Abstract
The aim of this study was to evaluate the phytochemical composition and the antioxidant properties of watercress (Nasturtium officinale R. Br., Brassicaceae) produced under organic production system. Fresh baby-leaf watercress samples were collected from local organic farms. High performance liquid chromatography with diode array detection (HPLC-DAD) and high performance liquid chromatography with diode array detection mass spectrometry (HPLC-MS) were used to assess the phytochemical composition, and spectrophotometric methods were used to assess the antioxidant capacity. Two major classes of healthier secondary plant metabolites were identified: phenolics and glucosinolates. The major phenolics determined were chlorogenic acid, quercetin-3-O-rutinoside, dicaffeoyltartaric acid, and isorhamnetin. The glucosinolates were composed exclusively of gluconasturtiin, the precursor of the anticarcinogenic, and antimicrobial compound 2-phenylethyl isothiocyanate. The extracts of organic young baby-leaf watercress presented high antioxidant capacity and this property was highly related with caffeic acid, quercetin-3-O-rutinoside, isorhamnetin, and glucosnastrutiin. The results achieved showed that baby-leaf watercress can provide high contents of antioxidant compounds at levels even superior to similar adult plant material.
El objetivo de este estudio fue evaluar la composición fitoquímica y propiedades antioxidantes de berros (Nasturtium officinale R. Br., Brassicaceae) producidos bajo el sistema de producción ecológico. Muestras frescas de hojas tiernas de berros se recolectaron en granjas ecológicas locales. HPLC-DAD y HPLC-MS fueron utilizados para evaluar la composición fitoquímica y métodos espectrofotométricos se utilizaron para evaluar la capacidad antioxidante. Se identificaron dos clases principales de metabolitos secundarios saludables de las plantas, compuestos fenólicos y glucosinolatos. Los principales compuestos fenólicos determinadas fueron: ácido clorogénico, quercetina-3-O-rutinósido, ácido dicafeoil tartárico y isoramnetina. Los glucosinolatos fueron compuestas exclusivamente por gluconasturtina, el precursor del compuesto anti-cancerígenos y antimicrobiana isotiocianato de 2-feniletilo. Los extractos de brotes jóvenes de berros ecológicos presentaron una alta capacidad antioxidante y esta propiedad fue muy relacionadas con el ácido cafeico, la quercetina-3-O-rutinósido, isorhamnetina y glucosnastrutiina. Los resultados obtenidos mostraron que los brotes de berro puede proporcionar un alto contenido de compuestos antioxidantes a niveles aún superiores del material similar de la planta adulta.
Introduction
Plant containing polyphenols and other related compounds is well accepted for antioxidant activity to scavenge reactive oxygen species and leads to the oxidative damage protection (Balasundram, Sundram, & Samman, Citation2006; Valko et al., Citation2007). Watercress belongs to the family of Brassicaceae (Syn. Cruciferae) and is regarded in some regions as a weed or aquatic vegetable. It is a hardy perennial native to Europe and America, sold as fresh and consumed in salads and soups. Like other Brassicacea vegetables (broccoli, cauliflower, cabbage, and turnip), the watercress is rich in glucosinolates (GSs), a class of sulfur and nitrogen organic compounds, which are enzymatically broken down via myrosinase (β-thioglucosidase, E.C. 3.2.1.147) after tissue disruption (e.g., cut, ground, or chewed) into numerous biologically active products such as isothiocyanates (ITCs), nitriles, and thiocyanates (TCs) (Bones & Rossiter, Citation2006). The breakdown products of certain GSs, particularly the ITCs, have been shown to protect against lung, colon, liver, stomach cancers, and redox stress (Lam et al., Citation2009). Over the past 20 years, it has been shown that many classes of phytochemicals are important in the diet and could be used as potential therapeutic tools (Johnson, Citation2007). However, the scientific investigation on the therapeutic use of watercress and other brassicas is often limited to primary nutrients, GSs, ITCs, and their role as antimutagenic, antigenotoxic, antiproliferative, and antitumoral agents (Boyd et al., Citation2006). Fewer reports have been published about their richness on phenolic acids, flavonoids, and antioxidant activity.
The studies of antioxidant activity are frequently limited to the total phenolics and total flavonoids, and the influence of individual phenolics on antioxidant activity if often forgotten in most studies of chemical composition and antioxidant activity. Also, the majority of the studies are about plants produced under conventional farming, fewer about low input production, and less about organic production, particularly of baby-leaf salads.
The preference for organic products is increasing all over Europe, due to the supposed absence of chemical contaminants within this mode of production. Despite this growing interest in organic production, there is insufficient information to state categorically that organic produce is better than conventional one. Among consumers, doubts still persist. Thus, studies that can evaluate the growing conditions on levels of nutrients and secondary metabolites are urgently required.
In view of the foregoing and importance of phenolics and GSs as antioxidants in baby-leaf watercress, the present study has been undertaken to identify the phytochemical composition (individual and total phenolics, total flanovoids, individual, and total GSs) and the antioxidant properties of fresh baby-leaf watercress produced under organic practices. We intend to increment the knowledge and discuss the chemical composition on secondary metabolites of organic baby-leaf watercress and discuss its potential as a source of bioactive compounds with beneficial effects in human health.
Material and methods
Plant material
Samples of young baby-leaf watercress (Nasturtium officinale R. Br., Brassicaceae) (500 g) were acquired directly from organic local farmers in Northern Region of Portugal. After purchase, the samples were transported to the Phytochemicals Laboratory of the University of Trás-os-Montes e Alto Douro (UTAD, Vila Real Portugal) and freeze-dried (Dura-DrTM μP – FTS Systems, UTAD, Vila Real, Portugal), ground to a fine powder using a blender (model BL41, Waring Commercial, Torrington, CT, USA), and weighed. The dry matter was determined. Then 0.5 g of powdered sample was then extracted with 10 mL of 700 mL L−1 methanol:water (v/v) and heated at 70°C for 30 minutes, agitated (vortex) every 5 minutes. All extracts were centrifuged (model 2100 Kubota, Japan) at 4000 rpm for 10 minutes, filtered, and dried in a rotary evaporator at 40°C until completely dried and kept in –20°C until use in biological assays. Methanolic extracts were used to quantify the total and individual phenolics and total flavonoids, and to evaluate the antioxidant activity. For individual and total GSs, the extraction procedure was different (see the respective section).
Individual phenolics content
The quantification of individual phenolics was performed by high performance liquid chromatography (HPLC) analysis, using a HPLC-mass spectrometry (MS) system (LCQ Advantage Max,ThermoFinnigan, brockport, NY, USA) equipped with diode array detector and reverse phase column (C18 Spherisorb ODS2, 250 mm of length and 4.6 mm diameter and 5 microns). After sample preparation, 200 µL of each diluted extracts were added to HCl (2M) in 500 mL L−1 methanol (methanol:water), and tert-butylhydroquinone (TBHQ) were placed in heater at 80°C for 2 hours and then centrifuged (model 2–16 K, Sigma) at 15,493 g for 20 minutes. After this, the supernatant was removed and stored at –80°C until its introduction in HPLC system. The eluent was constituted by water with 1 mL L−1(v/v) of trifluoroacetic acid (TFA) (solvent A) and acetonitrile with 100 mL L−1 TFA (solvent B). The TFA sharpens peaks and improves resolution. The TFA has low absorption within detection wavelengths, allows sensitive, nondestructive compounds detection at low UV wavelengths in HPLC separation systems. Elution was performed at a flow rate of solvent of 1 mL min−1, with a gradient starting with 100% of solvent A, and the injection volume of 10 μL. The identification was made comparing external standards (Extrasynthese, France), their retention times, and UV spectra. The LC-MS was used to confirm the results. The chromatograms were recorded at 270, 280, 320, and 370 nm for phenolics in general and more specifically 520 nm for anthocyanins.
Total phenolic content
Total phenolic content (TPC) was determined using Folin–Ciocalteu reagent (Panreac, Spain) and gallic acid as standard (Hakala, Lapvetelainen, Huopalahti, Kallio, & Tahvonen, Citation2003) with some modifications. In brief, 50 µL of extract or commercial standard were mixed with 2.5 mL of Folin & Ciocalteu's solution (1:10 v/v) and 2 mL of Na2CO3 (Sigma-Aldrich, Taufkirchen, Germany) solution 75 mL L−1 (w/v). After 15 minutes in the dark at 45°C temperature, absorbance was measured at 765 nm (U-2000, Hitachi, Japan). Standard gallic solutions (0–10 mg mL−1) were also assayed and calibration curve was obtained from the equation: y = 5.703x + 0.089; R 2 = 0.996. A blank assay was also prepared. The phenolic contents were expressed as mg gallic acid equivalent (GAE) kg−1 dry weight (dw).
Total flavonoid content
Total flavonoid determinations were made using colorimetric assays (Javanmardi, Stushnoff, Locke, & Vivanco, Citation2003) with small modifications. It was prepared a mixture of 1 mL of diluted extracts, 4 mL of bi-distilled water, and 0.3 mL of NaNO2 (Sigma-Aldrich) solution 5% (w/v). After shaking and 5 minutes at room temperature, 0.3 mL of AlCl3 (Sigma-Aldrich) solution 10 mg/100 mL (w/v) was added and allowed to stand for 6 minutes. After this, 2 mL of 1 M NaOH (Sigma-Aldrich) was added. Finally, volume was complete to 10 mL with bi-distilled water and thoroughly mixed. The absorbance of the pink mixture was measured at 510 nm (U-2000, serial 121–0120, Hitachi Ltd., Japan). Standard catechin (Sigma-Aldrich) solutions (0–10 mg mL−1) were also assayed and calibration curve was obtained from the equation: y = 0.029x + 0.188; R 2 = 0.993. A blank assay was also prepared. Total flavonoid contents (TFC) were expressed as mg catechin equivalent (CE) kg−1 dw. TFC determinations were made in triplicate.
Individual and total GSs
For individual and total GSs, the extraction procedure was made using the following methodology. Briefly, 0.2 g dw sample was extracted with boiling methanol (900 mL L−1 methanol:water (v/v), 3 mL) for 2 minutes. A solution of benzyl GLS (glucotropaeolin) 50 mg/100 mL L−1 (m/v) was used as internal standard. After re-extraction with boiling 700 mL L−1 methanol:water (v/v), the supernatant were combined to a final volume of 10 mL. An aliquot (2.5 mL) was evaporated to dry completely and re-suspended in water (2.5 mL), and 2 mL L−1 was applied to a small column of DEAE Sephadex A25. The desulpho-GSs were obtained using commercial aryl sulfatase (EC3.1.6.1) Type H1 from Helix pomatia (Sigma, St. Louis, MO, USA) at 14.6 units · g−1. The desulpho-GSs were eluted with water and analyzed using HPLC. The procedure adopted corresponds to the ISO 9167–1 method (EEC Regulation No. 9167–1, 1992).
The quantification of individual GSs was also performed by HPLC analysis. GSs peak identification and quantitative estimations were made using pure standard GSs as internal standard (benzyl GSs). GSs concentrations were expressed in μmol g−1 dw. All reagents were analytical or HPLC grade. The mobile phase consisted of two solvents A and B, being solvent A composed of ultra-pure water and solvent B by acetonitrile (CH3CN) at 200 mL L−1 acetonitril:water (v/v). Elution was performed with a flow rate of 1.5 mL minutes−1. The chromatograms were recorded at 229 nm and used to identify GSs. The GSs were quantified with the following formula: μmol of GS/100 g dw = AG/Api × FR × Cpi × 100 DW (μmol of GS 100 g−1 dw). GSs were expressed as μmol 100 g−1 dw, in which GS refers to GSs, AG refers to peak area of each GS to quantify, Api refers to peak area of the internal standard (glucotropaeolin), FR refers to response factor of each GSs identified, Cpi refers to the concentration of the internal standard (glucotropaeolin), and DW refers to dw of sample used in the extraction.
2,2-Diphenyl-1-picrylhydrazyl radical scavenging assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity was determined using a spectrophotometer (U-2000, Hitachi, Japan) according to the method described previously (Siddhraju & Becker, Citation2003) with small modifications. Briefly, stock solutions of crude extracts were prepared as 0.25, 0.5, 1.0, 1.25, 2.5, 5.0, and 10 mg mL−1. Antioxidant activity was measured using 300 µL of each hydrophilic extract and 2.7 mL of DPPH solution, 950 mL L−1 in ethanol: water (w/v), followed by agitation and reaction in dark at room temperature for 60 minutes. Absorbance values were measured at 517 nm. A blank assay, using 700 mL L−1 methanol:water, was also prepared. A positive control was made with standard solution Trolox (Sigma-Aldrich) in same concentrations than hydrophilic extract. The EC50, half maximal effective concentration, values were calculated based on a dose–response curve, where abscissa was concentration of plant sample extracts and ordinate was the average inhibition percentage. The experiment was carried out in triplicate.
The radical scavenging activity was calculated by the following formula:
The Absblank is the control reaction absorbance and Abssample (t) is the absorbance with the extract or standard solution.
Reducing power antioxidant assay
The reducing power of plant extracts was determined using the method described by Hinneburg, Dorman, and Hiltunen (Citation2006) with several modifications. Serial dilution of the extract was performed (0.25, 0.5, 1.0, 1.25, 2.5, 5.0, and 10 mg mL−1) in 2.5 mL of 0.2 M sodium phosphate buffer (pH 6.6) containing 2.5 mL 1% aqueous hexacyanoferrate [K3Fe(CN)6, Sigma] solution. The mixture was incubated at 50°C for 30 minutes. After, 2.5 mL of 100 mL L−1% trichloroacetic acid (TCA) (Sigma-aldrich):water (v/v) was added, and the mixture was mixed thoroughly in a vortex (Labinco, Netherlands) and centrifuged at 3000 rpm for 10 minutes. The supernatant was collected (2.5 mL), mixed with distilled water (2.5 mL) and 0.5 mL of 10 mL L−1 ferric chloride (FeCl3) (Sigma-aldrich) was added. The absorbance of this mixture was measured at 700 nm (U-2000, Hitachi, Japan). The intensity in absorbance is the measurement of antioxidant activity of the extract.
Ferric ion reducing antioxidant power
Ferric ion reducing antioxidant power (FRAP) is based on the ability of antioxidants to reduce Fe3+ to Fe2+ in the presence of 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), forming an intense blue Fe2+-TPTZ complex with an absorption maximum at 593 nm. The FRAP method used was based on a previous study (Stratil, Klejdus, & Kubán, Citation2006) with small modifications. Briefly, the FRAP reagent was prepared from sodium acetate buffer (300 mM, pH 3.6), 10 mM TPTZ solution (40 mM HCl as solvent), and 20 mM iron (III) chloride solution in a volume ratio of 10:1:1, respectively. The FRAP reagent was prepared freshly and warmed at 37 °C in a water bath before use. One hundred microliters of the diluted extracts (0.25, 0.5, 1.0, 1.25, 2.5, 5.0, and 10 mg mL−1) was added to 3 mL of the FRAP reagent. The absorbance of the reaction mixture was then detected at 593 nm (U-2000, Hitachi, Japan) after 4 minutes in room temperature. The standard curve was constructed using FeSO4 solution, and the results were expressed as μmol Fe(II) g−1 dw of plant material. Also, the EC50 values were calculated based on a dose–response curve. The experiment was carried out in triplicate.
Statistical analysis
SPPS for windows version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All experiments were performed in triplicate and the results were presented as the mean ± SEM (standard error of the mean). The data were analyzed using one-way ANOVA. The differences between the mean values were separated using Duncan test, at significance level of P < 0.05. Also a primary component analysis (PCA) and a Pearson correlation between phytochemicals content and antioxidant activity of organic baby-leaf watercress extracts were carried out.
Results and discussion
Phytochemicals
The phytochemical composition of organic baby-leaf watercress was measured and results are presented in –. Compared with other raw vegetables (Cieślik, Gręda, & Adamus, Citation2006; Heimler, Isolani, Vignolini, Tombeli, & Romani, Citation2007; Isabelle et al., Citation2010) including adult watercress (Tiveron et al., Citation2012), the organic baby-leaf watercress presented high average levels of TPC (14,000 ± 27 mg GAE kg−1 dw) and TFC (5600 ± 10 mg CAE kg−1 dw) representing between 1 and 2 times more than the values obtained by theses authors. The TFC represented 40% of the TPC. The major individual phenolics identified by HPLC () were gallic acid, chlorogenic acid, caffeic acid, quercetin-3-O-rutinoside, dicaffeoyltartaric acid, and isorhamnetin (). The phenolics quercetin-3-O-rutinoside and isorhamnetin (both flavonoids) represented 79% of the total individual phenolics identified, which is important since these two flavonoids are often associated with the decrease of imflammatory and cardiovascular risk and frequently reported as antagonizing agents of oxidation and apoptosis processes (Zhang et al., Citation2011; Russo, Spagnuolo, Tedesco, Bilotto, & Russo, Citation2012).
Figure 1. HPLC chromatogram of polyphenols standards (including phenolics, flavonoids, and anthocyanins) used for identification and quantification in organic baby-leaf watercress extracts at 270 nm (A and B) and 520 nm (C): 1, Gallic acid; 2, Chlorogenic acid; 3, Caffeic acid; 4, Cyanidin-3-glucoside; 5, Quercetin-3-O-rutinoside; 6, Luteolin-7-O-glucoside; 7, Dicaffeolyltartaric acid; 8, Quercetin-3-O-rhamnoside; 9, Isorhamnetin.
Figura 1. Cromatograma de HPLC de padrones de polifenoles (incluyendo fenólicos, flavonoides y antocianinas) utilizado para la identificación y cuantificación en extractos de brotes de berros ecológicos a 270 nm (A y B) y 520 nm (C): 1, Ácido gálico; 2, Ácido clorogénico; 3, Ácido cafeico; 4, Cianidina-3-glucósido; 5, Quercetina-3-O-rutinoside; 6, Luteolina-7-O-glucósido; 7, Dicaffeolyltartaric ácido; 8, Quercetina-3-O-rhamnósido; 9, Isorhamnetin.
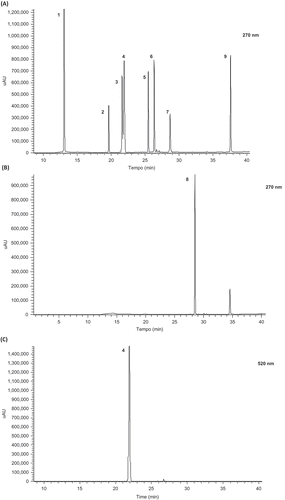
Table 1. Average levels of major individual phenolics quantified in organic baby-leaf watercress samples by HPLC, expressed as mg kg−1 of dry weight.
Tabla 1. Niveles medios de fenoles individuales en muestras de brotes de berros orgánicos cuantificados por HPLC, expresados como mg kg−1 de peso seco.
Table 2 Antioxidant and antiradical activity of organic baby-leaf watercress extracts at 1 mg mL−1concentration.
Tabla 2. Actividad antioxidante y antirradical de extractos de brotes de berros orgánicos a una concentración de 1 mg mL−1.
Table 3 Correlation coefficients between antioxidant activity measured with DPPH and FRAP and reducing power assays and phenolic, flavonoids, and hydroxycinnamic acid content.
Tabla 3. Coeficientes de correlación entre la actividad antioxidante medida con DPPH y FRAP y poder reductor y fenólicos, flavonoides y contenido en ácido hidroxicinámico.
Comparing with conventional production (Justesen & Knuthsen, Citation2001; Tiveron et al., Citation2012) and other physiological stages (adult and mature plants), the organic baby-leaf watercress presented higher diversity and levels of phytochemicals (1.5 times more), which means that this type of plant material is a good natural source of antioxidants compounds, particularly quercetin-3-O-rutinoside, a potent antioxidant compound. Thus, there is an advantage in consuming watercress as fresh and in a young physiological stage of development.
For the GSs, these were exclusively composed of the GS 2-phenylethyl (), normally known as gluconasturtiin, the most typical GS of this type of Cruciferae (Fahey, Zalcmann, & Talalay, Citation2001). The gluconasturtiin is an aromatic GS, whose enzymatic degradation product via myrosinase (β-thioglucoside glucohydrolase, EC 3.2.3.1), 2-phenylethylisothiocyanate (PEITC), is often associated with antimutagenic, anticarcinogenic, and antibacterial activity (Aires, Mota, Saavedra, Rosa, & Bennett, Citation2009; Hamilton & Teel, Citation1996; Lam et al., Citation2009).
The quantity determined in fresh baby-leaf watercress was 3308.7 ± 117.1 μmol 100 g−1 of dw, which is several times higher than the values presented for similar plant material but in different physiological (adult) stage and conventional production system (Engelen-Eigles, G., Holden, G., Cohen, J.Y.D., & Gardner, G., Citation2006; Kopsell, Barickman, Sams, & McElroy, Citation2007). These particular results show the importance of baby-leaf watercress as a source of gluconasturtiin.
Based on these results, it seems that organic baby-leaf watercress is a biological sample with an important richness in phytochemicals, with potential positives effect on human health. Thus, their consumption must be increased either in salads or in soups.
Antioxidant activity
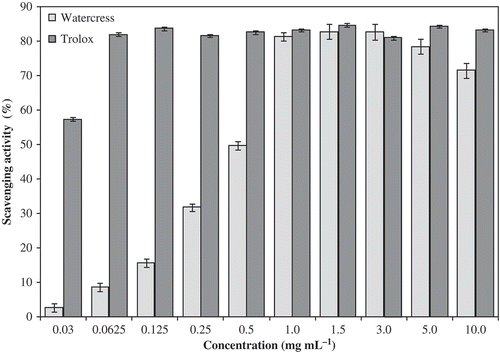
In , our results showed an effective antioxidant and antiradical activity which varied (P < 0.001) with concentration tested (–). The percentage of scavenging radicals of DPPH from 1 mg mL−1 showed high antiradical activity (81.4%) compared to the positive control (83.3%). The scavenging effect of organic baby-leaf watercress and commercial standards on the DPPH radical increased with concentration (). The effect of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen donating ability. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. The model of scavenging the stable DPPH is a widely used method to evaluate antioxidant activities compared to other methods (Gülçin, Citation2012). These results show that watercress is a free radical inhibitor, as well as an antioxidant that reacts with free radicals.
Figure 2. HPLC chromatogram for glucosinolates identified in baby-leaf watercress organic extract recorded at 229 nm.
Figura 2. Cromatograma de HPLC de glucosinolatos identificados en extracto de brotes de berros ecológicos, grabado a 229 nm.
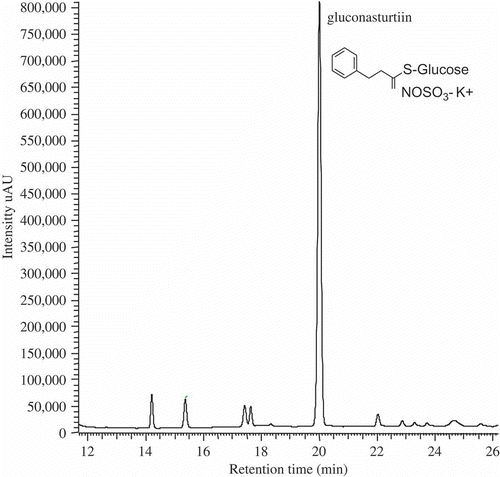
Figure 3. DPPH scavenging activity of organic baby-leaf watercress extracts at different concentrations. Each value represents mean ± SEM of three replicates.
Figura 3. Actividad de atrapamiento de radicales de DPPH de extractos de brotes de berros ecológicos en diferentes concentraciones. Cada valor representa la media ± error medio de tres repeticiones.
The extracts of organic baby-leaf watercress have strong antioxidant activity in reducing power method. The reducing power capacities of samples of watercress compared to Trolox are presented in and . As the same for percentage of scavenging radicals of DPPH by watercress hydrophilic extracts, the antioxidant activity measured by reducing power method was similar to positive control. Both varied with concentration tested (P < 0.001). With the FRAP method ( and ), the antioxidant activity detected at 1 mg mL−1 of watercress extracts was very different and was much lower than the antioxidant activity detected for the positive control ().We think that this method is not the most adequate to measure the antioxidant activity in this type of plant matrixes. Initially designed to determine the antioxidant activity of plasma, it was also been applied to other substrates; however, it seems not to have the same sensitivity, as we noted in the current study. Nevertheless, the same tendency was noted, e.g., the antioxidant activity increased with the extract concentration (). From the results obtained here and from results reported recently by other authors for adult watercress samples and other vegetables from conventional agricultural system including other baby-leaf vegetables (Gülçin, I., Citation2012; Ismail, Marjan, & Foong, Citation2004; Pinela, Barros, Carvalho, & Ferreira, Citation2012; Tiveron et al., Citation2012; Tomás-Callejas, López-Velasco, Artés, & Artés-Hernández, Citation2012), it was apparent that organic baby-leaf watercress extracts have higher in vitro antiradical and antioxidant capacity, which seems to be related with higher levels of phytochemicals.
Correlation between phytochemical composition and antioxidant activity
The antioxidant activity of fruits and vegetables has been related with the presence of bioactive compounds such as vitamins (A, C, and E), polyphenolic compounds (phenolic acids, flavonoids, and anthocyanins), and other compounds such β-carotene, tocopherol, and pigments, which have been considered to be a major dietary factor responsible for such protective effects (Shahidi, Liyana-Pathirana, & Wall, Citation2006).
To analyze this potential relation, a correlation analysis (Pearson) and a PCA were carried out between phytochemicals content and antioxidant activity of organic baby-leaf watercress extracts ( and ).
Figure 4. The reducing power capacities of baby-leaf watercress organic extracts at different concentrations. Each value represents mean ± SEM of three replicates.
Figura 4. La capacidad de poder reductor de extractos de brotes de berros ecológicos en diferentes concentraciones. Cada valor representa la media ± error medio de tres repeticiones.
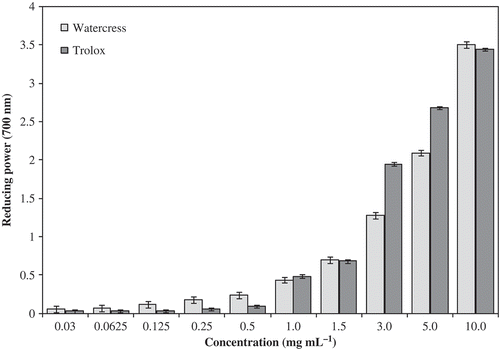
Figure 5. The reductive potential of baby-leaf watercress organic extracts at different concentrations. Each value represents mean ± SEM of three replicates.
Figura 5. El potencial reductor de brotes de berros ecológicos en diferentes concentraciones. Cada valor representa la media ± error medio de tres repeticiones.
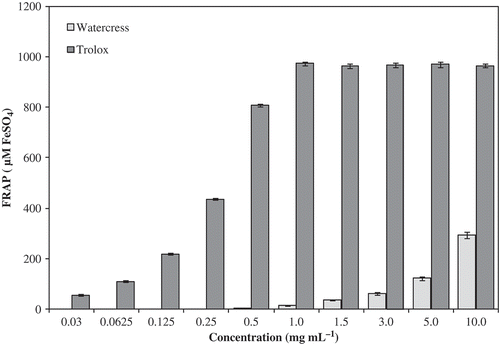
Figure 6. Principal component analyses results.
Figura 6. Resultados del análisis de componentes principales.
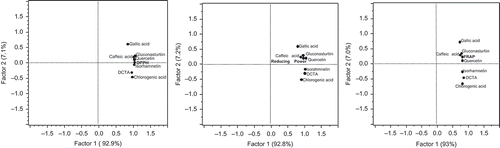
The Pearson correlation showed positive and significant (always lower than P < 0.05) correlation between antioxidant activity and the individual phytochemicals caffeic acid, quercetin-3-O-rutinoside, isorhamnetin, and gluconasturtiin (). Similar results were achieved with the PCA. The PCA analysis reveals that the antioxidant activity of watercress measured by three different methods was always explained by the presence of certain phytochemicals (100% of the variance). The shows the graphic representation of the relation between the antioxidant activity, measured by the three methods (DPPH, reducing power, and FRAP) and the concentration of phytochemicals. For DPPH method, the antioxidant activity was explained by the presence of caffeic acid, quercetin-3-O-rutinoside, gluconasturtiin, and isorhamnetin. Whilst for reducing power assay and FRAP method, the values for antioxidant activity were explained by the presence of caffeic acid, quercetin-3-O-rutinoside, and gluconasturtiin (). These three methods had in common the presence of caffeic acid, quercetin-3-O-rutinoside, and gluconasturtiin as the phytochemicals with higher relations with antioxidant activity. Thus, organic baby-leaf watercress seems to have high antioxidant activity due to the presence of caffeic acid, quercetin-3-O-rutinoside, and gluconasturtiin.
These results confirms the role of caffeic acid, quercetin-3-O-rutinoside, and gluconasturtiin as antioxidant compounds in watercress. The direct association between the levels of caffeic acid, querctin-3-O-rutinoside and antioxidant activity is not a surprise since other authors had reported it but for other food matrices. However this is the first report for gluconasturtiin, to our knowledge. No correlations between TPC and TFC were found, probably due to the nonselective effect of analytical methods used for their determination. The spectrophotometric methods used could extract other components than phenolics and flavonoids (such as ascorbic acid, tocopherol, and pigments (Chlorophylls a, b) which can interfere with the measurement of antioxidant activity. Thus, the HPLC methods are always preferable to determine the accurate levels of phenolics, GSs, and other phytochemicals than the spectrophotometric methods.
Based on our results, it seems that watercress could be an important source of phytochemicals with antioxidant properties which are important to human health, such as chlorogenic acid, caffeic acid, quercetin-3-O-rutinoside, and isorhamnetin frequently associated with antioxidant and anti-inflamatory properties (Valko et al., Citation2007). The gluconasturtiin, largely present in this type of plant material (Fahey et al., Citation2001), as we detected in baby-leaf watercress, is the precursor of the one of the most important ITCs, the PEITC (Fahey et al., Citation2001). This compound inhibits phase I enzymes, which are responsible for the activation of many carcinogens in animals and induces phase II enzymes, which are associated with enhanced excretion of carcinogens (Rose, Faulkner, Williamson, & Mithen, Citation2000). Thus, watercress may have exceptionally good antioxidant properties and anticarcinogenic potential. Therefore, the consumption of watercress, particularly the baby-leafs, must be incremented due to their beneficial properties for human health.
Conclusion
Majority of health-promoting phenolics and GSs are present in organic baby-leaf watercress at higher levels. From antioxidant point of view, it was showed that organic baby-leaf watercress possesses a substantial antioxidant capacity. The above results allows us to conclude that fresh and organic baby-leaf watercress can be used easily as natural source of bioactive compounds due to the presence of flavonoids and GSs, more precisely caffeic acid, quercetin-3-O-rutinoside, isorhamnetin, and gluconasturtiin. Further studies in order to identify new compounds and to compare samples from different production systems (organic, low input and conventional farming) are under progress in our laboratory.
Acknowledgments
The authors acknowledge the financial support provided by the Portuguese Foundation for Science and Technology, which is co-financed by FSE under QREN-POPH- Potencial Humano, Tipologia 4.1-Formação Avançada, da União Europeia (Alfredo Aires-SFRH/BPD/65029/2009).
The authors state that they have no conflict of interest to declare.
References
- Aires , A. , Mota , V. R. , Saavedra , M. J. , Rosa , E. A. S. and Bennett , R. N. 2009 . The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract . Journal of Applied Microbiology , 106 : 2086 – 2095 .
- Balasundram , N. , Sundram , K. and Samman , S. 2006 . Phenolic compounds in plants and agro-industrial by-products. Antioxidant activity, occurrence, and potential uses . Food Chemistry , 99 : 191 – 203 .
- Bones , A. M. and Rossiter , J. T. 2006 . The enzymatic and chemically induced decomposition of glucosinolates . Phytochemistry , 67 : 1053 – 1067 .
- Boyd , L. A. , McCann , M. J. , Hashim , Y. , Bennett , R. N. , Gill , C. I. R. and Rowland , I. R. 2006 . Assessment of the anti-genotoxic, anti-proliferative, and anti-metastatic potential of crude watercress extract in human colon cancer cells . Nutrition and Cancer , 55 : 232 – 241 .
- Cieślik , E. , Gręda , A. and Adamus , W. 2006 . Contents of polyphenols in fruit and vegetables . Food Chemistry , 94 : 135 – 142 .
- Engelen-Eigles , G. , Holden , G. , Cohen , J. Y. D. and Gardner , G. 2006 . The effect of temperature, photoperiod, and light quality on gluconasturtiin. concentration in watercress (Nasturtium officinale R. Br . Journal of Agricultural and Food Chemistry , 54 : 328 – 334 .
- Fahey , J. W. , Zalcmann , A. T. and Talalay , P. 2001 . The chemical diversity and distribution of glucosinolates and isothiocyanates among plants . Phytochemistry , 56 : 5 – 51 .
- Gülçin , I. 2012 . Antioxidant activity of food constituents: An overview . Archives of Toxicology , 86 : 345 – 391 .
- Hakala , M. , Lapvetelainen , A. , Huopalahti , R. , Kallio , H. and Tahvonen , R. 2003 . Effects of varieties and cultivation conditions on the composition of strawberries . Journal of Food Composition and Analysis , 16 : 67 – 80 .
- Hamilton , S. M. and Teel , R. W. 1996 . Effects of isothiocyanates on cytochrome P450 1A1 and 1A2 activity and on the mutagenicity of heterocyclic amines . Anticancer Research , 16 : 3597 – 3602 .
- Heimler , D. , Isolani , L. , Vignolini , P. , Tombeli , S. and Romani , A. 2007 . Polyphenol content and Antioxidative activity in some of freshly consumed salads . Journal of Agricultural and Food Chemistry , 55 : 1724 – 1729 .
- Hinneburg , I. , Dorman , H. J. D. and Hiltunen , R. 2006 . Antioxidant activities of extracts from selected culinary herbs and spices . Food Chemistry , 97 : 122 – 129 .
- Isabelle , M. , Bee Lan Lee , B. L. , Lim , M. T. , Koh , W. -T. , Huang , D. and Ong , C. N. 2010 . Antioxidant activity and profiles of common vegetables in Singapore . Food Chemistry , 120 : 993 – 1003 .
- Ismail , A. , Marjan , Z. M., & and Foong , C. W. 2004 . Total antioxidant activity and phenolic content in selected vegetables . Food Chemistry , 87 : 581 – 586 .
- Javanmardi , J. , Stushnoff , C. , Locke , E. and Vivanco , J. M. 2003 . Antioxidant activity and total phenolic content of Iranian Ocimum accessions . Food Chemistry , 83 : 547 – 550 .
- Johnson , I. T. 2007 . Phytochemicals and cancer . Proceedings of the Nutrition Society , 66 : 207 – 215 .
- Justesen , U. and Knuthsen , P. 2001 . Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes . Food Chemistry , 73 : 245 – 250 .
- Kopsell , D. A. , Barickman , T. C. , Sams , C. E., & and McElroy , J. S. 2007 . Influence of nitrogen and sulfur on biomass production and carotenoid and glucosinolate concentrations in watercress (Nasturtium officinale R. Br) . Journal of Agricultural and Food Chemistry , 55 : 10628 – 10634 .
- Lam , T. K. , Gallicchio , L. , Lindsley , K. , Shiels , M. , Hammond , E. , Tao , X. G. , Chen , L. , Robinson , K. A. , Caulfield , L. E. and Herman , J. G. 2009 . Cruciferous vegetable consumption and lung cancer risk: A systematic review . Cancer Epidemiology, Biomarkers & Prevention , 18 : 184 – 195 .
- Pinela , J. , Barros , L. , Carvalho , A. M., & and Ferreira , I. C. F. R. 2012 . Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens . Food and Chemical Toxicology , 50 : 829 – 834 .
- Rose , P. , Faulkner , K. , Williamson , G. and Mithen , R. 2000 . 7-Methylsulfinilheptyl and 8-methylsulfinilloctyl isothiocyanates from watercress are potent inducers of phase II enzymes . Carcinogenesis , 21 : 1983 – 1988 .
- Russo , M. , Spagnuolo , C. , Tedesco , I. , Bilotto , S. and Russo , G. L. 2012 . The flavonoid quercetin in disease prevention and therapy: Facts and fancies . Biochemical Pharmacology , 83 : 6 – 15 .
- Shahidi , F. , Liyana-Pathirana , C. M. and Wall , D. S. 2006 . Antioxidant activity of white and black sesame seeds and their hull fractions . Food Chemistry , 99 : 478 – 483 .
- Siddhraju , P. and Becker , K. 2003 . Antioxidant properties of various solvents extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam) leaves . Journal of Agricultural and Food Chemistry , 51 : 2144 – 2155 .
- Stratil , P. , Klejdus , B. and Kubán , V. 2006 . Determination of total content of phenolic compounds and their antioxidant activity in vegetables-evaluation of spectrophotometric methods . Journal of Agricultural and Food Chemistry , 54 : 607 – 616 .
- Tiveron , A. P. , Melo , P. S. , Bergamaschi , K. B. , Vieira , T. M. F. S. , Regitano-d'Arce , M. A. B. and Alencar , S. M. 2012 . Antioxidant activity of Brazilian vegetables and its relation with phenolic composition . International Journal of Molecular Sciences , 13 : 8943 – 8957 .
- Tomás-Callejas , A. , López-Velasco , G. , Artés , F. and Artés-Hernández , F. 2012 . Acidified sodium chlorite optimization assessment to improve quality of fresh-cut tatsoi baby leaves . Journal of the Science of Food and Agriculture , 92 : 877 – 885 .
- Valko , M. , Leibfritz , D. , Moncol , J. , Cronin , M. T. , Mazur , M. and Telser , J. 2007 . Free radicals and antioxidants in normal physiological functions and human disease . International Journal of Biochemistry & Cell Biology , 39 : 44 – 84 .
- Zhang , N. , Pei , F. , Wei , H. , Zhang , T. , Yang , C. , Ma , G. and Yang , C. 2011 . Isorhamnetin protects rat ventricular myocytes from ischemia and reperfusion injury . Experimental and Toxicologic Pathology , 63 : 33 – 38 .