Abstract
Casein hydrolysate with in vitro angiotensin-I-converting enzyme (ACE)-inhibitory activity of 48.2% was prepared from caseinate by Alcalase and modified by plastein reaction in propanol–water medium with extrinsic tyrosine or phenylalanine. By applying response surface methodology, suitable reaction temperature, Alcalase addition, substrate and propanol concentration were optimized as 46.8°C, 8.36 kU/g peptide, 56.8% (w/v) and 58.5% (v/v), respectively. A reaction time of 1 h conferred two modified hydrolysates the highest inhibitory activity (61.6–68.5%), while longer time (e.g. 6 h) brought them greater reaction extent but lower inhibitory activity (41.4–54.7%). Pepsic- and trypsic-digestion of the modified hydrolysates of the highest inhibitory activity (or of greater reaction extent) totally resulted in damaged (or enhanced) inhibitory activity. Twelve out of the obtained 16 digests had residual inhibitory activity about 48–70%, close or larger than casein hydrolysate. It is thus concluded that plastein reaction can enhance ACE inhibition and digestive stability of casein hydrolysate.
Se prepararon hidrolizados de caseína con actividad de inhibición ACE (siglas en inglés de enzima convertidora de la angiotensina-I) de 48,2% a partir de caseinato tratada con alcalasa, los cuales fueron modificados a través de una reacción plasteínica en un medio de agua con propanol al que se le agregó tirosina extrínseca o fenilalanina. Aplicando una metodología de superficie de respuesta y una temperatura de reacción apropiada, se optimizaron las concentraciones del agregado de alcalasa, del sustrato y del propanol como sigue: 46,8°C, 8,36 kU/g péptido, 56,8% (w/v) y 58,5% (v/v), respectivamente. Un tiempo de reacción de 1 hora brindó a los hidrolizados modificados una actividad inhibitoria más elevada (61,6–68,5%), mientras que un tiempo de reacción más extenso (por ejemplo, de 6 horas) les dio un grado de reacción mayor, pero una actividad de inhibición más reducida (41,4—54,7%). La digestión péptica y la tripsina de los hidrolizados modificados con la actividad inhibitoria más alta (o de mayor grado de reacción) determinaron una actividad inhibitoria dañada (o mejorada). De los dieciséis líquidos de digestión obtenidos, doce presentaron una actividad inhibitoria residual de entre 48% y 70%, cercana o superior a la de los hidrolizados de caseína. Por lo tanto, se concluye que, a través de la reacción plasteínica, se puede mejorar tanto la actividad de inhibición de la ACE como la estabilidad digestiva de los hidrolizados de caseína.
Introduction
Hypertension is a major controllable cause that induces cardiovascular disease events such as stroke, coronary heart disease, and kidney disease (Neutel, Smith, & Weber, Citation1999; Unger, Citation2002). Treatment of hypertension can effectively reduce the incidence of these diseases (Collins et al., 1990). Angiotensin-I-converting enzyme (ACE) plays an important physiological role in the regulation of blood pressure. ACE inhibitors can reduce blood pressure by decreasing the concentration of angiotensin II (Ehlers, Fox, Strydom, & Riordan, Citation1989). Synthetic drugs such as captopril and others have been used to lower high blood pressure in humans, but they are found to have some undesirable side effects including coughing, taste disturbances, skin rashes, and renal impairment (Brown & Vaughan, Citation1998). Some peptides or protein hydrolysates have been reported to have ACE inhibition. Milk proteins (Lopez-Fandiño, Otte, & Camp, Citation2006; Saito, Citation2008) and fermented dairies (González-Córdova et al., 2011; Sieber et al., 2010) are the main sources of ACE-inhibitory peptides. Other sources include soybean (Hang & Zhao, Citation2012), fish (Chen, Wang, Zhong, Wu, & Xia, Citation2012) and eggs (Yoshii et al., 2001). Proteolysis or microorganism fermentation of food proteins results in the release of some peptides with ACE inhibition; unfortunately, the sequences of the released ACE-inhibitory peptides are same as the peptide fractions held in the parent proteins. This means that it is impossible to obtain ACE-inhibitory peptides with primary structures different from the parent proteins. Digestive stability (or resistance) of ACE-inhibitory peptides is an important property, as digestive proteases may damage peptide structures, and consequentially the inhibitory activity. It has been found that some synthesized ACE-inhibitory peptides show resistance or sensitivity to the simulated digestion (Gómez-Ruiz, Ramos, & Recio, Citation2004).
Plastein reaction is considered as a reversal of the hydrolysis of proteins by proteases with two reactions, transpeptidation (Combes & Lozano, Citation1992) and condensation (Yamashita, Arai, & Fujimaki, Citation1976). The reaction results in the formation of new proteins or high molecular polypeptides from the original substrate of small peptides and amino acids (Fujimaki, Kato, Arai, & Yamashita, Citation1971). The reaction has been used to modify food proteins to improve their functional or nutritive property or mask the bitter taste of protein hydrolysates (Fujimaki et al., 1971; Yamashita et al., 1976). Recent researches also use plastein reaction in water medium to enhance ACE-inhibitory activity (Li, Li, & Zhao, Citation2010; Zhao & Li, Citation2009), digestive stability (Sun & Zhao, Citation2012), or scavenging activity (Zhao, Wu, & Li, Citation2010) of casein hydrolysate. Whether the reaction in other media consisting of water and one of the miscible organic solvents (e.g. methanol, ethanol, propanol or others) might also have a helpful effect on ACE inhibition of casein hydrolysate is not known thus far.
In the present study, a casein hydrolysate was prepared from caseinate by Alcalase and modified by Alcalase-catalyzed plastein reaction in propanol–water medium. Response surface methodology (RSM) was used to select suitable substrate and propanol concentration, Alcalase addition, and reaction temperature for the plastein reaction. ACE-inhibitory activities and digestive stability of some modified hydrolysates prepared with extrinsic phenylalanine and tyrosine addition were assayed and compared in vitro. The aim was to reveal the impacts of the plastein reaction in another medium other than water on ACE inhibition and digestive stability of the casein hydrolysate.
Materials and methods
Materials
Caseinate was purchased from Beijing Aoboxing Bio-Tech Co. Ltd. (Beijing, China) with a protein content of 86.0% on dry basis. Hippuryl-histidyl-leucine (HHL), rabbit lung acetone powder (as ACE source), and trypsin (89 kU/g) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Alcalase (118 kU/mL), pepsin (18 kU/g), and papain (29 kU/g) were obtained from Novozyme China (Tianjin, China), Hui Shi Biochem Reagent Co. (Shanghai, China), and Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China), respectively. l-Phenylalanine and l-tyrosine were obtained from Aladdin-Reagent Co. Ltd. (Shanghai, China). Other chemicals and reagents used were of analytical grade. Water used was highly purified water prepared by Milli-Q PLUS (Millipore Corporation, New York, NY, USA).
Preparation of casein hydrolysate
Caseinate solution of 10% (w/v on protein basis) was adjusted to pH 8.0 by 2 mol/L NaOH. The proteolysis was started by adding Alcalase to the solution at 1 kU/g protein and carried out in a water bath of 55°C with gently stirring. The solution of 15 mL was separated from the reaction system at 1, 2, 3, 4, 5, 6, 7, and 8 h, respectively, heated in a boiling water bath for 15 min, cooled to room temperature, and centrifuged at 11,000 g for 20 min. The separated supernatants (casein hydrolysates) were evaluated for ACE-inhibitory activities, degree of hydrolysis (DH), and protein recovery. The hydrolysate with relatively higher protein recovery and ACE-inhibitory activity was thus bulk prepared, spray-dried, and subjected for the plastein reaction.
Modification of casein hydrolysate by plastein reaction in propanol–water medium
Propanol was selected to prepare reaction medium for the plastein reaction as its higher boiling point or lower volatile than methanol and ethanol. Four suitable reaction conditions including substrate and propanol concentration, Alcalase addition, and reaction temperature were studied by the RSM and designed at five levels as 23.2, 30, 40, 50, and 56.8% (w/v) and 16.5, 25, 37.5, 50, and 58.5% (v/v), 1.64, 3, 5, 7, and 8.36 kU/g peptide, and 13.2, 20, 30, 40, and 46.8°C, respectively. The decrease of free amino groups of the modified hydrolysate was calculated by subtracting the content of free amino groups of the modified hydrolysate from that of the substrate and taken as the response to select the reaction conditions. The prepared casein hydrolysate was dispersed in the solvent of the selected propanol–water ratio to give a desired substrate concentration, together with a designed Alcalase addition. Then, the mixture was kept at the selected temperature for 6 h with gently stirring. After the reaction, the mixture was heated at 100°C for 15 min, diluted, and assayed for the content of free amino groups. The obtained responses were analyzed by Design Expert software version 7.0 (Stat-Ease Inc., Minneapolis, MN, USA) to generate response surface graphs and to select suitable reaction conditions.
With the selected reaction conditions, five modified casein hydrolysates (MCHs) with reaction time of 0.5–8 h were prepared and served as one group. Another two groups of MCHs were also prepared with the same conditions but with phenylalanine and tyrosine added at 0.54 mol per mol free amino groups of the hydrolysate as reported previously (Li, Li, & Zhao, Citation2010) and designed as MCHTs and MCHPs, respectively. ACE-inhibitory activity of each modified hydrolysate was assayed. Four modified hydrolysates of the highest activity or greater reaction extent were thus selected, bulk prepared, lyophilized, and subjected to enzymatic hydrolysis.
Enzymatic hydrolysis of the MCHs
The four modified hydrolysates were dissolved in water at peptide content of 10% (w/v). A portion of the solution was adjusted to the fixed pH by 2 mol/L NaOH or HCl solution and digested at 37°C by trypsin or pepsin (5 or 4 kU/g peptide, pH 8.1 or 2.0). The blank samples (zero time) were prepared by adding protease solutions pre-heated at 100°C for 15 min. After a hydrolysis time of 10, 30, or 60 min, the solutions were heated at 100°C for 15 min. The obtained digests were assayed for content of free amino groups and ACE-inhibitory activities.
Chemical analysis and evaluation of ACE inhibition
Protease activity was assayed as a reported method (Sarath, De La Motte, & Wangner, 2001). Nitrogen content of all samples was analyzed by the Kjeldahl method (IDF, 1993) and multiplied by 6.38 to give protein or peptide content. Content of free amino groups of the hydrolysates was assayed by o-pthaldialdehyde method (Church, Swaisgood, Porter, & Catignani, Citation1983) and used for DH calculation by the method of Adler-Nissen (Citation1979).
ACE inhibition in vitro of the hydrolysate was measured as per the method of Sarmadi, Ismail, and Hamid (Citation2011) with minor modifications. Briefly, 100 μL of the sample solution (0.3 mg/mL) and 250 μL of HHL solution (5 mmol/L in 100 mmol/L sodium borate buffer containing 300 mmol/L NaCl, pH 8.3) were incubated at 37°C for 5 min. Then, 150 μL of the ACE extract from rabbit lung acetone powder was added immediately, and the mixture was incubated at 37°C for 90 min. The reaction was stopped by adding 250 μL of 0.5 mol/L HCl. The mixture with adding the HCl solution before the ACE extract was served as zero-time blank. The hippuric acid formed in the assaying system was extracted by 3 mL ethyl acetate with vigorous shaking for 5 min. After standing for 3 min, 2 mL aliquot of the ethyl acetate layer was transferred to a clean tube and evaporated by heating at 85°C for 15 min. Hippuric acid left in the tube was re-dissolved in 3 mL of 1 mol/L NaCl and assayed for its absorbance at 228 nm. Water was added into the assaying system instead of the hydrolysate sample and served as a control. ACE-inhibitory activity (%) of the hydrolysate was calculated by the following equation.
Statistical analysis
All tests or analyses were carried out three times. The data were expressed as means ± standard deviations. Differences between the means of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan's multiple range tests. All tests were considered statistically significantly at p < 0.05. Design Expert software version 7.0 (Stat-Ease Inc., Minneapolis, MN, USA) and SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA) were used in data analysis.
Results and discussion
Preparation of casein hydrolysate
The DH, peptide recovery, and ACE-inhibitory activity of the casein hydrolysates prepared by Alcalase over a hydrolysis period of 8 h are listed in . As the hydrolysis time progresses from 1 to 6 h, the three indices show an increasing trend; then, only DH and peptide recovery increase as hydrolysis time prolongs but the inhibitory activity shows a decreasing trend. The casein hydrolysate with a hydrolysis time of 6 h having DH of 12.6% and ACE-inhibitory activity of 48.2% (at peptide content of 0.3 mg/mL) is selected as the substrate of the plastein reaction.
Table 1. Degree of hydrolysis (DH), peptide recovery, and ACE-inhibitory activities of the casein hydrolysates prepared at different times by Alcalase.
Tabla 1. Grado de hidrólisis (GH), recuperación de péptidos y actividades de inhibición de la ACE de los hidrolizados de caseína preparados en distintos momentos a partir de alcalasa.
Casein is an important source of the most potential ACE-inhibitory peptides (Otte, Shalaby, Zakora, Pripp, & El-Shabrawy, Citation2007). When yak casein is hydrolyzed by Alcalase, two separated peptides show IC50 values of 0.25 and 0.29 mg/mL, respectively (Mao, Ni, Sun, Hao, & Fan, Citation2007); i.e. they have ACE-inhibitory activity of 50% at 0.25 or 0.29 mg/mL, slightly higher than the present result. A soybean protein hydrolysate prepared by Alcalase for 6 h has the highest activity (Chiang, Tsou, Tsai, & Tsai, Citation2006), which shares same conclusion to the present study.
Selected plastein reaction conditions and ACE inhibition of the MCHs
Four reaction conditions for the plastein reaction were studied by RSM with central composite design. All obtained results are given in as six graphs. Each investigated condition shows impact on the plastein reaction, based on the obtained response. The optimized conditions by the software are reaction temperature of 46.8°C, Alcalase addition of 8.36 kU/g peptide, and substrate and propanol concentration of 56.8% (w/v) and 58.5% (v/v), respectively. The predicted decrease of free amino groups of the modified hydrolysate by the software is 327.8 μmol/g peptide, while the one observed is 344.5 μmol/g peptide.
Figure 1. Response surface graphs for the decrease of free amino groups of casein hydrolysates treated by Alcalase-catalyzed plastein reaction in propanol–water medium.
Figura 1. Gráficas de la superficie de respuesta para la disminución de grupos de amino libre en los hidrolizados de caseína tratados mediante una reacción plasteínica catalizada por alcalasa en un medio de agua con propanol.
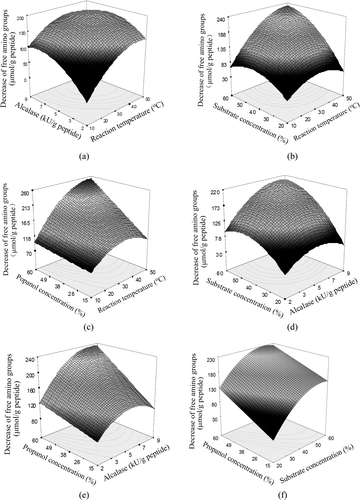
Five modified casein hydrolysates (MCH1–5) were thus prepared by applying these selected conditions and different reaction times. The evaluation results given in show that these MCHs have less free amino groups than the casein hydrolysate, indicating the occurrence of condensation during modification. MCH2 shows the highest inhibitory activity (63.8%). MCH5 has the longest reaction time (i.e. greatest reaction extent) but lowest inhibitory activity (32.5%), even lower than the casein hydrolysate. Other MCHs have inhibitory activity higher than the casein hydrolysate. It means that the plastein reaction of casein hydrolysate in propanol–water medium at lower reaction extent can enhance its inhibitory activity.
Table 2. ACE-inhibitory activities of the casein hydrolysate or modified casein hydrolysates treated in the propanol–water medium.
Tabla 2. Actividades de inhibición de la ACE de los hidrolizados de caseína o de los hidrolizados de caseína modificados, tratados en el medio de agua con propanol.
Phenylalanine and tyrosine, added at 0.54 mol per mol free amino groups of the hydrolysate, were used in the plastein reaction to obtain another two groups of the modified hydrolysates, MCHTs and MCHPs. Evaluation results in indicate that (1) the addition of the two acids totally results in much plastein reaction, i.e. much decrease in free amino groups; (2) suitable reaction extent confers the modified product higher inhibitory activity than the casein hydrolysate, e.g. a reaction time of 1 h brings MCHT2 or MCHP2 the highest inhibitory activity; (3) too longer reaction time (e.g. 6 or 8 h) leads to the modified product a damaged inhibitory activity, e.g. MCHT2 vs. MCHT4 (61.6% vs. 41.4%) or MCHP2 vs. MCHP4 (68.5% vs. 54.7%); (4) the addition of tyrosine or phenylalanine gives MCHT2 or MCHP2 an inhibitory activity slight lower or clear higher than the MCH2 (61.6 or 68.5% vs. 63.8%). Thus, MCHT2 and MCHP2 (highest activity) as well as MCHT4 and MCHP4 (greater reaction extent) are selected and investigated for their digestive stability.
Plastein reaction of casein hydrolysate in water results in an improved ACE inhibition (Zhao & Li, Citation2009). The addition of extrinsic amino acids into the reaction system in water may lead to the modified hydrolysate higher ACE-inhibitory activity (Li, Li, & Zhao, Citation2010; Zhao, Wang, & Li, Citation2012). Similar to the results in these researches, the present result shows that plastein reaction of casein hydrolysate in propanol–water medium can improves its ACE inhibition. In the three mentioned research works, the decrease of free amino groups of the modified products is about 185–195 mol/g peptide, less than that found in the present study. This partly owes to the partial replacement of water with propanol, which decreases water activity (a w) of the reaction system. The reaction equilibrium is thus shifted toward the condensation, and greater decrease of free amino groups is obtained. Higher substrate and propanol concentration are favorable to a lower a w, and higher Alcalase addition is also beneficial to the plastein reaction; consequentially, the selected three indices are all in higher levels to ensure the modified hydrolysate greater decrease of free amino groups. If the modified hydrolysate gets too greater decrease of free amino groups, the most active peptides previous formed might be blocked by the peptide fractions or amino acids during the condensation, resulting in itself a lower inhibitory activity. Therefore, only suitable reaction extent (e.g. reaction time of 1 h) can give the modified product (MCHT2 or MCHP2) higher inhibitory activity. Hydrophobic amino acids at C-terminal of the peptides are favorable to ACE inhibition (Lopez-Fandiño et al., 2006). MCHT2 or MCHP2 is prepared with extrinsic tyrosine (less hydrophobic) or phenylalanine (much hydrophobic). Some tyrosine or phenylalanine can be attached into the peptides by the condensation. MCHT2 or MCHP2 thus has an inhibitory activity slight lower or higher than the MCH2.
Digestive stability of the MCHs
The data in show that both pepsin and trypsin can induce proteolysis to the MCHT2, MCHP2, MCHT4, and MCHP4, reflected by the increase of free amino groups of the resulted digests. Longer hydrolysis time causes much proteolysis, i.e. greater increase of free amino groups of the digests.
Table 3. Impacts of proteolysis in vitro of the modified casein hydrolysates on the residual ACE-inhibitory activity.
Tabla 3. Impactos de la proteólisis in vitro de los hidrolizados de caseína modificados en la actividad de inhibición de ACE residual.
The assayed results in indicate that the carried out pepsic- and trypsic-digestion have different impacts on the inhibitory activities of the resulted digests. Seven digests from MCHT2 and MCHP2 show lower ACE inhibition than the parent substrates, especially when pepsin is applied in the digestion (41.6–48.6% vs. 61.6%, or 35.0–44.5% vs. 68.5%). At the same time, four digests from MCHT4 exhibit enhanced ACE inhibition (50.1–64.0% vs. 41.4%), especially when trypsin is applied. Two digests from MCHP4 by trypsin also have enhanced ACE inhibition (57.0–60.7% vs. 54.7%). These results indicate that digestion of the modified hydrolysates of the highest inhibitory activity results in activity damage, but digestion of the modified hydrolysates of greater reaction extent leads to activity enhancement. Among the resulted 16 digests, 12 digests show residual inhibitory activity larger than 48% (original inhibitory activity of the casein hydrolysate). On the contrary, further hydrolysis of the casein hydrolysate results in a lowered inhibitory activity (, hydrolysis time 6 vs. 7 or 8 h). It means that plastein reaction of the casein hydrolysate in propanol–water medium confers itself better digestive stability toward the two proteases.
Two peptides VRYL and KKYNVPQL from the Manchego cheese show impaired ACE inhibition upon digestion (Gómez-Ruiz et al., 2004). Same result is found for another two peptides YAEERYPIL and RADHPFL digested by pepsin or pancreatic extract (Miguel, Aleixandre, Ramos, & López-Fandiño, Citation2006). On the contrary, two peptides LHLPLP (Quirós, Contreras, Ramos, Amigo, & Recio, Citation2009) and ALPMHIR (Mullally, Meisel, & FitzGerald, Citation1997) have digestive stability. Digestion of fermented milks also has no impact on ACE-inhibitory activity (Hernández-Ledesma, Amigo, Ramos, & Recio, Citation2004). In the present study, digestion of MCHT2 and MCHP2 by pepsin and trypsin totally exhibits harmful effect on inhibitory activity, consistent with the results mentioned in the first two researches. MCHT4 and MCHP4 show digestive stability, sharing similar conclusion to the three researches mentioned later. This might be due to the release of peptide fractions or amino acids that previously blocked the active sites of the most active peptide, resulting in the digest higher inhibitory activity.
Conclusion
Plastein reaction of casein hydrolysate by Alcalase in propanol–water medium can enhance ACE inhibition of the modified hydrolysate, but too much reaction extent confers the modified hydrolysate a lower inhibitory activity. The addition of extrinsic phenylalanine but not tyrosine in the reaction system improves the activity of modified hydrolysate. Totally, the modified hydrolysate of the highest activity is sensitive to pepsic- and trypsic-digestion, while that of greater reaction extent has enhanced inhibitory activity upon digestion. Plastein reaction of casein hydrolysate in propanol–water medium can give itself higher inhibitory activity and better digestive stability.
Acknowledgements
This work was supported by the Innovative Research Team of Higher Education of Heilongjiang Province (Project No. 2010td11) and the National Natural Science Foundation of China (Project No. 30972132 and 31140009). The authors thank the anonymous reviewers and the editors for their help in this manuscript preparation.
References
- Adler-Nissen , J. 1979 . Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid . Journal of Agricultural and Food Chemistry , 27 : 1256 – 1262 .
- Brown , N.J. and Vaughan , D.E. 1998 . Angiotensin-converting enzyme inhibitors . Circulation , 97 : 1411 – 1420 .
- Chen , J. , Wang , Y. , Zhong , Q. , Wu , Y. and Xia , W. 2012 . Purification and characterization of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide derived from enzymatic hydrolysate of grass carp protein . Peptides , 33 : 52 – 58 .
- Chiang , W.D. , Tsou , M.J. , Tsai , Z.Y. and Tsai , T.C. 2006 . Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor . Food Chemistry , 98 : 725 – 732 .
- Church , F.C. , Swaisgood , H.E. , Porter , D.H. and Catignani , G.L. 1983 . Spectrophotometric assay using [#x03BF]-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins . Journal of Dairy Science , 66 : 1219 – 1227 .
- Collins , R. , Peto , R. , MacMahon , S. , Hebert , P. , Fiebach , N.H. , Eberlein , K.A. , Godwin , J. , … and Hennekens , C.H. 1990 . Blood pressure, strike, and coronary heart disease. Part 2, Short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context . The Lancet , 335 : 827 – 838 .
- Combes , D. and Lozano , P. 1992 . Alpha-chymotrypsin in plastein synthesis influence of water activity . Annals of the New York Academy of Sciences , 672 : 409 – 414 .
- Ehlers , M.R. , Fox , E.A. , Strydom , D.J. and Riordan , J.F. 1989 . Molecular cloning of human testicular angiotensin-converting enzyme: The testis isozyme is identical to the C-terminal half ofendothelial angiotensin-converting enzyme . Proceedings of the National Academy of Sciences of the Unite States of American , 86 : 7741 – 7745 .
- Fujimaki , M. , Kato , H. , Arai , S. and Yamashita , M. 1971 . Application of microbial proteinases to soybean and other materials to improve acceptability, especially through the formation of plastein . Journal of Applied Bacteriology , 34 : 119 – 131 .
- Gómez-Ruiz , J.A. , Ramos , M. and Recio , I. 2004 . Angiotensin-converting enzyme-inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion . International Dairy Journal , 14 : 1075 – 1080 .
- González-Córdova , A.F. , Torres-Llanez , M.J. , Rodríguez-Figueroa , J.C. , Espinosa-de-los-Monteros , J.J. , Garcia , H.S. and Vallejo-Córdoba , B. 2011 . Angiotensin converting enzyme inhibitory activity in milks fermented by Lactobacillus strains . CyTA – Journal of Food , 9 : 146 – 151 .
- Hang , M. and Zhao , X.H. 2012 . Fermentation time and ethanol/water-based solvent system impacted in vitro ACE-inhibitory activity of the extract of Mao-tofu fermented by Mucor spp . CyTA – Journal of Food , 10 : 137 – 143 .
- Hernández-Ledesma , B. , Amigo , L. , Ramos , M. and Recio , I. 2004 . Angiotensin converting enzyme inhibitory activity in commercial fermented products. Formation of peptides under simulated gastrointestinal digestion . Journal of Agricultural and Food Chemistry , 52 : 1504 – 1510 .
- IDF (International Dairy Federation) . 1993 . Determination of the nitrogen (Kjeldahl method) and calculation of the crude protein content (IDF Standard 20B) , Brussels , Belgium : International Dairy Federation .
- Li , Y.Y. , Li , T.J. and Zhao , X.H. 2010 . Preparation of Alcalase-catalyzed casein plasteins in the presence of proline addition and the ACE-inhibitory activity of the plasteins in vitro . European Food Research and Technology , 231 : 197 – 207 .
- Lopez-Fandiño , R. , Otte , J. and Camp , J. 2006 . Physiological, chemical and technological aspects of milk protein derived peptides with antihypertensive and ACE-inhibitory activity . International Dairy Journal , 16 : 1277 – 1293 .
- Mao , X.Y. , Ni , J.R. , Sun , W.L. , Hao , P.P. and Fan , L. 2007 . Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides . Food Chemistry , 103 : 1282 – 1287 .
- Miguel , M. , Aleixandre , M.A. , Ramos , I. and López-Fandiño , R. 2006 . Effect of simulated gastrointestinal digestion on the antihypertensive properties of ACE-inhibitory peptides derived from ovalbumin . Journal of Agricultural and Food Chemistry , 54 : 726 – 731 .
- Mullally , M.M. , Meisel , H. and FitzGerald , R.J. 1997 . Identification of a novel angiotensin-I converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine β-lactoglobulin . FEBS Letters , 402 : 99 – 101 .
- Neutel , J.M. , Smith , D.H. and Weber , M.A. 1999 . Is high blood pressure a late manifestation of the hypertension syndrome? . American Journal of Hypertension , 12 : 215 – 223 .
- Otte , J. , Shalaby , S.M. , Zakora , M. , Pripp , A.H. and El-Shabrawy , S.A. 2007 . Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: Effect of substrate, enzyme and time of hydrolysis . International Dairy Journal , 17 : 488 – 503 .
- Quirós , A. , Contreras , M.D.M. , Ramos , M. , Amigo , L. and Recio , I. 2009 . Stability to gastrointestinal enzymes and structure-activity relationship of β-casein-peptides with antihypertensive properties . Peptides , 30 : 1848 – 1853 .
- Saito , T. 2008 . Antihypertensive peptides derived from bovine casein and whey proteins: Bioactive components of milk . Advances in Experimental Medicine and Biology , 606 : 295 – 317 .
- Sarath , G. , De La Motte , R.S. and Wangner , F.W. 2001 . “ Protease assay methods ” . In Proteolytic enzyme: A practical approach , Edited by: Beynon , R.J. and Bond , J.S. 45 – 76 . Oxford , UK : IRL Press .
- Sarmadi , B. , Ismail , A. and Hamid , M. 2011 . Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of cocoa (Theobroma cacao L.) autolysates . Food Research International , 44 : 290 – 296 .
- Sieber , R. , Bütikofer , U. , Egger , C. , Portmann , R. , Walther , B. and Wechsler , D. 2010 . ACE-inhibitory activity and ACE-inhibiting peptides in different cheese varieties . Dairy Science and Technology , 90 : 47 – 73 .
- Sun , H. and Zhao , X.H. 2012 . ACE inhibition and enzymatic resistance in vitro of casein hydrolysate treated by plastein reaction and fractionated with ethanol/water or methanol/water . International Dairy Journal , 24 : 27 – 32 .
- Unger , T. 2002 . The role of the renin-angiotensin system in the development of cardiovascular disease . The American Journal of Cardiology , 89 ( 2A ) : 3A – 9A .
- Yamashita , M. , Arai , S. and Fujimaki , M. 1976 . Plastein reaction for food protein improvement . Journal of Agricultural and Food Chemistry , 24 : 1100 – 1104 .
- Yoshii , H. , Tachi , N. , Ohba , R. , Sakamura , O. , Takeyama , H. and Itani , T. 2001 . Antihypertensive effect of ACE inhibitory oligopeptides from chicken egg yolks . Comparative Biochemistry and Physiology C – Toxicology and Pharmacology , 128 : 27 – 33 .
- Zhao , H.H. , Wang , J.K. and Li , T.J. 2012 . In vitroACE-inhibitory and antioxidant activities of the casein hydrolysates subjected to plastein reaction with addition of three extrinsic amino acids . Biotechnology , 10 : 408 – 414 .
- Zhao , X.H. and Li , Y.Y. 2009 . An approach to improve ACE inhibitory activity of casein hydrolysates with Plastein reaction catalyzed by Alcalase . European Food Research and Technology , 229 : 795 – 805 .
- Zhao , X.H. , Wu , D. and Li , T.J. 2010 . Preparation and radical scavenging activity of papain-catalyzed casein plasteins . Dairy Science and Technology , 90 : 521 – 535 .