Abstract
Extraction of polyphenols of Laurus nobilis L. (Lauraceae), Coriandrum sativum L. (Apiaceae) and Amaranthus hybridus L. (Amaranthaceae) was carried out by solid–liquid extraction. The effect of ethanol concentration and the extraction time were determined to obtain the maximum of polyphenols. Three different methods, DPPH radical scavenging, ABTS radical cation scavenging and lipid oxidation inhibition, were tested to determine the antioxidant capacity in the extracts. The results indicate that L. nobilis has a high antioxidant potential: 94.73%, 47.71% and 76.86% for DPPH, ABTS and lipid oxidation inhibition, respectively. On the other hand, C. sativum and A. hybridus showed 13.69% and 10.16% in DPPH assay, and 9.22% and 14.96% in ABTS assay, while it did not show any antioxidant capacity in the lipid oxidation inhibition method under the experimental conditions. In addition, the phenolic compounds in the extracts were also characterized by High Performance Liquid Chromatography (HPLC) analysis.
La extracción de polifenoles de Laurus nobilis L. (Lauraceae), Coriandrum sativum L. (Apiaceae) y Amaranthus hybridus L. (Amaranthaceae) se llevó a cabo en una extracción sólido-líquido. El efecto de la concentración de etanol y el tiempo de extracción fueron evaluados para obtener el máximo de polifenoles. Tres diferentes métodos incluyendo el radical de barrido DPPH, radical catión de barrido ABTS y la inhibición de la oxidación lipídica fueron probados para determinar la capacidad antioxidante en los extractos. Los resultados indican que L. nobilis presentó mayor potencial antioxidante con un 94,73, 47,71 y 76,86% para DPPH, ABTS e inhibición de la oxidación lipídica respectivamente. Por otra parte, C. sativum y A. hybridus mostraron un 13,69 and 10,16% en el ensayo de DPPH, 9,22 y 14,96% en el ensayo de ABTS mientras que para la inhibición de la oxidación lipídica no tuvieron capacidad antioxidante bajo las condiciones probadas. Además, la caracterización de compuestos fenólicos en los extractos también fue estudiada por el análisis de Cromatografía Líquida de Alta Resolución (HPLC).
Palabras claves:
Introduction
Oxidation of lipid components in food due to the chain reaction of lipid peroxidation is a major problem for food manufacturers (Conforti, Statti, Uzunov, & Menichini, Citation2006). Food industries use synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), propyl gallate and tert-butylhydroquinone (t-BHQ) to overcome this problem. However, it is important to remember that these compounds are highly toxic and can have negative effects on human health (Santoyo, Lloria, Jaime, & Ibañez, Citation2006).
In this regard, scientific research has been carried out to identify biologically active molecules of natural origin, such as those from plants and vegetables. These molecules can be used as natural preservatives in food or as chemotherapeutic agents in order to prevent chronic degenerative diseases caused by the use of synthetic antioxidants or presence of free radicals in the body (Chattopadhyay & Bhattacharyya, Citation2007).
The extracts of many herbs and spices have become popular in recent years and attempts to characterize their bioactive principles have gained momentum for food applications as natural antioxidants (Shan, Cai, Brooks, & Corke, Citation2007). Polyphenols have been shown to exhibit antioxidant properties in vitro, and they also play a major role for their antimicrobial effects (Wang, Sun, Cao, Tian, & Li, Citation2008). A few studies have shown that the functional properties of some of the plant extracts is mainly due to the presence of phenolic compounds. Muñiz et al. (Citation2013) evaluated the antioxidant activity of Laurus nobilis extracts, obtaining more than 90% of antioxidant activity. Rahiman, Tantry, and Kumar (Citation2013) reported a high phenolic content in Coriandrum sativum extracts. On the other hand, the antioxidant capacity and phenolic content of Amaranthus hybridus have also been confirmed in previous studies (Chipurura, Muchuweti, Parawira, & Kasiyamhuru, Citation2011; Nana, Hilou, Millogo, & Nacoulma, Citation2012). The present study was aimed to define the optimum conditions of ethanol concentration and time for heat-reflux extraction of polyphenols from three spices, L. nobilis, C. sativum and A. hybridus, and to evaluate the respective antioxidant capacity. The phenolic profile of ethanolic extracts of these plants was also determined.
Materials and methods
Plant material
All plants were collected in the period from November 2010 to April 2011. Laurus nobilis and C. sativum were purchased from a local market in Saltillo city, Coahuila, Mexico, and A. hybridus was obtained from a local market in Valles city, San Luis Potosi, Mexico. Plant material was prepared according to a previously described methodology (Castillo et al., Citation2010; Osorio et al., Citation2010). Briefly, the plants were dried; ground and sieved through 20–30 meshes. Afterwards the samples were stored at room temperature in hermetically sealed black plastic bags under darkness to avoid possible oxidation of the compounds.
Chemical reagents
The chemicals and standards used were 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), 2,2-azino-bis 3 ethyl benzothiazoline-6-sulfuric acid (ABTS), linoleic acid, Folin–Ciocalteu reagent, pyrogallol, gallic acid, resorcinol, chlorogenic acid, methyl gallate, coumaric acid, catechin, 2-hydroxycinnamic acid, ellagic acid, quercetin and cinnamic acid; these were purchased from Sigma Aldrich (St. Louis, MO, USA).
Heat-reflux extraction (HRE)
The extraction was carried out under heat-reflux in a water bath maintained at 60°C using Felisa model Neslab RTE-111 cooler; ethanol was used as solvent at a solid liquid ratio of 1:4 (w/v). Extraction time and solvent concentration were determined in this study since earlier reports (Martins, Aguilar, De la Garza, Mussatto, & Teixeira, Citation2010) suggested that these parameters play an important role in the efficiency of the extraction of bioactive compounds.
Ten gram of each plant material was placed in an Erlenmeyer flask and covered with aluminium foil to avoid light exposure, added with 40 mL of ethanol (0%, 35% and 70% ethanol) and extraction was carried out for different durations (0, 2, 4, 6 and 8 h). The obtained extracts were passed through gauze, then filtered using Whatman No. 41 filter paper and centrifuged at 3500 rpm for 10 min (HERMLE Z 232 MKII). The filtered extracts were dehydrated at 60°C for 48 hours and stored in a refrigerator at 4°C. Three replications were maintained for all treatments. The dried extracts were re-suspended in water (1 mg of dried extract/L) for posterior studies.
Determination of total phenols content
The total phenols content in the obtained extracts of L. nobilis, C. sativum and A. hybridus was determined using Folin–Ciocalteu reagent by slightly modifying the procedure previously described (Makkar, Citation1999; Oliveira et al., Citation2008). Briefly, 800 µL of sample was mixed with 800 µL of Folin–Ciocalteu phenol reagent. After 5 min, 800 µL of Na2CO3 0.01 M and 5 mL of distilled water were added to the mixture. The absorbance was measured at 790 nm in a spectrophotometer (Varian 50 Bio). The amount of total phenolic compounds was expressed as mg gallic acid equivalents (GAE)/g of plant material using a regression equation that was obtained using gallic acid calibration curve (y = 0.0054x + 0.0383, r2 = 0.995). Extracts from each of the spices were taken for further studies based on the high phenolic content obtained.
Antioxidant capacity
DPPH radical scavenging activity
The free radical scavenging activity of plant extracts was measured by DPPH (Molyneux, Citation2004). Briefly, a 60 μM solution of DPPH in methanol was prepared and 2950 μL of this solution was added to 50 μL of extract solution in water (1000 mg/L). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then absorbance was measured at 517 nm (Varian 50 Bio spectrophotometer). Lower absorbance values of the reaction mixture indicated higher free radical scavenging capacity. The capability to scavenge the DPPH radical was calculated using the following equation:
ABTS radical cation scavenging activity
The antioxidant activity by ABTS radical cation scavenging was determined according to methodology previously described (Kumar, Mishra, Dubey, & Tripathi, Citation2007; Re et al., Citation1999). ABTS radical was freshly prepared by adding 5 mL of 2.45 mM potassium persulphate solution to 5 mL of a 7 mM ABTS solution and kept for 12 h in dark. These solutions were diluted with distilled water, later 50 μL of the extract was mixed with 1 mL of ABTS solution, homogenized in a vortex and the absorbance was recorded at 734 nm after 1 min by using a Varian 50 Bio UV-Vis spectrophotometer. ABTS–ethanol solution was used as blank. The total antioxidant capacity was calculated as the inhibition percentage of ABTS radical using the equation given above.
Lipid oxidation inhibition assay
The antioxidant capacity of all the plant extracts was determined based on the oxidation of linoleic acid (Martínez, Aguilera, Rodríguez, & Aguilar, Citation2011; Starzynska, Stodolak, & Jamroz, Citation2008). The linoleic acid solution was prepared by mixing 0.56 g of linoleic acid with 1.5 g of Tween 20 in 8 mL of 96% ethanol. For the assay, 50 μL of plant extract was mixed with 100 μL of linoleic acid and 1.5 mL of 0.02 M acetate buffer (pH 4.0), and homogenized in a vortex. The obtained emulsions were incubated at 37°C for 1 min, and added with 750 μL of 50 M FeCl2 solution (0.0994 g FeCl2 and 0.168 g EDTA diluted to 1 L with distilled water) to induce lipid oxidation. After 1 and 24 h, an aliquot of 250 μL of the reaction mixture was taken and 1 mL of 0.1 M NaOH in 10% ethanol was added to stop oxidation process; finally 2.5 mL of 10% ethanol was placed. The absorbance of samples was measured at 232 nm at their respective times of incubation (1 and 24 h); 50 μL of distilled water was used as control. Inhibition percentage of linoleic acid oxidation was calculated by the following equation:
where A is the difference between absorbance of control sample (distilled water) after 24 h and 1 h of incubation, and B is the difference between absorbance of each sample after 24 and 1 h of incubation.
High Performance Liquid Chromatography (HPLC) analysis
Identification of phenolic compounds in the selected extracts under the best extraction conditions was carried using Varian Pro-Star 330 HPLC fitted with DAD detector and by following the method described by Ruiz, Ascacio, Rodríguez, Morales, and Aguilar (Citation2011). The extracts selected in the previous step were filtered with a 0.22 µm nylon membrane and analysed using a Pursuit XRS C18 column, 5 µm (150 mm × 4.6 mm) at 30°C. The analytical conditions were: 10 µL injection volume, as the mobile phase was 3% acetic acid and acetonitrile and a flow rate of 1 mL/min during 25 min at a wavelength of 280 nm. Methanol was used as the washing phase.
Statistical analysis
Completely randomized design was employed with three replications for each case. Data were transformed with log x and analysed using analysis of variance (ANOVA) employing the software SAS V 9.0. Tukey (α = 0.5) was used for the multiple comparison tests.
Results and discussion
Total phenols content
Natural resources are an important source of potentially bioactive compounds for the development of new chemotherapeutic agents (AL-Saghir, Citation2009). There are many reports that state that parameters of extraction have a strong influence on the recovery of bioactive compounds (Xia et al., Citation2011).
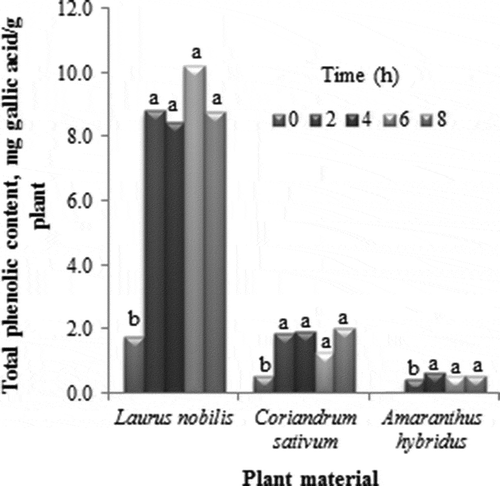
The concentration of total phenolic compounds present in the L. nobilis, C. sativum and A. hybridus extracts are shown in It can be observed that extraction time played an important role in obtaining bioactive compounds. Regardless of the plant material used, the highest yields of polyphenols were reported after 2 h of extraction and there was no significant difference in the yield for 8 h of extraction. However, a significant difference was observed among the yields recorded at time 0 (data not shown), where low values were observed in all cases. Our results are in agreement with the findings of Martins et al. (Citation2010) who studied the effects of longer extraction times for the recovery of nordihydroguaiaretic acid (NDGA) from Larrea tridentata by heat-reflux system. They reported that there was no significant difference between 1 and 3 h of extraction. This could be due to the fact that thermal extraction processes may accelerate rupture of cell membranes and affect the yield of polyphenols due to enhanced extraction of endocellular compounds that are soluble in the solvent (Ballard, Mallikarjunan, Zhou, & O´Keefe, Citation2010). Based on this report and our results, 2 h of extraction was chosen as the suitable time for obtaining the maximum yield of polyphenols from all the tested spices and this time period was also used in the evaluation of the second factor tested.
Figure 1. Effect of extraction time on the yield of phenolic compounds in the extracts of L. nobilis, C. sativum and A. hybridus.
Figura 1. Efecto del tiempo de extracción sobre el rendimiento de compuestos fenólicos en extractos de L. nobilis, C. sativum y A. hybridus.
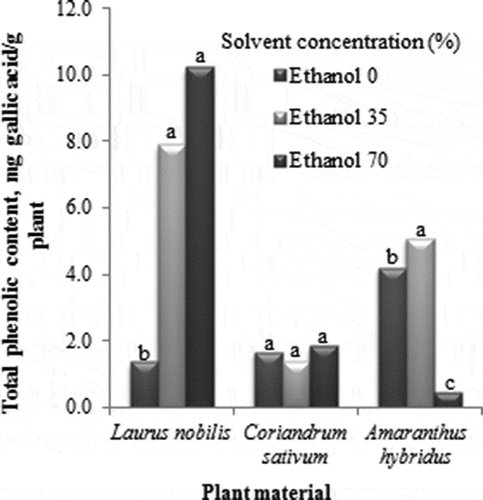
The second factor tested in this study was the concentration of the solvent on the efficiency of extraction. In general, aqueous alcohols and acetone have been widely used to extract phenolic components from plant materials, especially from herbs and spices. An extraction solvent system is generally selected according to the purpose of extraction, polarity of the interested components, polarity of undesirable components, the overall cost and safety (Wang et al., Citation2008). The effects of aqueous ethanol concentration on yields of phenolic compounds from L. nobilis, C. sativum and A. hybridus have been presented in In the three plants studied, a high yield was observed when an aqueous ethanol concentration of 35% was used. There was no significant difference (p < 0.05) in the efficiency of extraction between two different ethanol concentrations tested for L. nobilis and C. sativum. On the other hand, in the case of A. hybridus extract, it was observed that the yields of polyphenols from 0% and 70% aqueous ethanol were significantly lower than that from 35% ethanol. These results are in agreement with the data previously reported by Sultana, Anwar, and Ashraf (Citation2009), who studied the effect of different extracting solvents (absolute ethanol, absolute methanol, aqueous ethanol and aqueous methanol) on the extraction of phenolic compounds from medicinal plants and obtained higher yields with alcohol–water mixtures. As reported by Nana et al. (Citation2012), the phytochemical composition of A. hybridus indicated the presence of polyphenols and yields of 9.75, 8.30 and 7.75 GAE/100 mg were obtained in hydroacetonic, methanolic and aqueous extracts. Martins et al. (Citation2010) mentioned that addition of large amounts of water as part of the solvent resulted in enhancement of extraction efficiency, possibly due to increased swelling of plant material in the presence of water and by increasing contact surface between plant matrix and solvent. From the results obtained in this study, it can be observed that 35% aqueous ethanol concentration was suitable for the recovery of phenolic compounds from the three tested spices used in this study. Phenolic content from L. nobilis, C. sativum and A. hybridus extracts were 10.23, 1.38 and 5.08 mg of GAE/g plant material, respectively, under the best conditions of extraction.
Figure 2. Effect of ethanol concentration on the yield of phenolic compounds in the extracts of L. nobilis, C. sativum and A. hybridus.
Figura 2. Efecto de la concentración sobre el rendimiento de compuestos fenólicos en extractos de L. nobilis, C. sativum y A. hybridus.
Antioxidant capacity
Antioxidant capacity is widely used as a parameter for bioactive and functional components (Beyhan, Elmastas, & Gedikli, Citation2010). Due to differences among the large number of the systems available, the results of a single method can give only limited information about antioxidant properties of the extracts (Hayouni, Abedrabba, Bouix, & Hamdi, Citation2007). Hence in this study the antioxidant capacity of the selected extracts (2 h and aqueous ethanol concentration of 35%) by three different assays are given in along with polyphenols content of the extracts.
Table 1. Antioxidant capacity of three different plant extracts.
Tabla 1. Capacidad antioxidante de los tres diferentes extractos vegetales.
DPPH radical scavenging activity
Laurus nobilis extract recorded an effective antioxidant capacity, showing that the phenolic compounds present in this extract have a high degree of hydroxylation, which is manifested in the high capacity to donate protons and thus stabilize the DPPH radical. Elmastaş et al. (Citation2006) studied the antioxidant activity of ethanolic extracts of bay leaves (at 60 μg μL−1) and reported an inhibition of 92% of radical DPPH. This is consistent with the results reported by Muñiz et al. (Citation2013), who evaluated an antioxidant activity (94% inhibition DPPH radical) in L. nobilis extracts obtained by ultrasound. On the other hand, C. sativum and A. hybridus extracts showed only 13% and 10% of inhibition of the DPPH radical, respectively. Similarly, Wangensteen, Samuelsen, and Malterud (Citation2004) observed that the antiradical activity of extracts of coriander for the DPPH radical inhibition was only 15%. Rahiman et al. (Citation2013) studied the phenolic content in ethanolic extracts of C. sativum (70.24 mg/g) but did not find any correlation between the antioxidant activity and the phenolic content, which is in contrast with the report by Sulaiman, Sajak, Ooi, Supriatno, and Seow (Citation2011) who correlated the phenolic content with the antioxidant activity present in ethanolic extracts of C. sativum (r = 0.9998, p < 0.001); this variation probably could be influenced by environmental factors (Nana et al., Citation2012; Upadrasta, Mukhopadhyay, & Banerjee, Citation2011). On the other hand, Chipurura et al. (Citation2011) reported the activity of the antioxidant of A. hybridus and correlated this activity with the phenolic content present.
ABTS radical cation scavenging activity
It is interesting to note that in the case of both anti-radicals (DPPH and ABTS), L. nobilis extract recorded much higher antioxidant capacity (47%) than C. sativum and A. hybridus extracts (9% and 14%). The observed differences could be explained by the fact that the chemical composition of each plant is different and that is why different amounts of polyphenol compounds are present in the extracts of each plant. Özcan and Al Juhaimi (Citation2011) reported that the antioxidant activity of extracts depended on polyphenols, which may act as reductors.
Lipid oxidation inhibition assay
According to Huang, Ou, and Prior (Citation2005), use of linoleic acid as a lipid source in the LOI assay simulates the lipids present in a food system. In this study, lipid oxidation inhibition was determined in plant extracts. Like the other two previous assays, the maximum level of percentage of inhibition was exhibited by L. nobilis extract (76.8%), while no detectable activity was observed for the extracts of C. sativum and A. hybridus. These may be due to the low concentration of bioactive compounds in these plant extracts or presence of other molecules in these two spices that could be acting as pro-oxidants.
High Performance Liquid Chromatography (HPLC) analysis
HPLC analysis revealed the presence of four phenolic compounds in the L. nobilis extract: coumaric acid, gallic acid, pyrogallol and resorcinol (), which could be responsible for its high antioxidant activity. However, further studies more specific to each compound are necessary. Other authors have confirmed the presence of other phenolic compounds in the extracts of L. nobilis. Muchuweti et al. (Citation2007) reported the presence of cafeic, vanillic and ferulic acids in bay leaves extracts, while Lu, Yuan, Zeng, and Chen (Citation2011) confirmed the presence of rutinin and unknown phenolic acids. Literature demonstrated that environmental factors influence the variations between plants (Nana et al., Citation2012; Upadrasta et al., Citation2011). In the case of C. sativum and A. hybridus extracts, no phenolic compounds were detected under the conditions tested, which could be related to the low concentration as evident from low antioxidant activity recorded for these extracts.
Table 2. Phenolic profile of L. nobilis extract obtained by HPLC analysis.
Tabla 2. Perfil fenólico por análisis de HPLC del extracto de L. nobilis.
Conclusions
The results of the present study have revealed that 2 h of extraction by reflux-heat using aqueous ethanol at 35% concentration achieved high yields of polyphenols from L. nobilis, C. sativum and A. hybridus. It was observed that the antioxidant capacity of L. nobilis extract was higher than that of C. sativum and A. hybridus extracts. HPLC analysis revealed the presence of four phenolic compounds in L. nobilis, whereas no phenolic compounds were identified in C. sativum and A. hybridus extracts under the experimental conditions of this study. Finally, the phenolic compounds could be isolated from L. nobilis extracts due to their application as natural antioxidant compounds, which is important in view of the increasing use of synthetic antioxidants in the food industry.
Acknowledgements
D.B. Muñiz-Márquez thanks the Mexican Council for Science and Technology (CONACYT) for the graduate scholarship (No. 247493).
References
- AL-Saghir, M. G. (2009). Antibaterial assay of Cinnamomum cassia (Nees and Th. Nees) Ness ex Blume Bark and Thymus vulgaris L. leaf extracts against five pathogens. Journal of Biological Sciences, 9, 280–282.
- Ballard, S. T., Mallikarjunan, P., Zhou, K., & O´Keefe, S. (2010). Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chemistry, 120, 1185–1192.
- Beyhan, O., Elmastas, M., & Gedikli, F. (2010). Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae). Journal of Medicine Plants Reserch, 4, 1065–1070.
- Castillo, F., Hernández, D., Gallegos, G., Méndez, M., Rodríguez, R., Reyes, A., & Aguilar, C. N. (2010). In vitro antifungical activity of plant extracts obtained with alternative organic solvents against Rhizoctonia solani Kϋhn. Industrial Crops and Products, 32, 324–328.
- Chattopadhyay, R. R., & Bhattacharyya, S. K. (2007). Herbal spices as alternative antimicrobial food preservatives: An update. Pharmacognosy Reviews, 1, 239–247.
- Chipurura, B., Muchuweti, M., Parawira, W., & Kasiyamhuru, A. (2011). An assessment of the phenolic content, composition and antioxidant capacity of Bidens Pilosa, Cleome Gynandra, Corchorus olitorius, Galinsoga Parviflora and Amarantus hybridus. Acta Horticulturae, 11, 417–426.
- Conforti, F., Statti, G., Uzunov, D., & Menichini, F. (2006). Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp. piperitum (Ucria) Coutinho seeds. Biological & Pharmaceutical Bulletin, 29, 2056–2064.
- Elmastas, M., Gülcin, I., Isildak, Ö., Küfrevioglu, Ö. I., Ibaoglu, K., & Aboul-Enein, H. Y. (2006). Radical scavenging activity and antioxidant capacity of bay leaf extracts. Journal of the Iranian Chemical Society, 3, 258–266.
- Hayouni, E., Abedrabba, A. M., Bouix, M., & Hamdi, M. (2007). The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunistan Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chemistry, 105, 1126–1134.
- Huang, D., Ou, B., & Prior, R. L. (2005). The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry, 53, 1841–1856.
- Kumar, R., Mishra, A. K., Dubey, N. K., & Tripathi, Y. B. (2007). Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiflatoxigenic and antioxidant activity. International Journal of Food Microbiology, 115, 159–164.
- Lu, M., Yuan, B., Zeng, M., & Chen, J. (2011). Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Research International, 44, 530–536.
- Makkar, H. (1999). Quantification of tannins in tree foliage. A laboratory manual for the FAO/IAEA.
- Martínez, C. G., Aguilera, A. F., Rodríguez, R., & Aguilar, C. N. (2011). Fungal enhancement of the antioxidant properties of grape waste. Annals of Microbiology, 62, 922–930.
- Martins, S., Aguilar, C. N., De la Garza, I., Mussatto, S., & Teixeira, J. A. (2010). Kinetic study of nordihydroguaiaretic acid recovery from Larrea tridentata by microwave-assisted extraction. Journal of Chemical Technology and Biotechnology, 85, 1142–1147.
- Molyneux, P. (2004). The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin. Journal of Science and Technology, 26, 211–219.
- Muchuweti, M., Kativu, E., Mupure, C. H., Chidewe, C., Ndhlala, A. R., & Benhura, M. A. N. (2007). Phenolic composition and antioxidant properties of some spices. American Journal Food and Technology, 5, 414–420.
- Muñiz, D. B., Martínez, G. C., Wong, J. E., Belmares, R., Rodríguez, R., & Aguilar, C. N. (2013). Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrasonics Sonochemistry, 20, 1149–1154.
- Nana, F. W., Hilou, A., Millogo, J. F., & Nacoulma, O. G. (2012). Phytochemical composition, antioxidant and xanthine oxidase inhibitory activities of Amaranthus cruentus L. and Amaranthus hybridus L. extracts. Pharmaceuticals, 5, 613–628.
- Oliveira, I., Sousa, A., Ferreira, I., Bento, A., Estevinho, L., & Pereira, J. A. (2008). Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food and chemical toxicology, 46, 2326–2331.
- Osorio, E., Flores, M., Hernández, D., Ventura, J., Rodríguez, R., & Aguilar, C. N. (2010). Biological efficiency of polyphenolic extract from pecan nuts Shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Industrial Crops and Products, 31, 153–157.
- Özcan, M. M., & Al Juhaimi, F. Y. (2011). Antioxidant and antifungal activity of some aromatic plant extracts. Journal of Medicinal Plants Research, 5(1361), 2011).
- Rahiman, S., Tantry, B. A., & Kumar, A. (2013). Variation of antioxidant activity and phenolic content of some common home remedies with storage time. African Journal of Traditional Complementary and Alternative Medicines, 10, 124–127.
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237.
- Ruiz, J., Ascacio, J. A., Rodríguez, R., Morales, D., & Aguilar, C. N. (2011). Phytochemical screening of extracts from some Mexican plants used in traditional medicine. Journal of Medicinal Plants Research, 5, 2791–2797.
- Santoyo, S., Lloria, R., Jaime, L., & Ibañez, E. (2006). Supercritical fluid extraction of antioxidant and antimicrobial compounds from Laurus nobilis L. chemical and functional characterization. European Food Research and Technology, 222, 565–571.
- Shan, B., Cai, Y. Z., Brooks, J. D., & Corke, H. (2007). The in vitro antibacterial activity of dietary spice and medicinal herb extracts. International Journal of Food Microbiology, 117, 112–119.
- Starzynska, A., Stodolak, B., & Jamroz, M. (2008). Antioxidant properties of extracts from fermented and cooked seeds of polish cultivars of Lathyrus sativus. Food Chemistry, 109, 285–292.
- Sulaiman, S. F., Sajak, A. A., Ooi, K. L., Supriatno, & Seow, E. M. (2011). Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. Journal of Food Composition and Analysis, 24, 506–515.
- Sultana, B., Anwar, F., & Ashraf, M. (2009). Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules, 14, 2167–2180.
- Upadrasta, L., Mukhopadhyay, M., & Banerjee, R. (2011). Chemistry and biotechnology of polyphenols. Tannins: Chemistry, biological properties and biodegradation. Kerala, India: CIBET Publishers.
- Wang, J., Sun, B., Cao, Y., Tian, Y., & Li, X. (2008). Optimization of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chemistry, 106, 804–810.
- Wangensteen, H., Samuelsen, A. B., & Malterud, K. E. (2004). Antioxidant activity in extracts from coriander. Food Chemistry, 88, 293–297.
- Xia, E. Q., Ai, X. X., Zang, S. Y., Guan, T. T., Xu, X. R., & Li, H. B. (2011). Ultrasound-assisted extraction of phillyrin from Forsythia suspense. Ultrasonics Sonochemistry, 18, 549–552.
