Abstract
Enterococcus faecalis is frequently associated with raw milk cheeses of Mediterranean area. The genetic diversity of 38 E. faecalis obtained from raw milk products in Italy was assessed through Randomly Amplified Polymorphic DNA PCR (RAPD-PCR) and repetitive extragenic palindromic PCR (rep-PCR). The strains were screened for their antimicrobial activity against 5 food-borne spoilage and pathogenic bacteria and 13 lactic acid bacteria (LAB), commonly used as starter cultures. Investigation was made to identify the bacteriocinogenic potential by searching for bacteriocin-encoding genes. Inhibitory effects against undesirable bacteria, including Bacillus cereus (44.7% of strains), Escherichia coli (18.4%), Listeria monocytogenes (15.8%), Staphylococcus aureus (2.6%), and Clostridium sporogenes (21.1%), were detected. Moderate antagonism towards LAB was found. One strain producing enterocin AS-48 was identified, suggesting that the antimicrobial activity of the phenotypically positive isolates should be necessarily due to another non-enterocin factor. A deeper insight into biopreservation potential of dairy E. faecalis was provided, highlighting the influence of this species on cheese microbial community.
Frecuentemente, el Enterococcus faecalis es asociado con los quesos de leche cruda de la región mediterránea. En el presente estudio se valoró la diversidad genética de 38 E. faecalis obtenidos de productos de leche cruda de Italia, mediante RAPD-PCR y rep-PCR. Se estudió la actividad antimicrobiana de las cepas a partir de cinco bacterias patógenas y saprófitas de origen alimentario y de 13 bacterias de ácido láctico (BAL) utilizadas comúnmente como cultivos iniciadores. Con el objetivo de identificar el potencial bacteriocinagénico mediante la búsqueda de genes de codificación de bacteriocinas, se realizaron investigaciones, detectándose efectos inhibitorios contra bacterias indeseables, entre las que se incluyen Bacillus cereus (44.7% de las cepas), Escherichia coli (18.4%), Listeria monocytogenes (15.8%), Staphylococcus aureus (2.6%) y Clostridium sporogenes (21.1%). Asimismo, se constató un moderado antagonismo contra las BAL y se identificó una cepa que produce enterocina AS-48, lo cual sugiere que la actividad antimicrobiana de los aislados fenotípicamente positivos es consecuencia de otro factor diferente a la enterocina. El estudio arrojó una comprensión más profunda sobre el potencial de biopreservación de E. faecalis en lácteos, destacándose la influencia de esta especie dentro de la comunidad microbiana de los quesos.
Palabras claves: Enterococcus faecalis; biopreservación; enterocinas; quesos de leche cruda; rep-PCR; RAPD-PCR
Introduction
Micro-organisms are recognized to produce an abundant array of microbial defence systems, such as classical antibiotics, metabolic by-products, lytic agents, numerous types of protein exotoxins, and bacteriocins (Riley & Wertz, Citation2002). Bacteriocins are ribosomally synthesized proteins or peptides with antimicrobial properties, which are released by a broad variety of living organisms ranging from prokaryotes to higher eukaryotes, including Gram-positive and Gram-negative bacteria (Ghrairi, Frere, Berjeaud, & Manai, Citation2008). Generally, bacteriocins are characterized by a narrow killing spectrum and toxic effects restricted to bacteria closely related to the producing strain (Riley & Wertz, Citation2002).
Since food safety has received increasing attention, several studies have focused on bacteriocins in order to enhance the microbial quality and safety of fermented food (Alegría, Delgado, Roces, López, & Mayo, Citation2010). In fact, bacteriocins may serve as an alternative to chemical preservatives in foods (Casaus et al., Citation1997): their production may represent an additional functional property of value for selected food applications (Cleveland, Montville, Nes, & Chikindas, Citation2001), above all as a consequence of the increased demand by consumers for products that are less processed and are free of preservatives. They are also an interesting option for the food industry as their addition does not modify the taste or smell of the final products (Moraes et al., Citation2010). Furthermore, the application of bacteriocin-producing strains as starter or adjunct cultures in the production process of fermented food provides an attractive and economic alternative to the addition of bacteriocins, whose purification is expensive if compared to cultivation of strains for inoculation purposes (O’Sullivan, Ross, & Hill, Citation2002).
Among the Gram-positive bacteria, lactic acid bacteria (LAB) have been exploited as a reservoir for bacteriocins (Cleveland et al., Citation2001). Bacteriocins produced by LAB offer a number of desirable characteristics. First, they are commonly recognized as safe substances and have no activity or toxic effects on eukaryotic cells. They have also no activity on the gut microbial community, since they are inactivated by digestive enzymes during the transit through the gastro-intestinal channel. On the contrary, they show a relatively broad antimicrobial spectrum against food-borne spoilage and pathogenic bacteria, whose cytoplasmatic membrane represents the target of the bactericidal activity. They are suitable for genetic manipulation, as the genes for their expression are generally plasmid-encoded. Finally, they are usually pH and heat stable (Gálvez, Abriouel, López, & Ben Omar, Citation2007).
In particular, the Enterococcus genus produces bacteriocins (enterocins) with a broad inhibitory spectrum, showing inhibitory activity against not only Gram-positive but even Gram-negative pathogenic, toxigenic, and food-spoilage bacteria. This represents an unusual property for the bacteriocins produced by LAB (Abriouel, Ben Omar, Lucas, Martínez-Cañamero, & Gálvez, Citation2006; Khan, Flint, & Yu, Citation2010). However, the most frequently reported feature of enterocins is their specific and strong inhibition towards Listeria monocytogenes (Callewaert, Hugas, & De Vuyst, Citation2000). Numerous enterococcal species present in food systems are associated with the production of enterocins, mainly Enterococcus faecium and Enterococcus faecalis (Javed, Masud, Ain, Imran, & Maqsood, Citation2011). Even though these two species are often under discussion because of virulence traits, they also possess beneficial technological and biochemical features which make them as potential adjunct starters (Giraffa, Citation2003).
In a recent study, Chahad, El Bour, Calo-Mata, Boudabous, and Barros-Velàzquez (Citation2012) showed the presence of high bacteriocin-producing enterococci in farmed marine fish. The acquisition of these strains, already acclimated to seafood habitats, could be advantageous for biopreservation in aquatic foodstuffs (Chahad et al., Citation2012). Similarly, due to their implication in cheese ripening and aroma development, dairy enterococcal strains with bacteriocinogenic activity and a proved harmless nature could be included in starter cultures for many cheeses (Ogier & Serror, Citation2008).
Enterococci constitute a main component of the microflora in many traditional cheeses, especially those manufactured from raw milk in the Mediterranean area, where they occur as nonstarter lactic acid bacteria (Serio, Paparella, Chaves-López, Corsetti, & Suzzi, Citation2007).
The aim of this work was to screen phenotypically and molecularly for enterocin production towards representative dairy useful, spoilage, and pathogenic bacteria, E. faecalis, strains isolated from raw milk products in Northwest Italy, in order to assess their biopreservation potential within complex microbial community of raw milk cheeses and their usefulness as starter or protective cultures in dairy fermentation. Moreover, the genetic variability of the selected isolates was investigated with the purpose of revealing the possible correlation between the enterocin phenotypes and genotypes and the strain origin.
Materials and methods
Bacterial strains
A total of 38 E. faecalis isolates, previously selected in accordance with their origin, were included in this study. They had been isolated from raw milk samples (milk, curd, and cheese), obtained from different animal species (35 samples from cow and three from goat) and collected in three regions of the Northwest Italy (four from Aosta Valley, 28 from Lombardy, and six from Piedmont) during 1997–2009. Strains had been assigned to the species E. faecalis through species-specific PCR assay and partial 16S rRNA sequencing according to Morandi, Brasca, and Lodi (Citation2011), and then comprised into the CNR ISPA strain collection. Before use in assays, isolates were sub-cultured aerobically at 37°C in M17 broth (Scharlau Microbiology, Barcelona, Spain), and then kept at 4°C.
The 18 bacterial strains used as indicator organisms for the evaluation of antimicrobial activities are listed in . The strains were chosen in order to represent technologically useful, food-borne spoilage, and pathogenic bacteria. Throughout the experiments, the following media were used to culture these strains at 30°C: Brain Heart Infusion (BHI) broth (Scharlau Microbiology) for all pathogens, Reinforced Clostridium broth (RCM) (Scharlau Microbiology) for Clostridium sporogenes, MRS (de Man, Rogosa and Sharpe) broth (Scharlau Microbiology) for lactobacilli and pediococci, and M17 broth (Scharlau Microbiology) for Streptococcus, Leuconostoc, lactococci, and enterococci.
Table 1. Indicator organisms used in the antimicrobial activity assay.
Cepas de referencia utilizada para la identificacion de la actividad antimicrobiana.
RAPD-PCR analysis
RAPD-PCR was used to explore the genetic diversity of the E. faecalis strains. For RAPD-PCR, total DNA was extracted from the overnight cultures by the Microlysis kit (LaboGen, Rho, Italy) following the manufacturer’s instructions. The Quant-iT dsDNA High Sensitivity Assay kit and the Qubit fluorometer (Invitrogen, Minneapolis, MN, USA) were used for DNA quantification. After dilution to about 50 ng/μl, sample DNA underwent RAPD-PCR reactions performed with primers M13 and D11344, while amplification conditions as well as electrophoresis and analysis of the amplification products were as previously described (Andrighetto, Borney, Barmaz, Stefanon, & Lombardi, Citation2002; Morandi, Brasca, Andrighetto, Lombardi, & Lodi, Citation2006).
Rep-PCR typing
Rep-PCR was chosen as the second fingerprinting method. For rep-PCR analysis, genomic DNA was extracted from 1.8 ml of each enterococcal broth culture using the UltraClean Microbial DNA Isolation kit (Mo Bio Laboratories, Carlsbad, CA, USA). DNA was quantitated using the Quant-iT dsDNA High Sensitivity Assay kit and the Qubit fluorometer (Invitrogen) and was standardized to approximately 25–50 ng/μl. Two microlitres of Aliquots were amplified using the DNA fingerprinting DiversiLab kit (Bacterial Barcodes, Inc., Athens, GA, USA) in accordance with the manufacturer’s instructions. Thermal cycling parameters were as follows: initial denaturation at 94°C for 2 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 45°C for 30 s, and extension at 70°C for 90 s; and a final extension at 70°C for 3 min. The amplified product was stored at –20°C until detection. Analysis of rep-PCR products was implemented using the miniaturized electrophoresis system “DNA 7500 Lab-Chip” (Agilent Technologies, Santa Clara, CA, USA). This system separates pieces of DNA according to their size resulting in a plot of corresponding peaks (electropherogram), which can be monitored online on a personal computer and can be evaluated and translated into a pseudo-gel by particular software (Agilent Technologies). For rep-PCR results, two patterns were considered different if two and more peaks of the electropherogram differed in size.
Data analysis
Grouping of the RAPD-PCR and rep-PCR profiles was obtained with the BioNumeric 5.0 software package (Applied Maths, Sint-Martens-Latem, Belgium), using the UPGMA (unweighted pair group method with arithmetic averages) cluster analysis. The reproducibility value of the RAPD-PCR assay, calculated from two repetitions of independent amplification of enterococcal type strains, was higher than 90%. The reproducibility of rep-PCR patterns was assessed revealing a minimal similarity level of 90%, as reported by Jensen, Ardö, and Vogensen (Citation2009).
Antimicrobial activity assay
Antibacterial activity was evaluated parallel by standardized agar disc diffusion with extracellular extract of bacterial cells (Campos, Rodríguez, Calo-Mata, Prado, & Barros-Velázquez, Citation2006) and with overnight cultures against the indicator strains (). Twenty microlitres of each extracellular extract or 20 μl of an overnight culture of E. faecalis strains were spotted onto agar. Agar plates were seeded with indicator strains at 105 CFU/ml concentration, the plates were incubated at the optimal temperature of indicator strains for 24 h, and then the diameter (mm) of the growth inhibition zone was measured. To confirm the proteinaceous nature of the antimicrobial compound, cell free supernatant (CFS) from antimicrobial cultures was incubated (2 h, at 37°C) in the presence of proteinase K (1 mg/ml, Sigma-Aldrich, St. Louis, MO, USA), then the antimicrobial activity was tested using the standardized agar disc diffusion method (Sip, Wieçkowicz, Olejnik-Schmidt, & Grajek, Citation2012).
Detection of enterocin structural genes by PCR
The presence of bacteriocin-encoding genes was studied by PCR amplification with primers specific for the following enterococcal bacteriocins: enterocin A (entA), enterocin B (entB), and bacteriocin 31 (Bac31) (Du Toit, Franz, Dick, & Holzapfel, Citation2000); enterocin P (entP), enterocin Q (entQ), and enterocin L50A-B (entL50A-B) (Cintas et al., Citation2000); mundticin KS (MunKS) (Kawamoto et al., Citation2002); enterocin CRL35 (entCRL 35) (Saavedra, Minahk, Martins, de Ruiz Holgado, & Sesma, Citation2004); and enterocin AS-48 (entAS48) and enterocin 1071A-B (ent1071A-B) (Ben Omar et al., Citation2004). PCR products were separated by electrophoresis on 1.5% (w/v) agarose (GellyPhor, Euroclone, Milan, Italy) gels containing 0.09 μl/ml of SYBR Safe (Invitrogen). Electrophoretic separation was at 100 V for 90 min and, on each gel, a molecular size marker (100-bp DNA ladder, Euroclone) was included at two positions. The gels were photographed on an UVtransilluminator.
Results and discussion
Genetic diversity
In the present study, two DNA fingerprinting methods for comparative genome analysis, RAPD-PCR and rep-PCR, were performed in order to assess exhaustively the genetic diversity of the 38 E. faecalis strains. RAPD-PCR and rep-PCR seemed to reveal the same level of similarity among the isolates considered. In fact, both techniques grouped most E. faecalis isolates (37 out of 38 isolates) at a 50% similarity level (). Moreover, the very high genetic diversity possessed by strain AA4 was detected by both methods. At 80% similarity, arbitrarily chosen as a discriminating threshold to define the homogenous clusters and sub-clusters in each dendrogram, the cluster analysis of the combined RAPD-PCR patterns gave 7 distinct groups while rep-PCR dendrogram showed that at a similarity level of 80% only 4 isolates were grouped into different sub-clusters and the other 34 isolates showed enough similarity to each other, hence they were grouped together. However, the rep-PCR-derived dendrogram () depicted stronger relationship among the E. faecalis isolates, when compared with the RAPD-derived dendrogram (), hence higher inter-strain heterogeneity was obtained using the RAPD method. Only rep-PCR profiles highlighted that isolates from the same dairy source were often similar, since they clustered together and showed a closer genotypic relatedness (e.g. 92% for Valtellina Casera isolates and Formaggella della Val di Scalve cheese isolates). Moreover rep-PCR grouped together strains VC12, VC17, VC42, VC49, and SV70A harbouring entAS48 gene, while RAPD-PCR cluster analysis did not highlight any relationship with inhibition activity. Divergences of images in the microbial community between the two molecular techniques were detected. Some strains (i.e. VS368 and AA8 or VC17 and R221) had a high similarity coefficient by RAPD-PCR, while they had a dissimilar profile and could be considered distant from the genotypic point of view through rep-PCR. But considering other strains, both techniques clustered them together with a high similarity level (i.e. ID143A and ID160 or SV6 and SV56).
Figure 1. Dendrogram derived from profiles by the repetitive extragenic palindromic-PCR (rep-PCR) (a) and by the random amplification of polymorphic DNA (RAPD-PCR) generated with primers M13 and D11344 (b). The profile grouping was done with the BioNumeric 5.0 software package, using the unweighted pair group method with arithmetic averages (UPGMA) cluster analysis.
Dendrograma generado a partir de perfiles genéticos por PCR-palindrómico extragénico repetitivo (rep-PCR) (a) y por amplificación aleatoria de ADN polimórfico (RAPD-PCR) obtenidos con cebadores M13 y D11344 (b). Los perfiles fueron agrupados usando el software BioNumeric 5,0, usando el método de agrupación por media aritmética no ponderada (UPGMA).
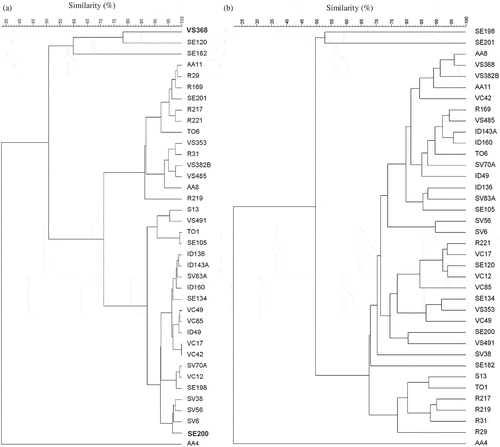
Actually, even though both molecular methods represent fast, reliable, and versatile typing tools to differentiate among related bacterial strains, they possess a different discriminatory potential. Rather, they were intentionally selected in this study because they provide strain characterization based on different discrimination principles. RAPD-PCR exploits a random primer able to anneal in several positions in the genome, thereby allowing amplification of random sequences (Iacumin, Comi, Cantoni, & Cocolin, Citation2006). Through RAPD-PCR, it is not possible to detect minor changes in the chromosome and amplification bands are strongly dependent from the conditions of the method and the primer used (Psoni, Kotzamanidis, Yiangou, Tzanetakis, & Litopoulou-Tzanetaki, Citation2007). Rep-PCR is able to produce fingerprints related to the presence of repeated elements within the genome considered (Iacumin et al., Citation2006). These repetitive elements, located in the intergenic regions of many bacterial genomes, are considered to be highly conserved and as such are useful for elucidating relationships within and between bacterial species (Mancuso, Avendaño-Herrera, Zaccone, Toranzo, & Margariños, Citation2007). Discordances between the two molecular techniques suggested that a polyphasic approach, combining two or more different methods, may represent an essential tool to obtain a more effective, accurate, and exhaustive investigation of the intra-specific typing.
Screening for bacteriocin production
The production of inhibitory compounds by E. faecalis strains against a selection of indicator organisms was first investigated by the disc diffusion method. Antibacterial activity may contribute to the microbiological safety of dairy end products, but also improve the competitiveness of strains that naturally occur in raw milk products (De Vuyst, Foulquié Moreno, & Revets, Citation2003). For that reason, 18 food-borne spoilage or pathogenic bacteria and technologically important LAB species, belonging to both Gram-positive and Gram-negative micro-organisms, were included in the test (). A variable number of the 38 strains inhibited the different indicators (). The presence of antibacterial activity towards just one indicator strain was reported within 11 isolates (28.9%). Interestingly, among these isolates, nine exclusively inhibited strain of Bacillus cereus. Strains harbouring activity against two genera or species were the most representative (36.8%). Of all these isolates, 21.1% inhibited contemporaneously Lactobacillus delbrueckii subsp. bulgaricus and Lactobacillus helveticus, 7.9% B. cereus and Cl. sporogenes, 5.3% B. cereus and Streptococcus thermophilus, and 2.6% two different E. faecalis strains. Isolates possessing activity against three bacteria were detected at percentages of 15.8%. All these narrow-spectrum inhibitors were equally distributed among E. faecalis strains of different geographical origin. Seven (18.4%) strains inhibited five or more of the tested micro-organisms. In detail, four, two, and one of these strains inhibited five, six, and eight indicator strains, respectively. Remarkably, five of these broad-spectrum isolates were obtained from Valtellina Casera cheese. Most of them exhibited inhibitory effects against L. monocytogenes, Escherichia coli, but also Lb. delbrueckii subsp. bulgaricus, Lb. helveticus, and Leuconostoc mesenteroides subsp. mesenteroides. The only strain (VC85) responsible for inhibition of eight different micro-organisms was the only one active against Lactobacillus fermentum and Lactobacillus reuteri. Since most strains possessing the broader spectrum of antibacterial activity were isolated from the same cheese, it was interesting to examine their possible genetic relatedness. Strains isolated from Valtellina Casera could be considered as genotypically close strains, because they were clustered at a cut-off of 92% and 72% similarity by rep-PCR and by RAPD-PCR, respectively.
Table 2. Prevalence of antimicrobial activity and enterocin genes in E. faecalis isolates in relation to their geographical and dairy origin.
Actividad antimicrobiana y genes para la producción de enterocinas de E. faecalis aisladas a partir de queso de leche cruda.
With regard to the selected pathogens or undesiderable bacteria, 28 (73.7%) strains exhibited antimicrobial activity against them. This feature hold by some enterococci explains their interest as food-grade preservatives to be used in dairy technology. Among these pathogenic isolates, B. cereus was the most sensitive micro-organism, since antimicrobial activity was revealed in 17 (44.7%) of the E. faecalis strains. Seven (18.4%) and six (15.8%) isolates showed activity against E. coli and L. monocytogenes, respectively. In contrast, Staphylococcus aureus was inhibited by just one strain. Interestingly, all strains owing antimicrobial activity against B. cereus inhibited no other pathogen. All five (13.2%) strains with inhibitory spectrum against two pathogens were simultaneously active against L. monocytogenes and E. coli. From the very beginning of enterococcal bacteriocins research, enterocins have been frequently reported as bacteriocins with antilisterial effect (Čanžek Majhenič, Citation2006). The strict phylogenetic relationship between Enterococcus and Listeria genera explains the high inhibitory effectiveness of Enterococcus against Listeria spp., particularly L. monocytogenes, as described by other authors (De Vuyst et al., Citation2003). Only one strain was demonstrated to inhibit the growth of three pathogenic indicators, whereas no E. faecalis was effective against all four pathogens. Clear inhibition haloes were observed in plates seeded with the Cl. sporogenes culture in presence of eight (21.1%) E. faecalis isolates, but weak inhibition haloes were also found (10.5%).
Considering LAB species, there was evidence of antibacterial activity in 25 (65.8%) of E. faecalis tested. In detail, all E. faecalis strains displayed a narrow range of antimicrobial activity towards other enterococci. In particular, three (7.9%) strains were revealed to be active against other E. faecalis isolates, but no one possessed activity against E. faecium (data not shown). The weak antagonism towards other enterococci recorded in this study is in agreement with findings of other researchers (Du Toit et al., Citation2000). Lb. helveticus, Lb. delbrueckii subsp. bulgaricus, Ln. mesenteroides subsp. mesenteroides, and St. thermophilus were inhibited by 21 (55.3%), 20 (52.6%), 6 (15.8%), and 5 (13.2%) strains, respectively. All 20 strains which were effective against Lb. delbrueckii subsp. bulgaricus displayed also antagonistic effect against Lb. helveticus. Actually, Lb. delbrueckii and Lb. helveticus are close relatives, since there is only one site in the middle of the 16S rDNA gene generating different DNA fragments allowing distinction between the two species (Germond et al., Citation2003). Additionally, in many cases, marked haloes were detected, particularly for Lb. helveticus and Lb. delbrueckii subsp. bulgaricus. Lb. fermentum and Lb. reuteri were inhibited by just one strain. On the contrary, no strain showed clear inhibitory effects against Lactococcus lactis subsp. cremoris, Pediococcus acidilactici, and Lactobacillus rhamnosus (data not shown). It is noteworthy that the 38 strains generally showed a very weak antagonistic effect towards E. faecium, E. faecalis, S. thermophilus, Lc. lactis, Lb. fermentum, Lb. reuteri, and Lb. rhamnosus. In fact, an antimicrobial activity against undesiderable bacteria but without effect on useful micro-organisms represents a potential, natural biopreservative for a variety of foods including dairy products (Ennahar & Deschamps, Citation2000). If the antagonistic strain is used in a mixed starter culture, no interference should occur with the acid and flavour production of the starter strain, otherwise a final product with reduced quality would be obtained (Khan et al., Citation2010). Thirteen (34.2%) strains could have a potential use in cheese technology, as they owned activity against detrimental or harmful bacteria, but were ineffective against LAB tested. However, 10 (26.3%) strains were described as unideal preservatives, showing antagonistic effects against beneficial micro-organisms, but not against pathogenic or spoilage genera.
Different results were obtained with the cell-free supernatant. Only the CFS of E. faecalis VC17 inhibited the growth of L. monocytogenes. No inhibition was observed against the remaining indicator strains. In order to identify the nature of the antimicrobial compound, a second test was carried out, removing proteins from CFS by proteinase K digestion. The anti-Listeria E. faecalis VC17 lost its potential after proteinase K treatment. The absence of inhibition zones confirmed the production of bacteriocins or bacteriocin-like compounds by this strain.
Consequently, the antimicrobial activity highlighted among E. faecalis strains was not related to production of enterocin, with exception of VC17. On the other hand, it is well known that complex microbial communities such as LAB (e.g. L. garvieae, Lactobacillus spp. and enterococci) can exert antimicrobial activity in cheese, not only by producing bacteriocins or decreasing pH but also by the synthesis of hydrogen peroxide, ethanol, and other organic acids (Delbes-Paus, Dorchies, Chaabna, Callon, & Montel, Citation2010).
Detection of enterocin structural genes
Molecular approach was applied with the aim of obtaining further information about the enterocin production capabilities of the E. faecalis examined (Gálvez et al., Citation2007). In fact, the lack of antimicrobial activity expression in phenotypical assay was previously reported. It was revealed as dependent on numerous factors, such as the interference of environmental conditions and the non-induction of the expression of genes encoding bacteriocins (Moraes et al., Citation2010). In particular, bacteriocins seem to be commonly regulated and subsequently produced only under appropriate growth conditions, such as incubation temperatures. Some bacteriocins are also produced on solid growth media but not in liquid cultures (Strompfová, Lauková, Simonová, & Marciňáková, Citation2008). Moraes et al. (Citation2010) assessed that the performance of the antimicrobial activity assay of LAB varies in relation to culture media and protocols too. E. faecalis strains were subsequently subjected to PCR analysis. Specific primers for genes of the most common enterocin structural genes were used in PCR protocol: enterocin A (entA), enterocin B (entB), bacteriocin 31 (Bac31), enterocin P (entP), enterocin Q (entQ), enterocin L50A-B (entL50A-B), mundticin KS (MunKS), enterocin CRL35 (entCRL 35), enterocin AS-48 (entAS48), and enterocin 1071A-B (ent1071A-B). The number of positive strains drastically decreased, as shown in . In fact, almost all genes were never detected among the isolates studied. Only 6 (15.8%) of the 38 strains produced an amplicon of the expected size (350 bp) for entAS-48, according to Ben Omar et al. (Citation2004). This was not surprising, since other authors reported similar results (Theppangna et al., Citation2007). In addition, an earlier study showed that entAS-48 was widely distributed throughout E. faecalis (Joosten, Rodríguez, & Nuñez, Citation1997). On the contrary, no equivalence in results was found with other authors, who detected the presence of genes encoding for ent1071 A-B and P in E. faecalis, whereas they never detected genes for entAS-48 (Martín-Platero, Valdivia, Maqueda, & Martínez-Bueno, Citation2009; Valenzuela et al., Citation2009). Moreover, a correlation can be established between the gene distribution and the strain origin, since four of the six positive strains were isolated from the same cheese (Valtellina Casera). As well as entA, entB, ent50, and Bac31, entAS-48 represents one of the well-characterized Enterococcus bacteriocin. The latter one is a plasmid-encoded, cyclic antibiotic peptide originally found in clinical samples, but it has been also detected in food samples (De Vuyst et al., Citation2003; Du Toit et al., Citation2000) and has been found at high proportion especially in goats’ and ewes’ milk (Čanžek Majhenič, Citation2006). Its broad activity spectrum comprising antilisterial properties together with antagonism against both Gram-positive (like S. aureus) and Gram-negative pathogens (like Salmonella spp. and E. coli) was previously assessed (Joosten, Nuñez, Devreese, Van Beeumen, & Marugg, Citation1996; Khan et al., Citation2010). In particular, entAS-48 was the first bacteriocin produced by Gram-positive bacteria which was demonstrated to possess antagonistic activity against Gram-negative bacteria, and the only one active against Listeria spp. (Gálvez et al., Citation2007; Joosten et al., Citation1996). Actually the VC17 strain, which was demonstrated to produce a protein substance inhibiting L. monocytogenes, harboured entAS-48.
In conclusion, this research contributed to get a deeper insight into antimicrobial activity possessed by E. faecalis. The selected isolates harboured antagonistic effects against well-recognized pathogens or spoilage bacteria. In particular, it is noteworthy that such a relevant number of strains showing antagonism towards B. cereus had never been previously reported. Some strains were demonstrated to hamper the growth of micro-organisms commonly employed in dairy production. The 13 strains described in this work, which are free of antibacterial effect towards technologically beneficial species, but owing activity against detrimental or harmful bacteria, may have an interesting dairy use. Only one strain was confirmed to be enterocinogenic, and the structural gene for enterocin AS-48 production was the only one detected. All strains possessing this gene inhibited the growth of L. monocytogenes and E. coli. The discrepancy between phenotypical and genotypical results highlighted the need of both approaches to investigate this enterococcal potential.
Finally, some interesting correlations were revealed between the geographical or dairy origin and both genetic diversity and antimicrobial activity, suggesting the deep relationship between the territory and some genotypic and metabolic traits of dairy microbiota. The complex ecosystem of raw milk cheeses includes strains characterized by a wide inter- and intra-specific genetic variation and bacterial biotypes with peculiar properties. These findings tend to substantiate the theory that the predominance of strains with particular capabilities is connected to a specific environment; therefore the autochthonous microflora of traditional cheeses represents a heritage that needs to be protected and conserved. Furthermore, this study corroborates the evidence that inhibition of pathogens in cheeses with complex microbial community is correlated with a high degree of microbial diversity in the community (Callon, Saubusse, Didienne, Buchin, & Montel, Citation2011).
Acknowledgement
This study was partly performed within the research project “Accordo Quadro CNR – Regione Lombardia”.
References
- Abriouel, H., Ben Omar, N., Lucas, R., Martínez-Cañamero, M., & Gálvez, A. (2006). Bacteriocin production, plasmid content and plasmid location of enterocin P structural gene in enterococci isolated from food sources. Letters in Applied Microbiology, 42, 331–337.
- Alegría, Á., Delgado, S., Roces, C., López, B., & Mayo, B. (2010). Bacteriocins produced by wild Lactococcus lactis strains isolated from traditional, starter-free cheeses made of raw milk. International Journal of Food Microbiology, 143, 61–66.
- Andrighetto, C., Borney, F., Barmaz, A., Stefanon, B., & Lombardi, A. (2002). Genetic diversity of Streptococcus thermophilus strains isolated from Italian traditional cheeses. International Dairy Journal, 12, 141–144.
- Ben Omar, N., Castro, A., Lucas, R., Abriouel, H., Yousif, N.M.K., Franz, C.M.A.P., … Gálvez, A. (2004). Functional and safety aspects of enterococci isolated from different Spanish foods. Systematic and Applied Microbiology, 27, 118–130.
- Callewaert, R., Hugas, M., & De Vuyst, L. (2000). Competitiveness and bacteriocin production of Enterococci in the production of Spanish-style dry fermented sausages. International Journal of Food Microbiology, 57, 33–42.
- Callon, C., Saubusse, M., Didienne, R., Buchin, S., & Montel, M.C. (2011). Simplification of a complex microbial antilisterial consortium to evaluate the contribution of its flora in uncooked pressed cheese. International Journal of Food Microbiology, 145, 379–389.
- Campos, C.A., Rodríguez, Ó., Calo-Mata, P., Prado, M., & Barros-Velázquez, J. (2006). Preliminary characterization of bacteriocins from Lactococcus lactis, Enterococcus faecium and Enterococcus mundtii strains isolated from turbot (Psetta maxima). Food Research International, 39, 356–364.
- Čanžek Majhenič, A. (2006). Enterococci: Yin–Yang microbes. Mljekarstvo, 56, 5–20.
- Casaus, P., Nilsen, T., Cintas, L.M., Nes, I.F., Hernández, P.E., & Holo, H. (1997). Enterocin B, a new bacteriocin from Enterococcus faecium TI36 which can act synergistically with enterocin A. Microbiology, 143, 2287–2294.
- Chahad, O.B., El Bour, M., Calo-Mata, P., Boudabous, A., & Barros-Velàzquez, J. (2012). Discovery of novel biopreservation agents with inhibitory effects on growth of food-borne pathogens and their application to seafood products. Research in Microbiology, 163, 44–54.
- Cintas, L.M., Casaus, P., Herranz, C., Håvarstein, L.S., Holo, H., Hernández, P.E., & Nes, I.F. (2000). Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. Journal of Bacteriology, 182, 6806–6814.
- Cleveland, J., Montville, T.J., Nes, I.F., & Chikindas, M.L. (2001). Bacteriocins: Safe, natural antimicrobials for food preservation. International Journal of Food Microbiology, 71, 1–20.
- Delbes-Paus, C., Dorchies, G., Chaabna, Z., Callon, C., & Montel, M.C. (2010). Contribution of hydrogen peroxide to the inhibition of Staphylococcus aureus by Lactococcus garvieae in interaction with raw milk community. Food Microbiology, 27, 924–932.
- De Vuyst, L., Foulquié Moreno, M.R., & Revets, H. (2003). Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. International Journal of Food Microbiology, 84, 299–318.
- Du Toit, M., Franz, C.M.A.P., Dick, L.M.T., & Holzapfel, W.H. (2000). Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. Journal of Applied Microbiology, 88, 482–494.
- Ennahar, S., & Deschamps, N. (2000). Anti-Listeria effect of enterocin A, produced by cheese-isolated Enterococcus faecium EFM01, relative to other bacteriocins from lactic acid bacteria. Journal of Applied Microbiology, 88, 449–457.
- Gálvez, A., Abriouel, H., López, R.L., & Ben Omar, N. (2007). Bacteriocin-based strategies for food biopreservation. International Journal of Food Microbiology, 120, 51–70.
- Germond, J.-E., Lapierre, L., Delley, M., Mollet, B., Felis, G.E., & Dellaglio, F. (2003). Evolution of the bacterial species Lactobacillus delbrueckii: A partial genomic study with reflections on prokaryotic species concept. Molecular Biology and Evolution, 20, 93–104.
- Ghrairi, T., Frere, J., Berjeaud, J.M., & Manai, M. (2008). Purification and characterisation of bacteriocins produced by Enterococcus faecium from Tunisian rigouta cheese. Food Control, 19, 162–169.
- Giraffa, G. (2003). Functionality of enterococci in dairy products. International Journal of Food Microbiology, 88, 215–222.
- Iacumin, L., Comi, G., Cantoni, C., & Cocolin, L. (2006). Molecular and technological characterization of Staphylococcus xylosus isolated from naturally fermented Italian sausages by RAPD, Rep-PCR and Sau-PCR analysis. Meat Science, 74, 281–288.
- Javed, A., Masud, T., Ain, Q., Imran, M., & Maqsood, S. (2011). Enterocins of Enterococcus faecium, emerging natural food preservatives. Annals of Microbiology, 61, 699–708.
- Jensen, M.P., Ardö, Y., & Vogensen, F.K. (2009). Isolation of cultivable thermophilic lactic acid bacteria from cheeses made with mesophilic starter and molecular comparison with dairy-related Lactobacillus helveticus strains. Letters in Applied Microbiology, 49, 396–402.
- Joosten, H.M.L.J., Nuñez, M., Devreese, B., Van Beeumen, J., & Marugg, J.D. (1996). Purification and characterization of enterocin 4, a bacteriocin produced by Enterococcus faecalis INIA 4. Applied and Environmental Microbiology, 62, 4220–4223.
- Joosten, H.M.L.J., Rodríguez, E., & Nuñez, M. (1997). PCR detection of sequences similar to the AS-48 structural gene in bacteriocin-producing enterococci. Letters in Applied Microbiology, 24, 40–42.
- Kawamoto, S., Shima, J., Sato, R., Eguchi, T., Ohmomo, S., Shibato, J., … Sameshima, T. (2002). Biochemical and genetic characterization of Mundticin KS, an antilisterial peptide produced by Enterococcus mundtii NFRI 7393. Applied and Environmental Microbiology, 68, 3830–3840.
- Khan, H., Flint, S., & Yu, P.-L. (2010). Enterocins in food preservation. International Journal of Food Microbiology, 141, 1–10.
- Mancuso, M., Avendaño-Herrera, R., Zaccone, R., Toranzo, A.E., & Margariños, B. (2007). Evaluation of different DNA-based fingerprinting methods for typing Photobacterium damselae ssp. Piscicida. Biological Research, 40, 85–92.
- Martín-Platero, A.M., Valdivia, E., Maqueda, M., & Martínez-Bueno, M. (2009). Characterization and safety evaluation of enterococci isolated from Spanish goats’ milk cheeses. International Journal of Food Microbiology, 132, 24–32.
- Moraes, P.M., Perin, L.M., Tassinari Ortolani, M.B., Yamazi, A.K., Viçosa, G.N., & Nero, L.A. (2010). Protocols for the isolation and detection of lactic acid bacteria with bacteriocinogenic potential. LWT – Food Science and Technology, 43, 1320–1324.
- Morandi, S., Brasca, M., Andrighetto, C., Lombardi, A., & Lodi, R. (2006). Technological and molecular characterisation of enterococci isolated from north–west Italian dairy products. International Dairy Journal, 16, 867–875.
- Morandi, S., Brasca, M., & Lodi, R. (2011). Technological, phenotypic and genotypic characterization of wild lactic acid bacteria involved in the production of Bitto PDO Italian cheese. Dairy Science & Technology, 91, 341–359.
- Ogier, J.-C., & Serror, P. (2008). Safety assessment of dairy microorganisms: The Enterococcus genus. International Journal of Food Microbiology, 126, 291–301.
- O’Sullivan, L., Ross, R.P., & Hill, C. (2002). Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie, 84, 593–604.
- Psoni, L., Kotzamanidis, C., Yiangou, M., Tzanetakis, N., & Litopoulou-Tzanetaki, E. (2007). Genotypic and phenotypic diversity of Lactococcus lactis isolates from Batzos, a Greek PDO raw goat milk cheese. International Journal of Food Microbiology, 114, 211–220.
- Riley, M.A., & Wertz, J.E. (2002). Bacteriocins: Evolution, ecology, and application. Annual Review of Microbiology, 56, 117–137.
- Saavedra, L., Minahk, C., Martins, P., de Ruiz Holgado, A.P., & Sesma, F. (2004). Enhancement of the enterocin CRL35 activity by a synthetic peptide derived from the NH2-terminal sequence. Antimicrobial Agents and Chemotherapy, 48, 2778–2781.
- Serio, A., Paparella, A., Chaves-López, C., Corsetti, A., & Suzzi, G. (2007). Enterococcus populations in Pecorino Abruzzese cheese: Biodiversity and safety aspects. Journal of Food Protection, 70, 1561–1568.
- Sip, A., Wieçkowicz, M., Olejnik-Schmidt, A., & Grajek, W. (2012). Anti-Listeria activity of lactic acid bacteria isolated from golka, a regional cheese produced in Poland. Food Control, 26, 117–124.
- Strompfová, V., Lauková, A., Simonová, M., & Marciňáková, M. (2008). Occurrence of the structural enterocin A, P, B, L50B genes in enterococci of different origin. Veterinary Microbiology, 132, 293–301.
- Theppangna, W., Murase, T., Tokumaru, N., Chikumi, H., Shimizu, E., & Otsuki, K. (2007). Screening of the enterocin genes and antimicrobial activity against pathogenic bacteria in Enterococcus strains obtained from different origins. Journal of Veterinary Medical Science, 69, 1235–1239.
- Valenzuela, A.S., Ben Omar, N., Abriouel, H., Lucas López, R., Veljovic, K., Martínez Cañamero, M., … Gálvez, A. (2009). Virulence factors, antibiotic resistance, and bacteriocins in enterococci from artisan foods of animal origin. Food Control, 20, 381–385.
