction scheme involving the conversion of glycerol into a coloured compound by the sequential action of GK, G3PO and peroxide enzymes.
Figura 1. Esquema de la reacción enzimática para la conversión de glicerol en un compuesto de color mediante la acción secuencial de GK, G3PO y enzimas de peróxido.
Due to the oxygen consumption in the enzymatic reactions shown in , it is possible to determine the glycerol content in beverages with an oxygen electrode like a Clark cell. This electrode is a special form of an electrochemical cell in which the current generated is proportional to the dissolved oxygen content present in solution. In principle, the cell consists of two electrodes: a central platinum disc and a silver ring. These electrodes are dipped into an electrolyte solution, e.g. KCl solution (Kelly & Christian, Citation1984; Montoya et al., Citation1993; Luetkmeyer et al., Citation2010). A thin film of this electrolyte solution separates the electrode surface from a membrane that is permeable to oxygen but impermeable to most catalytic poisons for the platinum present in the sample. Above this membrane, there is a compartment for adding the sample. An electrical potential is applied across the electrodes (approximately 0.7 V) so that the platinum electrode is negative with respect to the silver ring. Under appropriate conditions, the magnitude of the current will be linearly proportional to the oxygen concentration in the solution.
These enzymatic reactions can also be used to analyse glycerol with UV detection, in which case, a third enzyme, peroxidase, and the reagents 4-chlorophenol and 4-aminoantipyrine are added to the previous system in order to form a coloured compound (Quinoneimine Dye), as observed in reaction (3) presented in (Hwang, 2010).
In the present work, two methods based on an enzymatic assay with GK, G3PO with amperometric detection (EA) and a peroxidase assay with colorimetric detection (EC) were compared to determine the glycerol concentration in different samples of commercial beverages such as wine, sugar cane liquor, grape soda, cola soda and grape juice.
Materials and methods
Reagents
A commercial enzymatic kit from Laborlab including 2.0 mmol L−1 ATP, GK > 0.4 U mL−1, G3PO > 1.5 U mL−1, peroxidase > 2 U mL−1, 4.0 mmol L−1 4-chlorophenol, 0.5 mmol L−1 4-aminoantipyrine, 15 mmol L−1 magnesium chloride and pH 7.0 buffer was used for all measurements. All other chemicals were of analytical grade and were purchased from Merck (Darmstadt, Germany), including glycerol 87%.
Samples of commercial beverages
The glycerol content was analysed in different beverages: sugar cane liquor (Brazil), two commercial grape juices (Brazil), grape soda (Brazil), cola soda (Brazil) and four red wines (Chile, Argentine, Portugal and France).
Amperometric method
In this method, the Clark cell used was purchased from Rank Brothers. The grape soda, cola soda and the grape juices were analysed without dilution, and the red wines were analysed after a 10 × dilution. An aliquot (30 μL) of samples was added to the upper compartment of the Teflon membrane already containing 3 mL of the enzymatic kit. The enzymatic reaction involving the glycerol conversion to dihydroxyacetone phosphate began with the sample addition. The solution of the upper compartment was constantly agitated with a small magnetic stirrer to maintain a stable oxygen gradient across the membrane. A 3 mol L−1 KCl solution was added to the lower compartment of the Teflon membrane, where the platinum disk and the silver ring were present. The current was monitored with time, maintaining the platinum electrode at −0.7 V against the silver ring. The cleaning of the electrodes with 1 μm alumina paste and the exchange of the KCl solution were performed after approximately 40 analyses.
The calibration curve was constructed by adding glycerol standard solutions in the place of the sample, ranging from final concentrations of 3.95 × 10−6 to 6.32 × 10−5 mol L−1 in 3 mL of the enzymatic kit.
Colorimetric method
The grape soda, cola soda and the grape juices were analysed without dilution, and the red wines were analysed after a 50 × dilution. An aliquot (10 μL) of samples was added to a cuvette already containing 1 mL of the enzymatic kit.
The calibration curve for colorimetric analysis was constructed by adding glycerol standard solutions in place of the sample, ranging from final concentrations of 3.56 × 10−6 to 5.70 × 10−5 mol L−1 in 1 mL of the enzymatic kit.
A kinetic study was conducted at a wavelength of 505 nm correlating the final absorbance obtained for each analysis with the concentration of glycerol.
Instrumentations
All amperometric measurements were performed using a potentiostat PGSTAT 100 (Autolab, Eco-Chemie, the Netherlands) with a current-amplifying module controlled by GPES 4.8 software.
All colorimetric measurements were performed using a spectrophotometer from PerkinElmer Inc. (Waltham, MA, USA).
Statistical analysis
The detection limit (DL) of these two enzymatic methods was obtained from the experimental criteria and according to the 3 sb /b criterion, where b is the slope of the linear calibration plot and sb was estimated as the standard deviation of the blank analyses. The calibration curves were acquired by fitting the data obtained with glycerol standard samples: ΔI versus [glycerol] (amperometric method) and Absorbance versus [glycerol] (colorimetric method) to the linear regression model. These curves were submitted to the Cochran test to evaluate the bilateral deviation of the variances to a level of significance of 5%. The residue graphs for these two methods were obtained from the differences between the values calculated from the straight line of the calibration curves and the values obtained experimentally.
The recovery study for these two methods was performed after fortifying the sugar cane liquor sample with 0.0020, 0.0040, 0.0060, 0.0080 or 0.0100% (w/w) glycerol.
Results and discussion
Study of the enzymatic-amperometric method
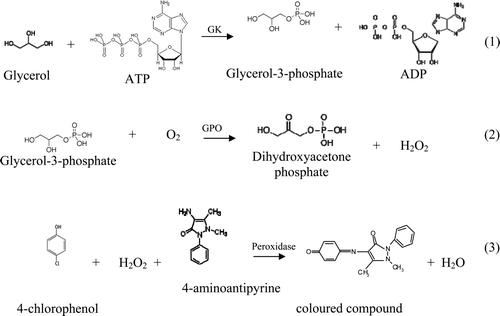
shows the current–time graph obtained in 3 mL of the commercial enzymatic kit containing a final concentration of glycerol of 63.2 μmol L−1 added approximately 100 s after the initiation of polarisation. The current response in the first 100 s is due to the spontaneously dissolved oxygen in the solution (from the ambient air), which reaches a constant value in less than 1 min. This first current plateau value is linearly proportional to the oxygen content in the solution. After the addition of the glycerol standard solution, the enzymatic reactions consume the oxygen present in the solution, showing a current decrease until the appearance of the second current plateau. The enzymatic reactions are fast, and the second current plateau is obtained in approximately 200 s. The change in the current plateau value (before and after adding this sample) is related to the oxygen consumed in the enzymatic reactions, which is proportional to the glycerol content present in the sample.
Figure 2. Current versus time plot obtained with an aliquot of glycerol standard solution in 3 mL of the commercial enzymatic kit with a final glycerol concentration of 63.2 μmol L−1.
Figura 2. Gráfico de corriente versus tiempo con una alícuota de glicerol estándar en 3 mL del botiquín enzimático comercial con una concentración final de glicerol de 63,2 μmol L–1.
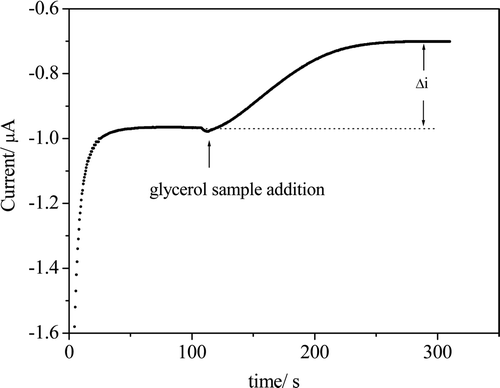
The enzymatic-amperometric method showed a good linearity with a 0.9975 linear correlation coefficient (r), and the response was linear in the concentration range from 3.95 μmol L−1 to 63.2 μmol L−1. The experimental detection limit was 1.1 μmol L−1, and 0.61 μmol L−1 was the detection limit estimated from the slope of the calibration curve. This curve was submitted to the Cochran test where the calculated value (0.4130) is lower than the tabulated value (0.6838), indicating homogeneous variances of the response with the change in the analyte concentration. presents a plot of the residuals in which a maximum residue of 4 nA is shown; this is equivalent to 0.35 μmol L−1, a value well below the detection limit. These results characterise the homoscedastic behaviour of the data.
Figure 3. Residue graph obtained from the differences between the values calculated from the straight line of the calibration curve by the enzymatic-amperometric method and the values obtained experimentally.
Figura 3. Gráfico de residuos obtenida de las diferencias entre los valores calculados de la línea recta de la curva de calibración mediante el método enzimático-amperométrico y los valores experimentales.
shows a comparison between several amperometric biosensors for glycerol determination in beverages (Gamella et al., Citation2008; Goriushkina et al., Citation2010; Monošík et al., 2012; Niculescu et al., Citation2003; Prodromidis et al., Citation1996) and in fermentation processes (Compagnone et al., Citation1998; Katrlik et al., Citation2006). From this table, it is clear that the detection limit and linearity range obtained in this work are comparatively similar to Gamella et al. and Compagnone et al. results (Gamella et al., Citation2008; Compagnone et al., Citation1998). However, it is important to notice that we did not use the redox mediator employed in the Gamella's study. An advantage of the amperometric method presented in this paper compared with Compagnone's method is that this author uses amperometric detection by H2O2 oxidation on the platinum electrode at very high potential ( + 650 mV vs. Ag/AgCl), which can be susceptible to other electroactive species during the analysis. In the present work, we use the Clark cell which employs an oxygen-permeable membrane that separates the enzymatic reaction compartment from the electrodes, avoiding any sort of interference during the amperometric detection. Only the dissolved oxygen reacts on the cathode surface.
Table 1. Amperometric biosensors reported in the literature for glycerol determination
Tabla 1. Biosensores amperométricos reportados en estudios para la determinación de glicerol.
Study of the enzymatic-colorimetric method
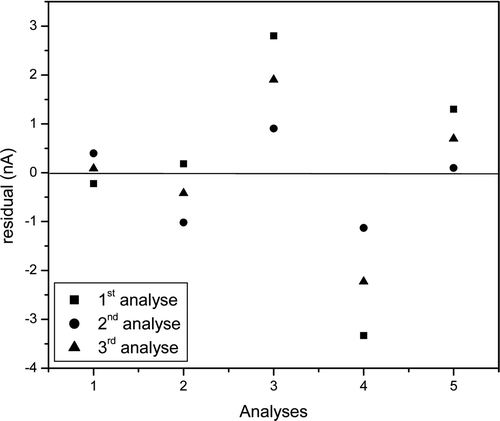
The calibration curve obtained by the enzymatic-colorimetric method showed a linear response in the concentration range from 3.56 μmol L−1 to 57.5 μmol L−1 and an excellent linear correlation coefficient of 0.9992 (r). The experimental detection limit was 0.77 μmol L−1, and 1.0 μmol L−1 was the detection limit estimated from the slope of the calibration curve. This curve was also submitted to the Cochran test, where the calculated value (0.4541) is lower than the tabulated value (0.6838); this indicates homogeneous variances of the response with the change in the analyte concentration. presents a plot of the residuals, where a maximum residue of 0.03 absorbance is shown; this is equivalent to 1.41 μmol L−1, a value very close to the detection limit. These results also characterise the homoscedastic behaviour of the data.
Figure 4. Residue graph obtained from the differences between the values calculated from the straight line of the calibration curve by the enzymatic-colorimetric method and the values obtained experimentally.
Figura 4. Gráfico de residuos obtenida de las diferencias entre los valores calculados de la línea recta de la curva de calibración mediante el método enzimático-colorimétrico y los valores experimentales.
The sugar cane liquor analysis by the enzymatic-amperometric and enzymatic-colorimetric methods showed a glycerol content lower than the detection limit, indicating very low glycerol content in this sample. Because of this result, the recovery study was performed after fortifying a sugar cane liquor sample with 0.0020, 0.0040, 0.0060, 0.0080 or 0.0100% (w/w) glycerol using the enzymatic-amperometric and enzymatic-colorimetric methods. shows the results of the recovery study. The average recovery for the EA method is 98 ± 2%, while for the EC method the average recovery is 96 ± 1%. These results show that the first method is more exact and that the second one is more precise. Both methods presented glycerol content values close to the theoretical values, indicating that both methods can be used for glycerol analysis. When comparing the averages obtained by the EA and EC methods for the sugar cane liquor sample fortified with 0.0020, 0.0040, 0.0060, 0.0080 or 0.0100% (w/w) glycerol, the calculated t-values were found to be lower than the critical t-value for the 99% confidence level. This indicates that the hypothesis was accepted and the averages for the two methods were equal.
Table 2. Comparison of the glycerol content in fortified sugar cane liquor samples with 0.0020, 0.0040, 0.0060, 0.0080 and 0.0100% (w/w) glycerol by the enzymatic-amperometric and enzymatic-colorimetric methods.
Tabla 2. Comparación del contenido de glicerol en muestras de licor fortificado de caña de azúcar con 0,0020, 0,0040, 0,0060, 0,0080, 0,0100% (w/w) glicerol, con los métodos amperométricos y enzimático-colorimétricos.
Commercial beverages analyses
The analyses of different commercial beverage samples showed good correlation between both enzymatic methods for glycerol content determination with a slope of 1.03 and a good linear correlation with r = 0.9899 (). shows the results of the glycerol content for different beverage samples with the average and the standard deviation. It is worth noting that for all beverage samples, the glycerol content observed is quite similar for both methods except for the grape juice sample, where half the glycerol content was observed when using the EC method. When comparing the averages obtained by the enzymatic-amperometric (EA) and the enzymatic-colorimetric (EC) methods, the calculated t-values were found to be lower than the critical t-value for the 99% confidence level. This indicates that the hypothesis was accepted and the averages were equal. The exception was the grape juice samples, which produced t cal values of 16.9 and 18.2. This result may be due to some interference in the EC method because of the intense colour of these samples.
Figure 5. Linear correlation between enzymatic-colorimetric (EC) and enzymatic-amperometric (EA) determinations of glycerol in different beverage samples.
Figura 5. Correlación lineal entre las determinaciones enzimático-colorimétricas (EC) y las enzimático-amperométricos (EA) de glicerol en distintas muestras de bebidas.
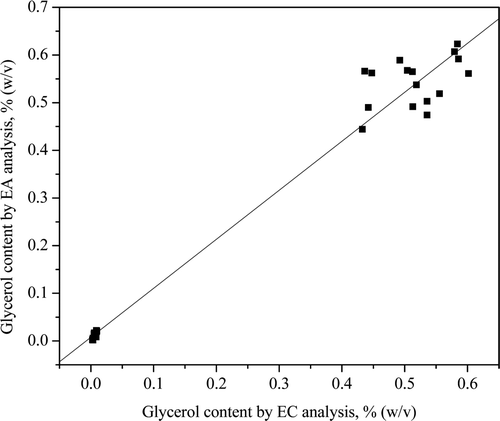
Table 3. Glycerol content obtained by the EA and EC methods for commercial beverages.
Tabla 3. Contenido de glicerol obtenido por los métodos EA y EC para bebidas comerciales.
Conclusions
In summary, two enzymatic assays based on two and three coupled activities with amperometric and colorimetric detection were compared for the analysis of glycerol in different commercial beverage samples. Both methods presented good linearity, short response times (5 min), low detection limits (approximately 1 μmol L−1) and a suitable working range (of 4 to 60 μmol L−1 glycerol approximately). The recovery studies in fortified sugar cane liquor samples showed 98 ± 2% recovery using the enzymatic-amperometric method and 96 ± 1% recovery using the enzymatic-colorimetric method. Good correlation was found between these two methods for glycerol determination in different commercial beverages with a slope of 1.03 and a linear correlation coefficient of 0.9899.
Acknowledgement
The authors wish to thank the Brazilian National Counsel for Scientific and Technological Development (CNPq) for their financial support.
References
- Balli , D. , Flari , V. , Sakellaraki , E. , Schoina , V. , Iconomopoulou , M. , Bekatorou , A. and Kanellaki , M. 2003 . Effect of yeast cell immobilization and temperature on glycerol content in alcoholic fermentation with respect to wine making . Process Biochemistry , 39 : 499 – 506 .
- Calderone , G. , Naulet , N. , Guillou , C. and Reniero , F. 2004 . Characterization of European wine glycerol: stable carbon isotope approach . Journal of Agricultural and Food Chemistry , 52 : 5902 – 5906 .
- Cañizares , P. and Castro , M.D.L. 1995 . Flow-through spectrofluorimetric sensor for the determination of glycerol in wine . Analyst , 120 : 2837 – 2840 .
- Compagnone , D. , Esti , M. , Messia , M.C. , Peluso , E. and Palleschi , G. 1998 . Development of a biosensor for monitoring of glycerol during alcoholic fermentation . Biosensors and Bioelectronics , 13 : 875 – 880 .
- Gamella , M. , Campuzano , S. , Reviejo , A.J. and Pingarrón , J.M. 2008 . Integrated multienzyme electrochemical biosensors for the determination of glycerol in wines . Analytica Chimica Acta , 609 : 201 – 209 .
- Goriushkina , T.B. , Shkotova , L.V. , Gayda , G.Z. , Klepach , H.M. , Gonchar , M.V. , Soldatkin , A.P. and Dzyadevych , S.V. 2010 . Amperometric biosensor based on glycerol oxidase for glycerol determination . Sensors and Actuators B , 144 : 361 – 367 .
- Greenhill, S.J. (2004). U.S. Patent Application No. 20040137546. Washington, DC: U.S. Patent and Trademark Office. Retrieved from http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1& Sect2=HITOFF&d=PG01&p=1&u=/netahtml/PTO/srchnum. html&r=1&f=G&l=50&s1=20040137546.PGNR.&OS=DN/2004 0137546&RS=DN/20040137546
- Katrlik , J. , Mastihuba , V. , Vostiar , I. , Sefcovicova , J. , Stefuca , V. and Gemeiner , P. 2006 . Amperometric biosensors based on two different enzyme systems and their use for glycerol determination in samples from biotechnological fermentation process . Analytica Chimica Acta , 566 : 11 – 18 .
- Kelly , T.A. and Christian , G.D. 1984 . Amperometric of glycerol and triglycerides using an oxygen electrode . Analyst , 109 : 453 – 456 .
- Luetkmeyer , T. , dos Santos , R.M. , da Silva , A.B. , Amado , R.S. , Vieira , E.C. and D'Elia , E. 2010 . Analysis of free and total glycerol in biodiesel using an electrochemical assay based on a two-enzyme oxygen-electrode system . Electroanalysis , 22 : 995 – 999 .
- Macedo , M.P. , Amado , R.S. , Castro , E.V. and D'Elia , E. 2010 . Analysis of free glycerol in biodiesel using an electrochemical assay based on a two-enzyme platinum microelectrode system . Journal of Applied Electrochemistry , 40 : 2061 – 2063 .
- Merchie , B. , Girard , A. , Maisterrena , B. , Michalon , P. and Couturier , R. 1992 . Reliable amperometric determination of glycerol and glycerol-3-phosphate with a bienzymatic nylon membrane electrode . Analytica Chimica Acta , 263 : 85 – 91 .
- Minakshi and Pundir , C.S. 2008 . Construction of an amperometric enzymic sensor for triglyceride determination . Sensors and Actuators B , 133 : 251 – 255 .
- Monošík, R., Stred'anský, M., Tkac, J., Šturdík, E. (2012). Application of enzyme biosensors in analysis of food and beverages. Food Analytical Methods, 5, 40–53
- Montoya , A. , March , C. , Mocholí , A. , Abad , A. , Manclús , J.J. and Ferrero , J.M. 1993 . Electrochemical assays based on enzyme-electrode systems to determine glycerol and propylene glycol in tobacco casing . Sensors and Actuators B , 15–16 : 429 – 434 .
- Niculescu , M. , Mieliauskiene , R. , Laurinavicius , V. and Csöregi , E. 2003 . Simultaneous detection of ethanol, glucose and glycerol in wines using pyrroloquinoline quinone-dependent dehydrogenases based biosensors . Food Chemistry , 82 : 481 – 489 .
- Prodromidis , M.I. , Stalikas , C.D.E. , Tzouwara-Karayanni , S.M. and Karayannis , M.I. 1996 . Determination of glycerol in alcoholic beverages using packed bed reactors with immobilized glycerol dehydrogenase and an amperometric FIA system . Talanta , 43 : 27 – 33 .
- Williams, S. (2010a). Method 968.09. In Official Methods of Analysis (pp. 12–13, 17th ed.). Virginia, USA: Association of Official Analytical Chemists
- Williams, S. (2010b). Method 972.10. In Official Methods of Analysis (pp. 13–14, 17th ed.). Virginia, USA: Association of Official Analytical Chemists