Abstract
Fresh-cut nectarines are very perishable product, and thus have a short shelf-life. In this study, minimally processed nectarines (cv Big Top) were treated with chitosan (20 g/L), sodium alginate (20 g/L) and Chitoplant® (20g/L), sealed in polypropylene plastic bags, and stored at 4°C for 5 days. Effects of the coatings on nectarine slices shelf-life and qualitative attributes were evaluated by investigating changes in colors, browning potential, total soluble solids content, and titratable acidity. Changes in quality parameters were lower in coated samples as compared with the control. The alginate coatings were effective on delaying the evolution of the parameters related to postharvest ripening, such as color (Hue, L*) and loss of acidity. The application of sodium alginate coating could be used to reduce deteriorative processes, maintain quality and improve the shelf-life of fresh-cut nectarine stored at 4°C.
Las nectarinas recién cortadas son muy perecederas y, por tanto, tienen una vida útil corta. En este estudio, se trataron nectarinas (cv Big Top) poco procesadas con recubrimientos de chitosán (20 g/L), alginato de sodio (20 g/L) y Chitoplant® (20 g/L). Luego fueron selladas en bolsas de plástico de polipropileno y almacenadas durante 5 días a 4°C. Se evaluaron los efectos de los recubrimientos en la vida útil y en los atributos cualitativos de gajes de nectarinas, para lo cual se analizaron los cambios de color, el potencial para el oscurecimiento, el contenido total de sólidos solubles y la acidez de titulación. Los cambios registrados en estos parámetros de calidad fueron menores en las muestras con recubrimiento que los que se observaron en la muestra de control. Los recubrimientos de alginato de sodio fueron efectivos para retrasar la evolución de parámetros relacionados con la maduración postcosecha, como el color (Tonalidad, L*) y la pérdida de acidez. Por lo cual, se concluye que el alginato de sodio puede aplicarse para reducir el proceso de deterioro, mantener la calidad y mejorar la vida útil de nectarinas recién cortadas y almacenadas a 4°C.
Introduction
Minimal processing has been defined as a combination of procedures, such as washing, sorting, trimming, peeling, and slicing or chopping, that do not affect the fresh-like quality of the food. The ready-to-eat fruit and vegetable market has rapidly grown in recent years due to the health benefits associated with these foods to increasingly health-conscious consumers with busy lifestyles and with increasing purchase power. Nevertheless, because the tissular integrity of fruits is more easily altered during processing, ready-to-use commodities are more perishable than the original materials (Oms-Oliu et al., Citation2010).
The main factors affecting the loss of consumer acceptability are discoloration, enzymatic browning, dryness, and texture loss (Pérez-Gago, González-Aguilar, & Olivas, Citation2005). These parameters determine the visual appearance of the fruits (Pace, Cefola, Renna, & Attolico, Citation2011). For this reason, the fruit processing industry requires the development of techniques capable of keeping safe shelf-life, preserving the original visual and organoleptic fresh-like characteristics of fresh-cut produces.
The commercial success of fresh-cut peach and nectarine slices has been limited due to their short shelf-life, because of cut surface browning, excessive flesh softening, and pit cavity breakdown (Gorny, Hess-Pierce, & Kader, Citation1998). For these reasons, it is essential to find treatments to slow down the browning and ripening process and prolong the shelf-life. Several treatments have been studied in order to maintain quality and to extend the shelf-life of fresh-cut peach and nectarine like heat treatment (Steiner et al., Citation2006), chemical treatments (Costa da Costa, Antunes, Valmor Rombaldi, & Arocha, Citation2011; Gorny, Hess-Pierce, & Kader, Citation1999; Zhu, Zhou, Zhu, & Guo, Citation2009), modified atmosphere packaging (Palmer-Wright & Kader, Citation1997; Zoffoli, Rodriguez, Aldunce, & Crisosto, Citation1997) and edible coatings application (Du, Citation1997; Ruoyi, Zhifang, & Zhaoxin, Citation2005).
Edible coatings, a new strategy used to extend shelf-life and to improve food quality of whole fruits and fresh-cut fruits, have been applied to many products. Coatings on minimally processed products create a semipermeable barrier to external elements that can reduce moisture loss, solutes migration, respiration and oxidative reaction rates (Barbosa, Dias de Mello Castanho & Rodrigues Monteiro, Citation2011; Vargas, Pastor, Chiralt, McClements & González- Martínez, Citation2008). Chitosan has been one of the most promising coating materials for fruits because of its good film-forming property, broad antimicrobial activity, and excellent compatibility with other substances. Furthermore, chitosan films are tough, highly transparent, long-lasting and flexible (Cé, Caciano, & Brandelli, Citation2012). Other popular edible coating formulations were polysaccharide-based like alginate or protein-based polymers (Dìaz-Mula, Serrano, & Valero, in press). Maintenance of fruit quality has been achieved by using chitosan in peach (Li & Yu, Citation2001), strawberries (Vu, Hollingsworth, Leroux, Salmieri, & Lacroix, Citation2011), and papaya (Asgar, Tengku, Kamaruzaman, & Yasmeen, Citation2011), pectin coating in melon (Ferrari, Sarantópoulos, Carmello-Guerreiro, & Hubinger, 2013), alginate in apple (Olivas, Mattinson, & Barbosa-Cánovas, Citation2007; Rojas-Graü, Tapia, Rodríguez, Carmona, & Martin-Belloso, Citation2007) and in papaya (Tapia et al., Citation2008).
The objective of this work was to evaluate the effectiveness of different coatings in order to prolong the shelf-life of nectarine slices, and to study the effect of the best treatment on the quality of fresh-cut nectarine slices stored in modified atmosphere packaging, as the commercial practice.
Materials and methods
Material
Nectarines (Prunus persica (L.) Batsch., cv. Big Top) were harvested at commercial maturity, and stored (4°C and 95% relative humidity, RH) before processing. The fruits were peeled, cored and cut into 5-mm-thick slices using a hand-operated slicer.
Methods
Sample preparation
Three different coating solutions were prepared: (1) 20 g/L chitosan (90–95% of deacetylated degree and 90–100 mPa.s of viscosity, Sigma-Aldrich Co., Steinhein, Germany), (2) 20 g/L sodium alginate (Sigma-Aldrich Co., Steinhein, Germany), (3) 20 g/L Chitoplant®, containing 95% chitosan, 2.5% boron and 2.5% zinc (Agritalia, Italy), a commercial chitosan formulation. Nectarines slices used as controls were not treated with coating.
| 1. | Film-forming solutions were prepared by dissolving chitosan (20 g/L) powders in an aqueous solution of 20 g/L citric acid. Then, the solution was added to 500 ml/L glycerol and 1.5 ml/L Tween 20 to improve the wettability (Duan, Wu, Strik, & Zhao, Citation2011). The mixture was homogenized for 1 min and stirred in a 60°C water bath for 30 min, followed by cooling to room temperature. | ||||
| 2. | Film-forming solutions were prepared by dissolving sodium alginate (20 g/L) powders in deionized water while heating on a stirring hot plate for 10 min at 70°C until the mixture became clear (Chiumarelli, Ferrari, Sarantópoulos, & Hubinger, Citation2011). Coating solutions also contained glycerol (15 ml/L) as an emulsifier and calcium chloride (20 g/L) (Sigma-Aldrich Co., Steinhein, Germany). Calcium chloride was used to induce crosslinking reaction (Rojas-Graü, Tapia, Rodríguez, Carmona, & Martin-Belloso, Citation2007; Tapia et al., Citation2008). | ||||
| 3. | Film-forming solutions were prepared by dissolving Chitoplant® (20 g/L) powders in deionized water with continuous shaking until the solution became clear. No plasticizer was added. | ||||
Slices were immersed in the coating solutions for 1 min at 20°C and were then allowed to drip off. Fruits dipped in sodium alginate-based coating, in addition, were immersed in calcium chloride for 30 s. Control fruits were dipped in distilled water.
Twenty-five slices were randomly selected and packaged in polypropylene plastic bags (20 cm × 30 cm size and 90 μm thickness) with 50 cm3 O2/m2/bar/day, 150 cm3 CO2/m2/bar/day and 2.8 g/m2/bar/day water vapor transmission rate (I.Plast, Italy). The packages were completely sealed (UNIMEC packaging systems, Italy) and then were stored at 4°C and 95% RH in darkness for 5 days.
Analysis
Gases composition
The concentrations of oxygen and carbon dioxide inside the packages were monitored daily by sampling (0.5 ml) the headspace using a CANAL 121 (Vizag, Gas Analysis, France). A syringe was inserted into the package through a rubber seal placed on the film. Gases were analyzed with an electrochemical sensor for O2 level and an infrared sensor for CO2 level. The instrument was calibrated towards air. Results were expressed as kPa of O2 and CO2 inside the bags.
Quality measurements
Color analysis was performed at 0, 1, 2 and 5 days of cold storage at 4°C.
L*, a*, and b* values were determined at two points along each side of the cut surface using a Minolta chroma-meter (CR400; Minolta, Ramsey, NJ, USA). The flesh color was also expressed as whiteness index (WI = 100 − [(100 − L*)2 + (a*)2 + (b*)2]1/2) according to Bolin and Huxsoll (Citation1991).
Quality analyses were performed at day 0 on the nectarines without coating and after 5 days of cold storage at 4°C in all the treatments. Total soluble solids (TSS) content was determined in the juice from 25 slices with a digital refractometer Atago PR-101 (Atago, Japan) at 20°C and results expressed as °Brix. Titratable acidity (TA) was determined by titration with 0.1 N NaOH up to pH 8.1, using 10 ml of diluted juice in distilled H2O and results were expressed as meq/L.
Browning potential
Browning potential was determined according to the method of Arias, Gonzalez, Lopez-Buesa and Oria (Citation2008) at day 0, and after 1 and 5 days of storage. The extract was obtained as follows: nectarine slices (25 g) from each treatment were homogenized for 2 min at 13,500 rpm with an Ultra-Turrax T25 (IKAs WERKE, Germany), the homogenates were centrifuged (Centrifuge AVANTITM J-25, Beckman Instruments Inc., Fullerton, CA, USA) at 4000 rpm for 10 min and the supernatant was filtered through Whatman 4 filter paper (Whatman Intl., UK). The absorbance of the clear juice was then measured spectrophotometrically (Hitachi, U-5100, Japan) at 440 nm to determine browning potential (BP). This measurement was replicated three times.
Statistical analysis
The basic experimental design consisted of three coating treatments, each having three replicates. In sliced nectarine measures, a package contained 25 slices, which was equivalent to two nectarines, was considered a replicate. Data were analyzed by analysis of variance using statistical procedures of the STATISTICA ver. 6.0 (Statsoft Inc., Tulsa, OK, USA). The sources of variance being coating treatments. Tukey's test HSP (honestly significant differences) was used to determine significant differences among treatment means. Means values were considered significantly different at P ≤ 0.05.
Results and discussion
Gases composition
Significant differences were observed between coated and uncoated nectarine slices regarding the composition of O2 and CO2 in the head space along the evaluated period ( and ). Higher O2 levels were found in alginate coating slices whereas lower levels were found in chitosan coating slices. This result, probably depend on the different barrier effect and mechanical properties of the coatings solutions used (Chiumarelli & Hubinger, Citation2012). Moreover, the application of coatings in fresh-cut products can create a modified atmosphere around each piece, reducing the respiration rate and the metabolic processes (Rojas-Graü et al., Citation2008).
Figure 1. Evolution of O2 (KPa) during cold storage on nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L).
Figura 1. Evolución de O2 (KPa) durante el almacenamiento en frío sobre las nectarinas recubiertas con Chitoplant® (20 g/L), quitosán (20 g/L) y alginato (20 g/L).
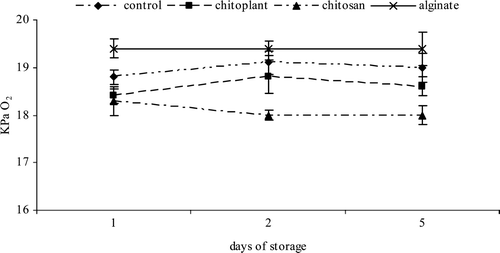
Figure 2. Evolution of CO2 concentration (KPa) during cold storage on nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L).
Figura 2. Evolución de CO2 (KPa) durante el almacenamiento en frío sobre las nectarinas recubiertas con Chitoplant® (20 g/L), quitosán (20 g/L) y alginato (20 g/L).
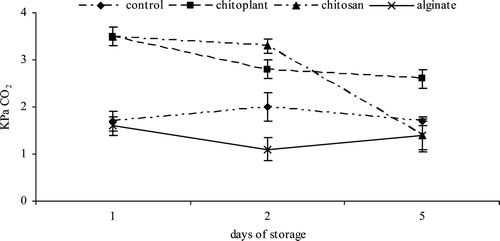
CO2 production was lower in alginate-coated slices with respect to control ones, especially in the first 2 days of storage (), in agreement with previous reports in peaches coated with chitosan, methylcellulose or alginate (Li & Yu Citation2001; Maftoonazad, Ramaswamy, & Marcotte, Citation2008). Alginate coating acts as barrier to gas exchange, so there is less oxygen available for plant tissues respiration, resulting in lower release of carbon dioxide by the product, and, consequently, reducing the metabolic processes (Rojas-Graü, Tapia, Rodríguez, Carmona & Martin-Belloso, Citation2007). On the contrary, CO2 production was significantly higher in Chitoplant® treated slices.
Quality measurements
Cut surface browning is a major problem for several minimal processed fruits. On non-treated samples a darkening effect on the color of cut slices was noted, when compared to treated samples, in according with instrumental color evaluation (Hue angle).
During storage, a reduction in Hue angle was observed in all the treatments () and in control slices. The highest values were obtained in slices treated with sodium alginate, with final value of 95.12, showing a reduction of the ripening process.
Figure 3. Evolution of color (Hue angle) during cold storage on nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L). Data are the mean ± SE (n = 25).
Figura 3. Evolución de color (ángulo de tonalidad) durante el almacenamiento en frío sobre las nectarinas recubiertas con Chitoplant® (20 g/L), quitosán (20 g/L) y alginato (20 g/L). Los datos son medias ± desviación estándar (n = 25).
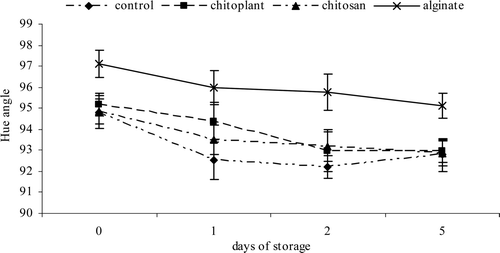
No significant differences were observed in those slices treated with 2% chitosan, 2% chitoplant and control respect to color evolution during cold storage. A sharp decrease in Hue angle would indicate an over-ripe and senescence process of fruit, which is considered as detrimental (Rojas-Graü et al., Citation2008). On the contrary, the alginate-coated fruits (with higher Hue angle) maintained the typical bright color of recently processed fruits.
Fruit color is crucial in purchase decisions, especially if the product is packaged and cannot be touched or smelled. During storage the L* value decreased for all the control and treated fruits (). However, a smaller decrease was observed for the fresh-cut fruit treated with sodium alginate and control. Lightness was better preserved by the treatment with sodium alginate coating.
Figure 4. Evolution of color (L*) during cold storage on nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L). Data are the mean ± SE (n = 25).
Figura 4. Evolución de color (L*) durante el almacenamiento en frío sobre las nectarinas recubiertas con Chitoplant® (20 g/L), quitosán (20 g/L) y alginato (20 g/L). Los datos son medias ± desviación estándar (n = 25).
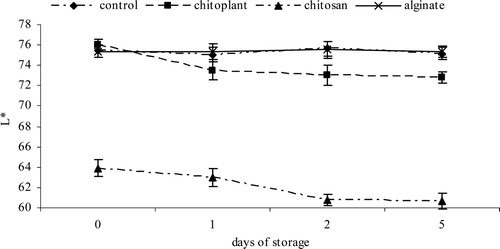
On the contrary, chitosan-coated slices showed the lowest L*. The lower L* level could be also correlated with the reduction of O2 and increase of CO2 inside the packages and with a greater deterioration of the fruit (). A rapid deterioration of the fruit involved an increase in respiration rate and enzymatic metabolic processes that led to a loss of quality of fresh-cut produce (Gonzalez-Aguilar et al., Citation2009).
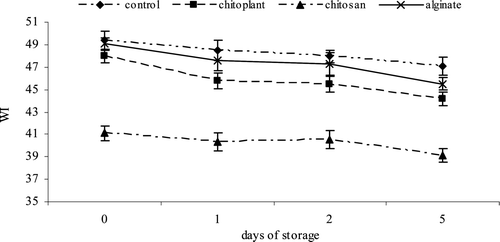
The b* value was used to estimate changes in the yellow color during the storage time (Gonzalez-Aguilar et al., Citation2009). Control fruit showed the lowest b* values, and high values of coating fruits indicated that chitosan and alginate treatments were able to maintain the highest b* values during the storage time (Figure 5). The severity of surface whitening for the coated and uncoated nectarine slices was quantitatively expressed by WI scores at different treatments (). Higher WI scores indicated greater development of surface whiteness (Gounga, Xu, Wang, & Yang, Citation2008). For uncoated slices, the WI score ranges from 49.4 to 47.1 while the chitosan coating showed the lowest WI score of 39.11, at the end of storage period. This result is related with L* values and with the result of previous work (Raybaudi-Massilia, Mosqueda-Melgar, & Martín-Belloso, Citation2008; Vargas, Chiralt, Albors, & González-Martínez, Citation2009) and could be due to the film forming effect of chitosan on surface pores of the samples, which limits water losses. Changes in WI were also reported by Aguayo, Allende, and Artés (Citation2003) in “Piel de sapo” melon and others varieties, indicating that WI decreased when translucency injury increased on fresh-processed melon, as a consequence of a physiological disorder characterized by dark and glassy flesh.
Figure 5. Evolution of color (b*) during cold storage on nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L). Data are the mean ± SE (n = 25).
Figura 5. Evolución de color (b*) durante el almacenamiento en frío sobre las nectarinas recubiertas con Chitoplant® (20 g/L), quitosán (20 g/L) y alginato (20 g/L). Los datos son medias ± desviación estándar (n = 25).
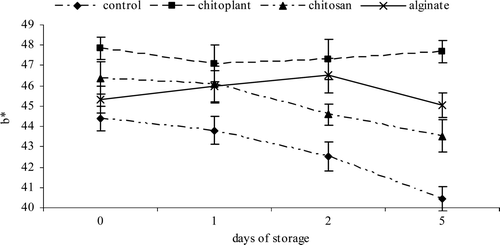
Figure 6. Evolution of color (Whiteness Index) during cold storage on nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L). Data are the mean ± SE (n = 25).
Figura 6. Evolución de color (índice de blancura) durante el almacenamiento en frío sobre las nectarinas recubiertas con Chitoplant® (20 g/L), quitosán (20 g/L) y alginato (20 g/L). Los datos son medias ± desviación estándar (n = 25).
Total soluble solids at harvest was 8.2° Brix with significant effect (P ≤ 0.05) of coating treatments during storage on nectarine slices. The Chitoplant® coating induced a significant (P ≤ 0.05) decrease in TSS content and titratable acidity values compared to chitosan-treated slices. The faster decrease in acidity gave rise to a faster senescence (Asgar et al., Citation2011). On the contrary, the chitosan and alginate coatings were significantly (P ≤ 0.05) effective in delaying the loss of acidity, which occurred during cold storage (titratable acidity at harvest was 97.98 meq/l).
The levels of titratable acidity were correlated with the antibrowning efficacy of the treatments. Han, Zhao, Leonard, and Traber (Citation2004) reported that in raspberry and strawberry, the chitosan coatings slowed down the changes in titratable acidity, effectively delaying fruit ripening. The effects of edible coating on decreasing acidity losses have been also found in chitosan- and alginate-coated peaches (Li & Yu Citation2001; Maftoonazad et al., Citation2008). The effect of coating on acidity retention could be a result of the lower respiration rate found in coated fruits, since organic acids are substrates for many reactions during aerobic respiration in plant cell. In chitosan coating significant (P ≤ 0.05) higher acidity values are also due to the effect of citric acid utilized for film-forming solution.
shows pH variations during storage for control and coated samples. As expected, since coated fruits showed less variation in titrable acidity, the associated variation in their pH was also relatively lower and not significant (pH at harvest was 5.48). At the end of storage, control nectarines had significant higher pH than coated fruits, confirming some previous results (Togrul & Arslan, Citation2004).
Table 1. Changes in total soluble solid content (°Brix), titratable acidity (meq/l) and pH of nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L) after cold storage (4°C and 95% RH).
Tabla 1. Cambios en el contenido total de sólidos solubles (°Brix), acidez valorable (meq/L) y pH de las nectarinas recubiertas con Chitoplant® (20 g/L), quitosán (20 g/L) y alginato de sodio (20 g/L) después de almacenamiento en frío (4°C y 95% RH).
Browning potential
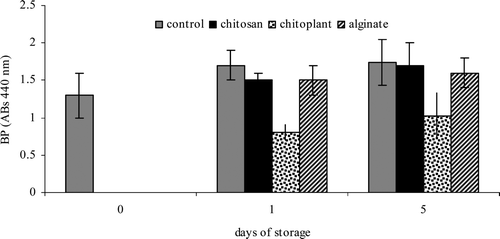
Browning potential was monitored in all the samples. An increase in browning potential was found throughout storage (Figure7) in accordance with other works (Castaner, Gil, Ruíz & Artes, Citation1999; Martin-Diana et al., Citation2007). Control samples presented the highest and Chitoplant® the lowest values (). The application of Chitoplant® coating proved to be the treatment that was the most effective in reducing browning index of nectarine slices. This could be due to the inactivation of the enzymes by the use of coating and to the lower viscosity of Chitoplant® (Martin-Diana et al., Citation2007; Romanazzi, Mlikota Gabler, Margosan, Mackey, & Smilanick, Citation2009).
Figure 7. Browning potential values during cold storage on nectarines coated with Chitoplant® (20 g/L), chitosan (20 g/L) and sodium alginate (20 g/L). Data are the mean ± SE (n = 25).
Figura 7. Potential de oscurecimiento (Abs 440 nm) durante el almacenamiento en frío de las nectarinas recubiertas de Chitoplant® (20 g/L), quitosano (20 g/L) y alginato de sodio (20 g/L). Promedio ± error estándar (n = 25).
Chitosan coatings were able to decrease browning of fruit by reducing polyphenol oxidase and peroxidase activities; these changes were directly related to color changes of the flesh of different fruits and vegetables. A chitosan coating form a protective barrier on the surface of the fruit and reduce the supply of oxygen for enzymatic oxidation of phenolics (Zhang & Quantick, Citation1998).
Conclusions
The use of edible coatings can help maintain desirable quality characteristics of fresh-cut Big Top nectarine slices. The coated slices maintained their initial color during the refrigerated storage, in particular, the samples treated with alginate coating. Moreover, coatings reduced browning potential and decay of fresh-cut nectarine, in particular with the use of Chitoplant®. The alginate coatings were effective on delaying the evolution of the parameters related to postharvest ripening, such as color and loss of acidity, which could be explained by the gas barrier provided by the coatings creating a modified atmosphere in the fruit. On the contrary, chitosan coating was not effective at delaying color browning, in particular the lightness and the whitness. According to the results obtained in this study, sodium alginate (20 g/L) and Chitoplant® appear to be a promising conservation alternative for fresh-cut nectarine.
References
- Asgar , A.M. , Tengku , M.M. , Kamaruzaman , S. and Yasmeen , S. 2011 . Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage . Food Chemistry , 124 : 620 – 626 .
- Aguayo , E. , Allende , A. and Artés , F. 2003 . Keeping quality and safety of minimally fresh processed melon . European Food Research and Technology , 216 : 494 – 499 .
- Arias , E. , Gonzalez , J. , Lopez-Buesa , P. and Oria , R. 2008 . Optimization of processing of fresh-cut pear . Journal of the Science of Food and Agriculture , 88 : 1755 – 1763 .
- Barbosa , L.N. , Dias de Mello Castanho , A.R. and Rodrigues Monteiro , A. 2011 . Influence of temperature and edible coating on the physical and chemical parameters and sensory acceptance of fresh-cut organic carrots . CyTA – Journal of Food , 1 : 31 – 36 .
- Bolin , H.R. and Huxsoll , C.C. 1991 . Control of minimally processed carrot (Daucus carota) surface discolouration caused by abrasion peeling . Journal of Food Science , 56 : 416 – 418 .
- Castaner , M. , Gil , M.I. , Ruíz , M.V. and Artes , F. 1999 . Browning susceptibility of minimally processed Baby and Romaine lettuces . European Food Research and Technology , 209 : 52 – 56 .
- Cé , N. , Caciano , P.Z. and Brandelli , N.A. 2012 . Antimicrobial activity of chitosan films containing nisin, peptide P34, and natamycin . CyTA – Journal of Food , 10 ( 1 ) : 21 – 26 .
- Chiumarelli , M. , Ferrari , C.C. , Sarantópoulos , C.I.G.L. and Hubinger , M.D. 2011 . Fresh cut ‘Tommy Atkins’ mango pre-treated with citric acid and coated with cassava (Manihot esculenta Crantz) starch or sodium alginate . Innovative Food Science and Emerging Technologies , 12 : 381 – 387 .
- Chiumarelli , M. and Hubinger , M.D. 2012 . Stability, solubility, mechanical and barrier properties of cassava starch e Carnauba wax edible coatings to preserve fresh-cut apples . Food Hydrocolloids , 28 : 59 – 67 .
- Costa , da Costa, A. , Antunes , P.L. , Valmor Rombaldi , C. and Arocha , G.M. 2011 . Control of enzymatic browning and flesh firmness in fresh-cut peaches . Ciencia Rural , 41 : 1094 – 1101 .
- Díaz-Mula , H.M. , Serrano , M. and Valero , D. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit . Food Bioprocess Technology , in press 5, 2990–2997
- Du , J. 1997 . Effects of chitosan coating on the storage of peach, Japanese pear and kiwifruit . Journal of the Japanese Society for Horticultural Science , 66 : 15 – 22 .
- Duan , J. , Wu , R. , Strik , B.C. and Zhao , Y. 2011 . Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions . Postharvest Biology and Technology , 59 : 71 – 79 .
- Ferrari , C.C. , Sarantópoulos , C. , Carmello-Guerreiro , S.M. and Hubinger , M.D. 2013 . Effect of osmotic dehydration and pectin edible coatings on quality and shelf life of fresh-cut melon . Food Bioprocess Technology , 6, 80–91
- Gonzalez-Aguilar , G.A. , Valenzuela-Soto , E. , Lizardi-Mendoza , J. , Goycoolea , F. , Martinez-Tellez , M.A. , Villegas-Ochoa , M.A. … and Ayala-Zavala , J.F. 2009 . Effect of chitosan coating in preventing deterioration and preserving the quality of fresh-cut papaya ‘Maradol . Journal of the Science of Food and Agriculture , 89 : 15 – 23 .
- Gorny , J.R. , Hess-Pierce , B. and Kader , A.A. 1998 . Effects of fruit ripeness and storage temperature on the deterioration rate of fresh-cut peach and nectarine slices . HortScience , 33 : 110 – 113 .
- Gorny , J.R. , Hess-Pierce , B. and Kader , A.A. 1999 . Quality changes in fresh-cut peach and nectarine slices as affected by cultivar, storage atmosphere and chemical treatments . Journal of Food Science , 64 : 429 – 432 .
- Gounga , M.E. , Xu , S.Y. , Wang , Z. and Yang , W.G. 2008 . Effect of whey protein isolate–pullulan edible coatings on the quality and shelf life of freshly roasted and freeze-dried chinese chestnut . Journal of Food Science , 73 : 155 – 161 .
- Han , C. , Zhao , Y. , Leonard , S.W. and Traber , M.G. 2004 . Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria ananassa) and raspberries (Rubus idaeus) . Postharvest Biology and Technology , 33 : 67 – 78 .
- Li , H. and Yu , T. 2001 . Effect of chitosan coating on incidence or brown rot, quality and physiological attributes for postharvest peach fruit . Journal of the Science of Food and Agriculture , 81 : 269 – 274 .
- Maftoonazad , N. , Ramaswamy , H.S. and Marcotte , M. 2008 . Shelflife extension of peaches through sodium alginate and methyl cellulose edible coatings . International Journal of Food Science & Technology , 43 : 951 – 957 .
- Martin-Diana , A.B. , Rico , D. , Barry-Ryan , C. , Frias , J.M. , Henehan , G.T.M. and Barat , J.M. 2007 . Efficacy of steamer jet-injection as alternative to chlorine in fresh-cut lettuce . Postharvest Biology and Technology , 45 : 97 – 107 .
- Olivas , G.I. , Mattinson , D.S. and Barbosa-Cánovas , G.V. 2007 . Alginate coatings for preservation of minimally processed ‘Gala’ apples . Postharvest Biology and Technology , 45 : 89 – 96 .
- Oms-Oliu , G. , Rojas-Graü , M.A. , González , L. , Varela , P. , Soliva-Fortuny , R. , Hernando , M.I. … and Martín-Belloso , O. 2010 . Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: a review . Postharvest Biology and Technology , 57 : 139 – 148 .
- Pace , B. , Cefola , M. , Renna , F. and Attolico , G. 2011 . Relationship between visual appearance and browning as evaluated by image analysis and chemical traits in fresh-cut nectarines . Postharvest Biology and Technology , 61 : 178 – 183 .
- Palmer-Wright , K. and Kader , A.A. 1997 . Effect of controlled-atmosphere storage on the quality and carotenoid content of sliced persimmons and peaches . Postharvest Biology and Technology , 10 : 89 – 97 .
- Pérez-Gago , M.B. , González-Aguilar , G.A. and Olivas , G.I. 2005 . Edible coatings for fruits and vegetables . Stewart Postharvest Review , 6 : 1 – 14 .
- Raybaudi-Massilia , R.M. , Mosqueda-Melgar , J. and Martín-Belloso , O. 2008 . Edible alginate- based coating as carrier of antimicrobials to improbe shelf-life and safety of fresh-cut melon . International Journal of Food Microbiology , 121 : 313 – 327 .
- Rojas-Graü , M.A. , Tapia , M.S. and Martín-Belloso , O. 2008 . Using polysaccharidebased edible coatings to maintain quality of fresh-cut Fuji apples . Food Science & Technology , 41 : 139 – 147 .
- Rojas-Graü , M.A. , Tapia , M.S. , Rodríguez , F.J. , Carmona , A.J. and Martin-Belloso , O. 2007 . Alginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples . Food Hydrocolloids , 21 : 118 – 127 .
- Romanazzi , G. , Mlikota Gabler , F. , Margosan , D.A. , Mackey , B.F. and Smilanick , J.L. 2009 . Effect of acid used to dissolve chitosan on its film forming properties and its ability to control postharvest grey mold of table grape . Phytopathology , 99 : 1028 – 1036 .
- Ruoyi , K. , Zhifang , Y. and Zhaoxin , L. 2005 . Effect of coating and intermittent warming on enzymes, soluble pectin substances and ascorbic acid of Prunus persica (cv. Zhonghuashoutao) during refrigerated storage . Food Research International , 38 : 331 – 336 .
- Steiner , M. , Abreu , L. , Correia , S. , Beirao-da-Costa , E. , Leitao , M. , Beirao-da-Costa , J.E. and Moldao-Martins , M. 2006 . Metabolic response to combined mild heat pre-treatments and modified atmosphere packaging on fresh-cut peach . European Food Reserch and Technology , 222 : 217 – 222 .
- Tapia , M.S. , Rojas-Graü , M.A. , Carmona , A. , Rodríguez , F.J. , Soliva-Fortuny , R. and Martin-Belloso , O. 2008 . Use of alginate- and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya . Food Hydrocolloids , 22 : 1493 – 1503 .
- Togrul , H. and Arslan , N. 2004 . Extending shelf-life of peach and pear by using CMC from sugar beet pulp cellulose as a hydrophilic polymer in emulsions . Food Hydrocolloids , 18 : 215 – 226 .
- Vargas , M. , Chiralt , A. , Albors , A. and González-Martínez , C. 2009 . Effect of chitosan-based edible coatings applied by vacuum impregnation on quality preservation of fresh-cut carrot . Postharvest Biology and Technology , 51 : 263 – 271 .
- Vargas , M. , Pastor , C. , Chiralt , A. , McClements , D.J. and González- Martínez , C. 2008 . Recent advances in edible coatings for fresh and minimally processed fruits . Critical Reviews in Food Science and Nutrition , 48 : 496 – 511 .
- Vu , K.D. , Hollingsworth , R.G. , Leroux , E. , Salmieri , S. and Lacroix , M. 2011 . Development of edible bioactive coating based on modified chitosan for increasing the shelf life of strawberries . Food Research International , 44 : 198 – 203 .
- Zhang , D.L. and Quantick , P.C. 1998 . Antifungal effects of chitosan coating on fresh strawberries and raspberries during storage . The Journal of Horticultural Science and Biotechnology , 73 : 763 – 767 .
- Zhu , L.Q. , Zhou , J. , Zhu , S.H. and Guo , L.H. 2009 . Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid . Food Chemistry , 114 : 174 – 179 .
- Zoffoli , J.P. , Rodriguez , J. , Aldunce , P. and Crisosto , C.H. Development of high concentration carbon dioxide modified atmosphere packaging systems to maintain peach quality. In . Proceedings of the International Controlled Atmosphere Research Conference . pp. 37 – 45 . Davis, CA : University of Davis .