Abstract
The objective of this study was to investigate the antibacterial effect of caffeine on the behavior and changes in cell morphology of E. coli O157:H7 in a liquid medium and in skim milk. The inhibitory effect of caffeine at different concentrations was determined by inoculating E. coli O157:H7 in laboratory medium and skim milk samples. Samples were incubated at 37ºC for 48 h, and E. coli O157:H7 population was enumerated. Our results showed that caffeine significantly (P < 0.05) inhibited the growth of E. coli O157:H7 in laboratory medium and milk samples. A greater than 3.0 log CFU ml−1 inhibition was observed in milk containing 5.0 g/L caffeine within 12 h of incubation. Moreover, using flow cytometry, marked changes in the morphology of E. coli O157:H7 were also observed. Caffeine has potential as an antimicrobial agent and could be used as an effective natural additive to improve the safety of food products.
El objetivo del presente estudio fue investigar el efecto antibacteriano que tiene la cafeína sobre el comportamiento y los cambios de la morfología celular de E. coli O157:H7 en un medio líquido y en leche descremada. Se determinó el efecto inhibitorio de distintas concentraciones de cafeína, inoculando E. coli O157:H7 en muestras de un medio de laboratorio y de leche descremada. Dichas muestras fueron incubadas a 37°C durante 48 horas, enumerándose posteriormente la población de E. coli O157:H7. Los resultados demostraron que la cafeína inhibió significativamente (P < 0,05) el crecimiento de E. coli O157:H7 tanto en las muestras del medio de laboratorio como en la muestra de leche. En la leche descremada que contenía 5,0 g/L de cafeína, se observó una inhibición mayor a 3,0 log CFU ml–1, ocurrida dentro de las 12 horas de incubación. Asimismo, a partir del uso de una citometría de flujo se observaron cambios significativos en la morfología de E. coli O157:H7. La cafeína tiene el potencial de ser un agente antimicrobiano, por lo cual podría utilizarse como un aditivo natural efectivo para mejorar la seguridad de los productos alimenticios.
Palabras Claves:
Introduction
Escherichia coli O157:H7, which is responsible for causing hemorrhagic colitis and hemolytic uremic syndrome, is considered as a significant foodborne pathogen (Doyle, Citation1991; Riley et al., Citation1983). E. coli O157:H7 has been implicated in outbreaks from a variety of foods including raw milk, undercooked ground beef, fermented meat, and lettuce (Ackers et al., Citation1998; Armstrong, Hollingsworth, & Morris, Citation1996; Hancock, Besser, Rice, Herriott, & Tarr, Citation1997; Mao, Doyle, & Chen, Citation2001). Milk contamination occurs usually during milking, although it is possible during storage and transportation. Pathogenic microorganisms such as Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes in raw milk have been responsible for several outbreaks of foodborne illnesses (Omiccioli, Amagliani, Brandi, & Magnani, Citation2009; Proctor & Davis, Citation2000). Even though pasteurization is the established method of ensuring the safety of milk, foodborne pathogens have also been reported from the pasteurized milk (Ackers et al., Citation2000; Mazumdar, Hartmann, Kämpfer, & Keusgen, Citation2007; Olsen et al., Citation2004). There is ever-increasing consumer demand for fresher and minimally processed food. This demand has prompted the search for more effective non-thermal processing technologies for the treatment of different food products including milk (Ross, Griffiths, Mittal, & Deeth, Citation2003).
The use of chemical agents with antimicrobial activity is one of the most traditional methods to control foodborne pathogens (Kim & Fung, Citation2004). Nowadays, the content levels of chemical preservatives in food products are an important factor influencing the consumer’s choice. Therefore, interest in naturally occurring compounds as antimicrobial agents has increased as the popularity of natural ingredients or foods has increased among consumers. Plants are rich in antimicrobial agents (Gyawali & Ibrahim, Citation2012). Plant products such as coffee and tea are the two non-alcoholic beverages consumed commonly all over the world and known to possess various biological properties including antibacterial activities (Arora, Kaur, & Kaur, Citation2009). Caffeine (1, 3, 7-trimethyl xanthine) is one of the three methylated xanthine alkaloid derivatives present in many plant species (Ibrahim, Salameh, Phetsomphou, Yang, & Seo, Citation2006) and has been shown to have antimicrobial activity against E. coli O157:H7, Salmonella, Pseudomonas sp., Staphylococcus aureus, and several strains of enterobacteria (Almeida, Farah, Silva, Nunan, & Glória, Citation2006; Daglia, Cuzzoni, & Dacarro, Citation1994; Dash & Gummadi, Citation2008; Esimone, Okoye, Nworu, & Agubata, Citation2008; Ibrahim et al., Citation2006; Ramanaviciene, Mostovojus, Bachmotova, & Ramanavicius, Citation2003). Esimone et al. (Citation2008) reported the antibacterial effectiveness of amoxicillin in combination with caffeine. This suggests that caffeine could also enhance the effectiveness of antibiotics. Caffeine containing products such as coffee, tea, cola, and chocolates are among the most widely consumed foods. (Ibrahim et al., Citation2006). The antimicrobial activity of coffee and tea are accredited mainly to their polyphenolic contents (flavonoids, catechins, tannins) and caffeine (Arora et al., Citation2009). To the best of our knowledge, the effect of caffeine against foodborne pathogens in a liquid food model has not been well studied. Therefore, the objective of this study was to determine the antibacterial effect of caffeine on the behavior and changes in cell morphology of E. coli O157:H7 in laboratory media and milk samples.
Materials and methods
Bacterial culture, media and preparation of the test inocula
Three different strains of E. coli O157:H7 (Strain ATCC E0019, ATCC 43895, and ATCC H1730) from our culture collection were used in this study. Each strain was grown separately in 10 ml of Tryptic Soy Broth (Becton, Dickinson and Company, Sparks, MD) with 6.0 g/L of Yeast Extract (TSBYE) at 37ºC for 24 h with agitation (150 rpm). For the growth study in laboratory media, each active strain was harvested by centrifugation (4°C, 8000g for 10 min), washed in peptone water and 1 ml of each culture was then re-suspended in 9 ml of sterile peptone water. The bacterial population in each culture was determined by plating 0.1-ml portions of appropriately diluted culture on duplicate Tryptic Soy Agar with 6.0 g/L Yeast Extract (TSAYE), with incubation at 37°C for 24 h. The initial inoculum of each strain was ~3.0 log CFU ml−1. Similarly, for the study using skim milk sample, a mixture of each individual strain was inoculated. The bacterial count of the three strain mixtures of pathogen was also confirmed by plating 0.1 ml portions of appropriate dilutions on TSAYE plates.
Sample preparation and inoculation
To determine the effect of caffeine (1, 3, 7–trimethylxanthine, Sigma Chemicals, St. Louis, MO, USA) in laboratory media, TSBYE broth containing caffeine (Cf) at 0, 3.0, 4.0, and 5.0 g/L were individually inoculated with each strain of ~3.0 log CFU ml−1. Turbidity readings were measured at 0, 12, 24, and 48 h at 610 nm by spectrophotometer (Model Genesys 10 Vis, Thermospectronic, Rochester, NY, USA). Skim milk sample (pH 6.7) was selected as a liquid food model. Skim milk powder (Difco) was used to prepare milk samples of 8 g milk powder in 100 ml water (w/v). Fluid milk was kept at 4°C for 40 min to allow for hydration. Batches of 10 ml milk sample were prepared with Cf (0, 4.0, and 5.0 g/L, w/v), and sterilized at 110°C for 10 min. A volume of 100 μl of the appropriately diluted three-strain mixture of E. coli O157:H7 was added to each 10-ml of milk sample containing different levels of Cf to obtain an inoculation level of approximately 2.5 log CFU ml−1. The inoculated samples without any added treatment (0 g/L) served as controls. The inoculated samples were incubated at 37°C for 48 h.
Microbiological analysis
To determine the effect of Cf against E. coli O157:H7 in laboratory media, at the end of the incubation period (48 h), samples were serially diluted in 1.0 g/L peptone water, and bacterial populations were determined by surface plating on TSAYE. Plates were incubated at 37ºC for 24 h before colonies were counted. Similarly, the population of mix E. coli O157:H7 strain in milk sample was enumerated at 0, 6, 12, 24, and 48 h by plating (0.1 ml) after serial dilutions (1:10) on duplicate TSAYE plates.
Flow cytometry analysis
Flow-cytometric measurements were made using an Accuri C6 Flow Cytometer (BD biosciences, San Jose, California). Unstained E. coli O157:H7 cells, grown in TSBYE broth and treated with caffeine (0, 3.0, 4.0, and 5.0 g/L) for 48 h, were acquired. At least 10,000 cells per sample were collected using medium flow rate (35 µL/min, 16 µm core) and each sample was collected in triplicate. Cells were gated using side scatter (SSC) vs. forward scatter (FSC), each with logarithmic amplification. To exclude debris, the SSC-H threshold was set to a value of 10,000. Data and graphs were prepared with FCS express (De Novo Software, Los Angeles, CA).
Statistical analysis
The experimental comparisons were performed twice. The data were analyzed using the general linear model of Statistical Analysis Software (the SAS system for Windows, version 9.2, SAS institute, Cary, N.C.). The model included the treatment concentrations as the major effect. Significant differences (P < 0.05) between average pathogen populations were determined using Duncan’s Multiple Range Test.
Results
Growth of E. coli O157:H7 in liquid medium (TSBYE broth)
In TSBYE broth, Cf (3.0–5.0 g/L) significantly (P < 0.0001) inhibited the growth of tested strains. Antimicrobial activity of Cf was tested in TSBYE broth by monitoring the turbidity readings during 48 h of incubation and enumerating bacterial population at the end of incubation period by standard plate count technique. ((a)–(c)) shows the growth of E. coli O157:H7 strains in the presence of different concentration of Cf in TSBYE broth over 48 h at 37°C. In the samples without Cf, the turbidity readings reached absorbance of 1.0–1.1. The addition of Cf to TSBYE broth caused growth inhibition to all tested strains when compared to the control sample. shows the final population (log CFU ml−1) of E. coli O157:H7 strains after 48 h of incubation. The average initial count for E. coli O157:H7 was approximately 3.0 log CFU ml−1. In TSBYE broth without Cf, the population of E. coli O157:H7 reached to 8.96 log CFU ml−1 on an average. The addition of Cf at 3.0 g/L retarded the growth of all tested strains. There was an average log suppression of 1.42 when compared to the control. The addition of Cf at 4.0 g/L caused significant (P ≤ 0.0001) growth inhibition of E. coli O157:H7 strains by an average of 3.05 log compared to the control sample. The addition of Cf at 5.0 g/L caused further reduction by an average of 5.0 log CFU ml−1 compared to the control. Overall, a similar pattern of growth was observed with strain E0019 and 43895 during incubation for 48 h. However, strain H1730 showed very low growth in the presence of Cf at 5.0 g/L, thus showing more sensitivity to caffeine’s effect.
Figure 1. Growth of E. coli O157:H7 (a) strain E0019 (b) strain 43895, and (c) strain H1730 in TSBYE at 37°C for 48 h with different concentrations of caffeine.
Figura 1. Crecimiento de E. coli O157:H7 (a) cepa E0019, (b) cepa 43895, y (c) cepa H1730 en TSBYE a 37°C durante 48 horas con distintas concentraciones de cafeína.
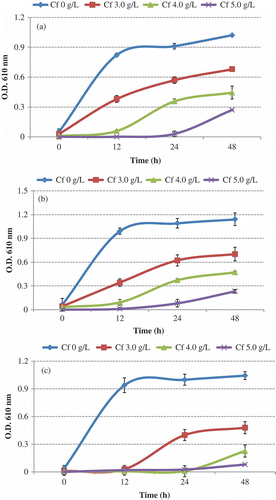
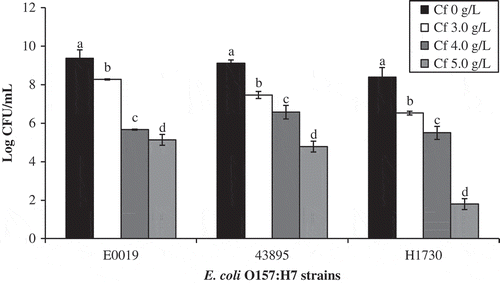
Figure 2. Population of E. coli O157:H7 in TSBYE in the presence of caffeine after incubation at 37°C for 48 h. Means (± standard error) with different letters within the groups are significantly different (P < 0.05).
Figura 2. Población de E. coli O157:H7 en TSBYE en presencia de cafeína tras ser incubada a 37°C durante 48 horas. Medias (± error estándar) con distintas letras dentro del grupo son significativamente diferentes (P < 0.05).
Growth of E. coli O157:H7 in skim milk
As seen from and (in TSBYE), Cf at 4.0 and 5.0 g/L showed the strongest (P ≤ 0.0001) inhibitory activity against all tested strains of E. coli O157:H7. Based on these results, we selected Cf 4.0 and 5.0 g/L for further study in skim milk. shows the inhibition of E. coli O157:H7 in the presence of caffeine in milk sample during 48 h of incubation at 37ºC. The number of E. coli O157:H7 in the milk samples increased from the initial inoculum level of 2.5 log CFU ml−1 to 8.35 CFU ml−1 in the control sample after 12 h of incubation. In the presence of 4.0 and 5.0 g/L Cf, the E. coli O157:H7 population grew to 5.87 and 4.93 log CFU ml−1 within 12 h of incubation showing 2.48–3.42 log suppression (P = 0.0002) of E. coli O157:H7. After 12 h, the population grew and reached the final level of 8.84 log CFU ml−1 in the control sample. In the presence of 4.0 and 5.0 g/L Cf, inhibition of 1.06 and 1.53 log was observed after 48 h of incubation, respectively. However, the results showed higher suppression (>3.0 log) of E. coli O157:H7 within 12 h of incubation when compared to the control sample. Unlike the TSBYE medium, the same concentration of Cf (5.0 g/L) had a less pronounced effect in milk. Increasing Cf concentration in milk did not achieve the same antimicrobial effects as that in broth. Milk used as a food model for this study may be nutritious enough to overcome the inhibitory effect of caffeine against E. coli O157:H7 strains.
Table 1. Population of E. coli O157:H7 (log CFU ml−1) in milk sample treated with caffeine (Cf) for 48 h at 37°C.
Tabla 1. Población de E. coli O157:H7 (log CFU ml–1) en muestra de leche tratada con cafeína (Cf) durante 48 horas a 37°C.
Bacterial morphology
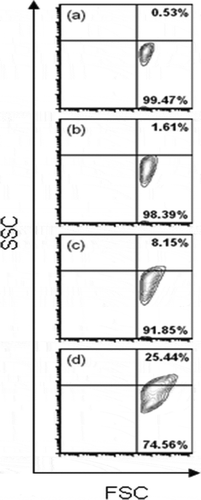
By comparing side scatter (SSC), a measure of cell granularity with forward scatter (FSC), a measure of relative cell size, we were able to evaluate the morphology of E. coli O157:H7 treated with different concentrations of caffeine. By comparing untreated sample ((a)), with caffeine-treated E. coli O157:H7 ((b)–(d)), we found that as the concentration of caffeine increased, E. coli O157:H7 exhibited changes in size and granularity as indicated by shifts in both the X and Y axes, respectively. At Cf 0 g/L (control), 99% of the cell population assumed to be alive was present in the lower quadrant of the histogram. At Cf 3.0 g/L, a little change in the profile of bacterial population was observed showing a slight shift (1.61%). However, at 4.0 and 5.0 g/L Cf, the movement of cell population to the upper quadrant was clearly visible (about 8.15% and 25.44%).
Figure 3. Contour plots demonstrating changes in the morphology of E. coli O157:H7 (strain 43895) treated with caffeine (a) Cf 0 g/L (b) Cf 3.0 g/L (c) Cf 4.0 g/L, and (d) Cf 5.0 g/L.
Figura 3. Gráficas de contorno mostrando cambios en la morfología de E. coli O157:H7 (cepa 43895) tratada con cafeína (a) Cf 0 g/L, (b) Cf 3.0 g/L, (c) Cf 4.0 g/L, y (d) Cf 5.0 g/L.
Discussion
Microbiological safety of liquid food including milk and juice has been of considerable concern in relation to foodborne pathogens, particularly E. coli O157:H7 (Baskaran, Amalaradjou, Hoagland, & Venkitanarayanan, Citation2010; Enache et al., Citation2011; Omiccioli et al., Citation2009). In this paper, we reported the antimicrobial activity of caffeine against E. coli O157:H7 in liquid media. Caffeine is a commonly used ingredient that not only imparts flavor, but has also been reported to have antimicrobial activity. Almeida et al. (Citation2006) indicated that caffeine, trigonelline, and protocatechuic acid are potential natural antimicrobial agents against enterobacteria, and therefore could be used in foods as a natural preservative to control their growth.
Caffeine is the most comprehensively studied natural ingredient found in food supplies, pharmacological preparations, and over-the-counter medicines (Nawrot et al., Citation2003). Caffeine is a food additive that has been used as a flavoring agent in foods and beverages for many years. Products such as tea, coffee, and cocoa are among the most widely consumed caffeine-based foods in the world. Depending on the serving size, the type of product, and preparation method, the amount of caffeine in these food products also varies. A typical 8-ounce cup of coffee and tea has 24–120 mg of caffeine; a 12-ounce soft drink of cola contains 30–60 mg of caffeine; and chocolate contains 1–35 mg of caffeine per ounce of serving (IFIC, 2008).
The American Medical Association, the US Food and Drug Administration (FDA) and numerous regulatory agencies have reported that consuming caffeine in moderate amount is safe (Heckman, Weil, & Gonzalez de Mejia, Citation2010). A review of the literature suggests that moderate caffeine consumption of 300 mg/day is safe and does not cause adverse health effects (IFIC, 2008; Knight, Knight, Mitchell, & Zepp, Citation2004). Nawrot et al. (Citation2003) reported that daily caffeine consumption at levels up to 400–450 mg/day was likewise not associated with any adverse effects. Moreover, caffeine provides a pleasant taste and aroma and is a popular stimulant in most developed countries (Almeida et al., Citation2006; IFIC, 2008). However, some potential negative health effects including those related to heart disease, hydration, addiction, fertility, pregnancy, miscarriage, and consumption by children/kids and teens still persist (IFIC, 2008). Many products that claim to boost energy due to the presence of large quantities of caffeine are gaining popularity, especially among young people. More recently, new beverages such as energy drinks with high-caffeine content have been developed. These energy drinks are heavily marketed to young people, and are quickly becoming the leading alternative to soft drinks. At the same time, consumption of milk by younger people is decreasing. Since several milk-containing products such as chocolate and pudding contain caffeine, caffeinated milk could also gain popularity among young people who might be more inclined to buy milk if it were caffeinated.
Our results showed significant inhibition of tested strains in the presence of caffeine in both laboratory medium and in skim milk. These results are in agreement with those of Ibrahim et al. (Citation2006), who reported significant growth inhibition of E. coli O157:H7 with concentration levels of 0.50% and higher. The antimicrobial effect of Cf at 2.0 mg/mL against enterobacteria (E. coli and S. enterica) has been reported by Almeida et al. (Citation2006). Authors have demonstrated that the concentrations of caffeine found in coffee extracts are enough to warrant 50% of the antimicrobial effect against Salmonella enteric. In another study, Cf at 10 g/L has shown to inhibit the growth of E. coli DH5α and Psedomonas fluorescens 5443 in the culture medium. However, in our study a significant growth inhibition was obtained at 5.0 g/L in liquid and skim milk samples. Similarly, 1 Log reduction of Salmonella Typhimurium and Salmonella Enteritidis was observed in raw ground chicken breast meat with coffee filtrate, 0.93 mg glyoxal, and 1 mg caffeine/g chicken (Maletta & Were, Citation2012). In vitro antimicrobial activity of caffeine was evaluated against several bacterial strains including Escherichia coli, Pseudomonas aeruginosa, pseudomonas fluorescens, and Staphylococcus aureus (Kascatan-Nebioglu et al., Citation2006; Ramanaviciene et al., Citation2003). Thus, the addition of caffeine as a natural antimicrobial to milk, juice, and various beverages has potential, as supported by these studies showing antimicrobial activities on pathogens.
In previous studies, the antimicrobial effect of tea extracts was evaluated against several foodborne pathogens including E. coli O157:H7 in a laboratory medium and in various foods, especially liquid foods (Kim & Fung, Citation2004; Kim, Ruengwilysup, & Fung, Citation2004). However, after reviewing the literature, we did not find adequate information on the use of caffeine in food systems as an antimicrobial agent. There are several flavored milk products in the market. It is known that many flavored ingredients contain caffeine. Since, milk is one of the most balanced nutritious foods and a highly perishable commodity, we selected milk as our food model to determine the effect of caffeine against E. coli O157:H7. In our earlier study (Ibrahim, Salameh, & Lloyd, Citation2001), we found that E. coli O157:H7 survived well in skim milk. The presence of fat globules and other nutrients in high-fat milk could have interfered with the ability of pathogen to grow during food processing.
Bacterial survival and death can be observed with traditional culture methods. However, these culture and colony count methods can be time-consuming. Recently, flow cytometry has become a powerful tool in microbiology that allows the treatment effect on various foodborne pathogens to be detected more rapidly. In addition, flow cytometry allows for the determination of morphological changes and investigation of the mechanisms of antimicrobial action in foodborne pathogens. Flow cytometry can be used to evaluate the effects of antimicrobial compound on microbial viability, vitality, and used to determine the mechanisms leading to cell damage (Álvarez-Barrientos, Arroyo, Cantón, Nombela, & Sánchez-pérez, Citation2000). We observed changes in the integrity of the bacterial cell after treatment with different concentrations of caffeine. Our findings are consistent with the morphological changes of ampicillin-treated E. coli detected by flow cytometry previously reported (Gant, Warnes, Phillips, & Savidge, Citation1993). We speculate that the mode of action of caffeine involves the inhibition of cell wall synthesis leading to bacterial filamentation, which could be an indication of the initial process of cell lysis. More studies in this area are underway. Dash & Gummadi (Citation2008) also observed changes in cell morphology in E. coli and various bacterial strains in the presence of caffeine that could bring about cell disintegration. Similarly Sandlie, Solberg, & Kleppe (Citation1980) and Esimone et al. (Citation2008) have shown that caffeine inhibits the synthesis of DNA and impairs RNA and protein synthesis. One possible explanation for this effect of caffeine could be the inhibition of bacterial cell wall synthesis by the influx of caffeine into the cells and ultimately damage to the DNA that could lead to the lysis of cells.
Conclusion
In our study, our results revealed that caffeine has antibacterial effect against E. coli O157:H7 in liquid medium and skim milk. Prominent morphological change was observed for E. coli O157:H7, which formed enlarged cells when treated with caffeine as compared to the original size of the control sample. Caffeine at 5.0 g/L caused inhibition of E. coli O157:H7 population by an average of 5.0 log CFU ml−1 in 48 h of incubation in liquid medium. Similarly, 3.42 log suppression of E. coli O157:H7 was obtained in milk sample within 12 h of incubation when compared to the control sample. Further research using flow cytometry is required to determine how caffeine leads to the inhibition of growth of E. coli O157:H7. We expect low concentration of caffeine in combination with other natural ingredients could produce synergistic antimicrobial activity. Moreover, additional studies are also required to test the antimicrobial activity of caffeine in different food models as well as its impact on the sensory characteristics of foods. Application of caffeine as a flavoring agent in food products could be useful in controlling pathogenic bacteria as well as supporting physically active lifestyle.
Acknowledgment
This publication was made possible by grant number NC.X-267-5-12-170-1 from the National Institute of Food and Agriculture and its contents are solely the responsibility of the authors and do not necessarily reflect the official view of the National Institute of Food and Agriculture.
References
- Ackers, M. L., Mahon, B. E., Leahy, E., Goode, B., Damrow, T., Hayes, P. S., … Hutwagner, L. (1998). An outbreak of Escherichia coli O157: H7 infections associated with leaf lettuce consumption. Journal of Infectious Disease, 177, 1588–1593.
- Ackers, M. L., Schoenfeld, S., Markman, J., Smith, M. G., Nicholson, M. A., Dewitt, W., … Slutsker, L. (2000). An outbreak of Yersinia enterocolitica O: 8 infections associated with pasteurized milk. Journal of Infectious Disease, 181, 1834–1837. doi:10.1086/315436
- Almeida, A. A. P., Farah, A., Silva, D. A. M., Nunan, E. A., & Glória, M. B. A. (2006). Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. Journal of Agricultural and Food Chemistry, 54, 8738–8743. doi:10.1021/jf0617317
- Álvarez-Barrientos, A., Arroyo, J., Cantón, R., Nombela, C., & Sánchez-pérez, M. (2000). Applications of flow cytometry to clinical microbiology. Clinical Microbiology Reviews, 13, 167–195. doi:10.1128/CMR.13.2.167-195.2000
- Armstrong, G. L., Hollingsworth, J., & Morris Jr, J. G. (1996). Emerging foodborne pathogens: Escherichia coli O157: H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiologic Review, 18, 29–51.
- Arora, D. S., Kaur, G. J., & Kaur, H. (2009). Antibacterial activity of tea and coffee: Their extracts and preparations. International Journal of Food Properties, 12(2), 286–294. doi:10.1080/10942910701675928
- Baskaran, S. A., Amalaradjou, M. A. R., Hoagland, T., & Venkitanarayanan, K. (2010). Inactivation of Escherichia coli O157:H7 in apple juice and apple cider by trans-cinnamaldehyde. International Journal of Food Microbiology, 141, 26–129. doi:10.1016/j.ijfoodmicro.2010.04.002
- Daglia, M., Cuzzoni, M. T., & Dacarro, C. (1994). Antibacterial activity of coffee: Relationship between biological activity and chemical markers. Journal of Agricultural and Food Chemistry, 42, 2273–2277. doi:10.1021/jf00046a036
- Dash, S. S., & Gummadi, S. N. (2008). Inhibitory effect of caffeine on growth of various bacterial strains. Research Journal of Microbiology, 3, 457–465.
- Doyle, M. P. (1991). Escherichia coli O157: H7 and its significance in foods. International Journal of Food Microbiology, 12, 289–301. doi:10.1016/j.bbr.2011.03.031.
- Enache, E., Mathusa, E. C., Elliott, P. H., Black, D. G., Chen, Y., Scott, V. N., & Schaffner, D. W. (2011). Thermal resistance parameters for Shiga toxin-producing Escherichia coli in apple juice. Journal of Food Protection, 74, 1231–1237. doi:10.4315/0362-028X.JFP-10-488
- Esimone, C., Okoye, F., Nworu, C., & Agubata, C. (2008). In vitro interaction between caffeine and some penicillin antibiotics against Staphylococcus aureus. Tropical Journal of Pharmaceutical Research, 27, 969–974.
- Gant, V. A., Warnes, G., Phillips, I., & Savidge, G. F. (1993). The application of flow cytometry to the study of bacterial responses to antibiotics. Journal of Medical Microbiology, 39, 147–154. doi:10.1099/00222615-39-2-14
- Gyawali, R., & Ibrahim, S. A. (2012). Impact of plant derivatives on the growth of foodborne pathogens and the functionality of probiotics. Applied Microbiology and Biotechnology, 95, 29–45. doi:10.1007/s00253-012-4117-x
- Hancock, D., Besser, T., Rice, D., Herriott, D., & Tarr, P. (1997). A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiology and Infection, 118, 193–195.
- Heckman, M. A., Weil, J., & Gonzalez de Mejia, E. (2010). Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. Journal of Food Science, 75, R77–R87. doi:10.1111/j.1750-3841.2010.01561.x
- Ibrahim, S. A., Salameh, M. M., & Lloyd, T. A. (2001). Growth and survival of Escherichia coli O157:H7 in solids fortified skim milk stored at low temperatures. Food Protection Trends (Formerly Dairy, Food and Environmental Sanitation), 21, 897–900.
- Ibrahim, S., Salameh, M., Phetsomphou, S., Yang, H., & Seo, C. (2006). Application of caffeine, 1, 3, 7-trimethylxanthine, to control Escherichia coli O157: H7. Food Chemistry, 99, 645–650. doi:10.1016/j.foodchem.2005.08.026
- IFIC. (2008). Caffeine & health: Clarifying the controversies. Washington, DC: International Food Information Council Foundation, 1–16.
- Kascatan-Nebioglu, A., Melaiye, A., Hindi, K., Durmus, S., Panzner, M. J., Hogue, L. A., … Crosby, S. D. (2006). Synthesis from caffeine of a mixed N-heterocyclic carbene-silver acetate complex active against resistant respiratory pathogens. Journal of Medicinal Chemistry, 49, 6811–6818. doi:10.1021/jm060711t
- Kim, S., & Fung, D. (2004). Antibacterial effect of water-soluble arrowroot (Puerariae radix) tea extracts on foodborne pathogens in ground beef and mushroom soup. Journal of Food Protection, 67, 1953–1956.
- Kim, S., Ruengwilysup, C., & Fung, D. (2004). Antibacterial effect of water-soluble tea extracts on foodborne pathogens in laboratory medium and in a food model. Journal of Food Protection, 67, 2608–2612.
- Knight, C. A., Knight, I., Mitchell, D. C., & Zepp, J. E. (2004). Beverage caffeine intake in US consumers and subpopulations of interest: Estimates from the Share of Intake Panel survey. Food and chemical toxicology, 42, 1923–1930. doi:10.1016/j.fct.2004.05.002
- Maletta, A. B., & Were, L. M. (2012). Effect of coffee filtrate,methylglyoxal, glyoxal, and caffeine on Salmonella Typhimurium and S. Enteritidis survival in ground chicken breasts. Journal of Food Science, 77(2), M135-M141. doi:10.1111/j.1750-3841.2011.02554.x
- Mao, Y., Doyle, M. P., & Chen, J. (2001). Insertion mutagenesis of wca reduces acid and heat tolerance of enterohemorrhagic Escherichia coli O157: H7. Journal of bacteriology, 183, 3811–3815. doi:10.1128/JB.183.12.3811-3815.2001
- Mazumdar, S., Hartmann, M., Kämpfer, P., & Keusgen, M. (2007). Rapid method for detection of Salmonella in milk by surface plasmon resonance (SPR). Biosensensors and Bioelectronics, 22, 2040–2046. doi:10.1016/j. bios.2006.09.004
- Nawrot, P., Jordan, S., Eastwood, J., Rotstein, J., Hughenholtz, A., & Feeley, M. (2003). Effects of caffeine on human health. Food Additives and Contaminants, 20, 1–30. doi:10.1080/0265203021000007840
- Olsen, S. J., Ying, M., Davis, M. F., Deasy, M., Holland, B., Iampietro, L., … Gormley, B. (2004). Multidrug-resistant Salmonella Typhimurium infection from milk contaminated after pasteurization. Emerging Infectious Diseases, 10, 932–935. doi:10.3201/eid1005.030484
- Omiccioli, E., Amagliani, G., Brandi, G., & Magnani, M. (2009). A new platform for Real-Time PCR detection of Salmonella spp., Listeria monocytogenes and Escherichia coli O157 in milk. Food Microbiology, 26, 615–622. doi:10.1016/j.fm.2009.04.008
- Proctor, M. E., & Davis, J. P. (2000). Escherichia coli O157: H7 infections in Wisconsin, 1992–1999. Wisconsin Medical Journal, 99, 32–37.
- Ramanaviciene, A., Mostovojus, V., Bachmotova, I., & Ramanavicius, A. (2003). Anti-bacterial effect of caffeine on Escherichia coli and Pseudomonas fluorescens. Acta Medica Lituanica, 10, 185–188.
- Riley, L. W., Remis, R. S., Helgerson, S. D., Mcgee, H. B., Wells, J. G., Davis, B. R., … Hargrett, N. T. (1983). Hemorrhagic colitis associated with a rare Escherichia coli serotype. New England Journal of Medicine, 308, 681–685.
- Ross, I. V. A., Griffiths, M. W., Mittal, G. S., & Deeth, H. C. (2003). Combining nonthermal technologies to control foodborne microorganisms. International Journal of Food Microbiology, 89, 125–138. doi:10.1016/S0168-1605(03)00161-2
- Sandlie, I., Solberg, K., & Kleppe, K. (1980). The effect of caffeine on cell growth and metabolism of thymidine in Escherichia coli. Mutation research, 73, 29–41.
