Abstract
Information looking at the interaction mechanism of chitosan–gelatin is scarce. The objective of this work was to analyze the interactions between chitosan and bovine (BG) and salmon gelatins using a chemometrics approach based on the multivariate statistical analysis, partial least square regression (PLSR), with data collected from amino acid profiling and differential scanning calorimetry.
Gelatins showed differences in imino acid profile (higher amount of proline and hydroxyproline in BG). Chitosan modified the microstructure of films as indicated by variations in glass transition temperature (Tg), heat capacity (∆Cp), melting temperature (Tm), and enthalpy (∆H), in partially crystalline and amorphous films due to the effect of chitosan on gelatin chain renaturation and moisture content. PLSR showed that thermal properties of gelatin–chitosan films were affected by presence of amino acids Cysteine, Tyrosine, Valine, Arginine, Hydroxyproline, and Glycine, which would modify the orientation of side chains of polymer, while charged amino acids would encourage gelatin renaturation rather than gelatin–chitosan interaction.
Existe poca información en torno al mecanismo de interacción entre el quitosano y la gelatina. El presente estudio tuvo como objetivo analizar las interacciones existentes entre el quitosano y las gelatinas de bovino (BG) y de salmón (SG), empleando para ello un enfoque de quimiometría basado en el análisis estadístico multivariante (regresión del mínimo cuadrático parcial, PLSR) y utilizando los datos recabados por medio del perfilado de aminoácidos y de la calorimetría diferencial de barrido. Se observó que las gelatinas mostraron diferencias en el perfil de aminoácidos detectándose concentraciones más elevadas de Pro y Hyp en BG. Al mismo tiempo, debido al efecto del quitosano en la renaturalización de la cadena de gelatina y del contenido de humedad, éste modificó la microestructura de las películas, lo cual fue indicado por las variaciones de la temperatura de transición vítrea (Tg), de los cambios en la capacidad calorífica (∆Cp), de la temperatura de fusión (Tm) y cambios de entalpía, en las películas parcialmente cristalinas y amorfas. La PLSR demostró que las propiedades térmicas de las películas gelatina-quitosano fueron afectadas por la presencia de los aminoácidos cisteína, tirosina, valina, arginina, hidroxiprolina y glicina, que modificaron la orientación de las cadenas laterales de polímeros, a la vez que los aminoácidos con carga favorecían la renaturalización de la gelatina en vez de la interacción gelatina-quitosano.
1. Introduction
Gelatin is a fibrillar protein derived from collagen by partial thermal hydrolysis under acidic or alkaline conditions (Ahmad & Benjakul, Citation2011). It is a very versatile hydrocolloid widely used in different industries due to its well-recognized gelling and viscoelastic properties based on its helix to coil transition. In the food industry, gelatin has been utilized in confectionery, low-fat spreads, dairy and meat products, whereas in pharmaceutical and biomedical applications gelatin has been used as a matrix for implants, in manufacturing of hard and soft capsules, as plasma expanders, in wound care, as injectable drug delivery microspheres, and in intravenous infusions (Gómez-Guillén et al., Citation2009; Karim & Bhat, Citation2009, Citation2008). Novel applications of gelatin are related with its use as scaffolds building in tissue engineering mainly in combination with other polymers such as chitosan and hyaluronic acid (Acevedo et al., Citation2013; Enrione et al., Citation2013).
Gelatin has a specific amino acid profile, which determines many of its physical properties and how it interacts with water and other polymers. Gelatin is characterized by the presence of the amino acid glycine (Gly) every third residue generating a repetitive pattern (Gly–X–Y)n (Bella, Brodsky, & Berman, Citation1995; Brodsky & Ramshaw, Citation1997). The X and Y positions can accommodate any amino acid, but to form a stable triple-helix configuration, a significant fraction of these positions (about 20%) requires imino acids to stabilize the extended nature of the individual chains (Persikov, Ramshaw, Kirkpatrick, & Brodsky, Citation2000). Indeed, X and Y positions are most frequently occupied by the imino acids proline (Pro) and hydroxyproline (Hyp). This special pattern determines the secondary structure of gelatin as polyprolyne II (PPII) conformation, where all residues in the X and Y positions are highly exposed to the solvent, making the helical structure well suited for interacting with other molecules and for self-association (Berman, Kramer, Bella, Mayville, & Brodsky, Citation1999).
Differences in the content of imino acids Pro and Hyp have been reported between gelatins from different origins, which generate marked differences in their rheological properties (Haug, Draget, & Smidsrød, Citation2004; Leuenberger, Citation1991). Gelatin from mammalian origin (e.g. bovine or pig) shows higher amount of imino acids than gelatin from marine origin (Haug et al., Citation2004; Joly-Duhamel, Hellio, & Djabourov, Citation2002; Karim & Bhat, Citation2009). This would explain differences in thermal properties such as helix-to-coil transition temperature (or melting temperature (Tm)) and glass transition temperature, which are generally higher in mammal gelatins (Díaz, López, Matiacevich, Osorio, & Enrione, Citation2011; Joly-Duhamel et al., Citation2002). Also differences in imino acid content may explain differences observed in values of gel strength (higher Bloom strength in mammal gelatins) as suggested by several studies reported in the literature (Eysturskarð, Haug, Ulset, & Draget, Citation2009; Gómez-Estaca, Montero, Fernández-Martín, & Gómez-Guillén, Citation2009). A higher Bloom strength reflects a higher level of protein renaturation (Bigi, Panzavolta, & Rubini, Citation2004; Eysturskarð et al., Citation2009).
On the other hand, demand for fish gelatin has increased significantly in communities with religious or cultural considerations as an alternative source of mammal gelatin (Chiou et al., Citation2008).
Chitosan is a natural polymer widely distributed in nature. Moreover, it is considered the second most abundant polymer after cellulose (Jayakumar, Nwe, Tokura, & Tamura, Citation2007; Tharanathan & Kittur, Citation2003). Chitosan is obtained after partial N-acetylation of chitin, the main component of outer skeleton of insects and crustacean shells (Tharanathan & Kittur, Citation2003; Vishu Kumar, Varadaraj, Gowda, & Tharanathan, Citation2005). Despite significant antimicrobial activity of chitosan toward various fungi, gram-positive, and gram-negative bacteria (Goy, de Britto, & Assis, Citation2009; No, Meyers, Prinyawiwatkul, & Xu, Citation2007; Rabea, Badawy, Stevens, Smagghe, & Steurbaut, Citation2003), it uses in food are still uncommon due to its low solubility in water, requiring deacetylation and low pH conditions using organic acids (e.g. acetic acid, lactic acid, malic acid, etc.) for solubilization (Park, Marsh, & Rhim, Citation2002; Rabea et al., Citation2003). Commercial chitosan is available with 85% or more deacetylated units and molecular weights between 100 and 1000 kDa (Park et al., Citation2002). Although there is not a specific standard value to classify the molecular weight of chitosan, normally is accepted that Mw < 50 kDa is considered low, 50 < Mw < 150 kDa is medium and Mw > 150 kDa is considered high (Goy et al., Citation2009).
Along with the well-known antimicrobial activity, chitosan has been widely used to improve the mechanical properties of polymeric materials used in different industrial applications. For instance, Chen, Embree, Brown, Taylor, and Payne (Citation2003) studied the transglutaminase catalysis of a gelatin–chitosan gel, showing that gels obtained by this method were stronger if the reaction occurred in the presence of chitosan. Baruch and Machluf (Citation2006) were able to produce microcapsules of chitosan–alginate for the encapsulation of mammalian cells with an extremely high mechanical resistance. Nada, El-Sakhawy, Kamel, Eid, and Adel (Citation2006) showed that chitosan enhanced the strength properties of paper sheets before and after an aging step at high temperature. More recently de Moura et al. (Citation2009) studied how chitosan/tripolyphosphate nanoparticles affected the mechanical properties of hydroxypropyl methylcellulose (HPMC) films, concluding that the incorporation of chitosan nanoparticles improved significantly their mechanical and film barrier properties, due to the tendency of chitosan nanoparticles to occupy empty or free spaces in the HPMC matrix.
Although a number of studies are available looking at the mechanical or physical properties of gelatin–chitosan-based systems, little information is available looking at the interaction mechanism between both the biopolymers. Taravel and Domard (Citation1995, Citation1993) studied the interaction between collagen and chitosan by Fourier transform infrared spectroscopy (FTIR) and circular dichroism, concluding that interactions are produced by electrostatic and hydrogen bonds. In a later work, Sionkowska, Wisniewski, Skopinska, Kennedy, and Wess (Citation2004) characterized the molecular interaction between collagen and chitosan using viscometry, wide angle x-ray, and FTIR showing that chitosan alters the helical structure of collagen by the formation of a hydrogen bonding network, which increased the viscosity of the blend and produced a loss of helical structure encouraging the presence of a “gelatin” like phase. More recently, Gómez-Estaca, Gómez-Guillén, Fernández-Martín, and Montero (Citation2011) determined that interactions between gelatin and chitosan were stronger in blends with fish gelatin compared to those with bovine gelatin (BG), which was explained by the lower imino acid content in marine gelatins. Imino acids Pro and Hyp act in the formation of junction zones during the gelling mechanism (Ledward, Citation1986); hence, lower imino acids content in fish gelatin would lead to a less extensive self-aggregation of the polymer chains, which would facilitate gelatin–chitosan interactions (Gómez-Estaca et al., Citation2011). Chitosan would also interfere in the ability of gelatin to refold into triple-helix chains on cooling (Gómez-Estaca et al., Citation2011). Indeed Arvanitoyannis, Nakayama, and Aiba (Citation1998) showed a decrease in the renaturation fraction of gelatin when chitosan content increased.
Although significant effects of chitosan on gelatin’s molecular configuration have been established, information related to possible driving mechanisms for these interactions is scarce. The characterization of interactions that take place in a gelatin–chitosan mixture might define the structural organization at sub-nano scale, and determine some relevant functional properties at meso- and macroscale such as mechanical and physical properties of the system (e.g. increase in modulus and changes in water diffusivity). Moreover, a better understanding of polymer–polymer interaction could also be important in the structural stability of system during storage. All these properties are crucial and should be considered when any biopolymer mixture is designed to be used as protectant for functional compounds (microencapsulation) or for controlled release systems, both applications highly relevant in food and pharmaceutical industries.
This putative study aims to provide new insights into the interactions mechanisms between chitosan and gelatin from two different origins (bovine and salmon) by applying a multivariable statistical analysis such as partial least square regression (PLSR) with data collected from amino acid profiling and differential scanning calorimetry (DSC), as part of exploratory study about modulation of physical properties and structural stability in biopolymer mixtures and its theoretical prediction by statistical tools.
2. Materials and methods
2.1. Gelatin and chitosan
In this study, gelatin samples from two different origins were used. BG corresponded to a commercial product (Rousselot, Brazil; type B, Bloom 220) extracted from hide and bones and salmon gelatin (SG) extracted from the skin of Salmo salar species (kindly supplied by Salmon Oil Chile) by an acid-alkaline method following the protocol proposed by Zhou and Regenstein (Citation2005). The salmon skins were chopped and immersed in a 0.1 mol/l NaOH solution at 10°C for 1 h with constant agitation; then immersed in a 0.05 mol/l acetic acid solution for 1 h at 10°C with constant agitation in order to eliminate impurities. The skins were then submerged again in a 0.1 mol/l NaOH solution at 10°C for 1 h with constant agitation. The gelatin extraction was carried out at 64°C and pH ~4.0, for 3.5 h adjusting the pH with a 0.05 mol/l acetic acid solution. The supernatant liquid was vacuum filtered and dried at 50°C for 24–48 h. The gelatin obtained was ground, hermetically sealed and stored at ambient temperature (20°C) until further use.
The chitosan (β(1–4)-2-amine-2-deoxy-D-glucopyranose) purchased from Sigma-Aldrich (USA) used in this study was of low molecular weight (~50 kDa) and with an acetylation degree of 92%.
2.2. Biochemical characterization
2.2.1. Proximal analysis
For both gelatins and chitosan powders, a composition analysis was carried out following the well-established methodologies (AOAC, Citation1995). Moisture content (MC) was determined gravimetrically by oven drying at 105°C for 24 h. Crude fat content (CFC) was determined by solvent extraction using the Soxhlet method, protein content (PC) was assessed by the Kjeldhal methodology (conversion factor for gelatin 5.55 (Rivero, García, & Pinotti, Citation2009)), whilst total ash content (AC) was determined gravimetrically by a furnace at 550°C. The carbohydrate content (CC) was determined as following: CC = 100 – MC – CFC – PC – AC in line with the recommendation given by INFOODS (http://www.fao.org/infoods/infoods/standards-guidelines/food-component-identifiers-tagnames/en/).
2.2.2. Amino acid profile
The amino acid profile of each gelatin was determined by high performance liquid chromatography (HPLC) following the methodology described by Janssen et al. (Citation1986). Ten milligram of sample was hydrolyzed for 24 h with 0.25 N HCL at 110°C. The hydrolysate obtained was derivatized with 20 μL of phenyltiocyanate at 10% to generate phenylthiocarbamil amino acids, which were separated and quantified by HPLC at a wavelength of 254 nm. A HPLC (Waters 600 Controller, USA) with diode array detector (Waters 996) and Phenomenex Luna RP 18 column (150 × 4.6 mm) with particle size of 5 µm was used in this study. Gradient separation was performed using two solvents: (A) an anhydrous sodium acetate solution 0.14 mol/l (pH 5.9)/acetonitrile in proportion 94:6 (v/v) and (B) HPLC-grade acetonitrile/water solution in proportion 60:40 (v/v). The injection volume was 20 μl, the column temperature was 40°C, and the analysis time was 30 minutes. Amino acid quantification was carried out using external standards of each amino acid (Sigma-Aldrich, Germany).
2.3. Films preparation
Gelatin–chitosan films were prepared by cold casting. Suspensions of gelatin with different fractions of chitosan were prepared using distilled water as solvent. Chitosan was previously dissolved using acetic acid (glacial, Merck, Germany) in a proportion 1:1. The solid fraction in all suspensions was kept constant at 70 g/l, thus compositions of film forming solutions were 70 g/l gelatin–0 g/l chitosan, 65 g/l gelatin–5 g/l chitosan, and 60 g/l gelatin–10 g/l chitosan. All suspensions were kept at 50°C for 1 h under constant agitation (~500 rpm) and then poured onto rectangular Teflon flat containers and maintained at 5 ± 1°C and 65 ± 5% equilibrium relative humidity (ERH) until films with uniform thickness of 0.25 ± 0.1 mm were formed. Films were shaped to dimensions of 85 mm in length and 9 mm in width and maintained under ~0% ERH using P2O5 for 1 week. Then, the films were conditioned under ~54% ERH using a saturated salt solution (MgNO3) at 20°C until an equilibrium was achieved (difference between two consecutives weight determinations in 24 h lower than 0.5%). The MC of the equilibrated films was determined gravimetrically following the method explained in Section 2.2.1.
2.4. Thermal properties
Thermal properties of the films were analyzed by DSC (Pyris Diamond DSC, Perkin Elmer, USA). Approximately, 18 mg of each sample were hermetically sealed in aluminum pans of 30 μL. The method was: heating from −10°C to 90°C at 10°C/min, holding at that temperature for 5 min, cooling from 90°C to −10°C at 40°C/min, holding at that temperature for 5 min and reheating to 90°C at 10°C/min. Prior the measurements, the DSC was calibrated using indium (Tm = 156.6 ± 1.56°C, ΔH = 28.45 ± 1 J/g). An empty pan was used as reference.
The Tm was determined as the onset of the endothermic transition observed on the first heating scan. The energy of this transition was determined as the change in enthalpy (∆H), defined as the area under the curve. The glass transition temperature (Tg) was determined as the midpoint of the change in heat capacity observed on the first heating scan (partially crystalline sample) and on second heating scan (amorphous sample). The difference in heat capacity (∆Cp) associated to each transition is also reported. All samples were analyzed in triplicate.
2.5. Chemometric modeling
In order to explore putative factor interactions between the factors called “amino acids” (18 amino acids measured, see ) and the factor called “chitosan” and the effects of these factor interactions on changes in thermal properties a chemometrics approach was used.
The factor interaction between amino acidic profile from each gelatin and chitosan, and changes in thermal properties were modeled defining two variable matrices (X and Y).
The X matrix was defined as the factor interaction between amino acids and chitosan. Each Xj variable is calculated as the product (multiplication) of a “j” amino acid concentration and a chitosan concentration in the film system. This is repeated for all amino acids, obtaining one Xj variable per each amino acid. Since two chitosan concentrations (5 g/l and 10 g/l) and two types of gelatin (BG and SG) were used, we calculated four data per each Xj variable. Besides, we used and additional data to represent the system without interaction, calculated of the film without chitosan (0 g/l chitosan). Finally, we obtained five data per each variable.
The Y matrix was defined as the absolute change in thermal properties produced by chitosan addition in the film. Thus, the thermal property value of gelatin film (BG or SG) without chitosan is subtracted of the thermal property value of gelatin–chitosan film. This is repeated for all thermal properties, obtaining one Yi variable per each property.
The X and Y variables were modeled by PLSR. The PLSR allows establish a system to represent Y variables as a function of X variables, Y = BX (where B is a matrix containing the “bij” coefficients):
The significant “bij” coefficients (p < 0.10) were used to represent the dependence between X and Y variables. Nonsignificant coefficients were considered zero. Importance of the X variables was represented by mean of variable importance on projection (VIP) and shown as a VIP plot. The calculations were done using the software SIMCA-P (UMETRICS v12, Sweden) (Eriksson et al., Citation2006).
2.6. Statistical analysis
Two-way analysis of variance (ANOVA) was used to evaluate the effect of the factors type of gelatin (bovine and salmon), chitosan amount and interaction between the two over the value of each thermal property obtained from the DSC.
3. Results and discussion
3.1. Biochemical characterization
3.1.1. Proximal analysis
The composition of BG, SG, and chitosan are presented in . The PC was significantly higher in BG compared to SG (98.2% and 93.6% db, respectively). Fat and AC were higher in SG possibly due to differences in the extraction and purification methods used for commercial gelatins. Rahman, Al-Saidi, and Guizani (Citation2008) reported a higher level of protein (~88% and ~78%) but lower levels of fat (~1% and ~5%) and ash (~1% and ~8%) in commercial BG compared with gelatin extracted from tuna skin, respectively.
Table 1. Proximal analysis results of BG, SG, and chitosan. In all samples the variation coefficient (cv) was lower than 5%.
Tabla 1. Resultados del análisis proximal de BG, SG y quitosano. El coeficiente de variación (cv) fue menos de 5% en todas las muestras.
Chitosan composition showed a high level of carbohydrates (~99%, db). Also 6.62% of nitrogen was obtained in chitosan samples, corresponding to nitrogen of amine groups present in the chemical structure of the polymer.
3.1.2. Amino acid profile
The amino acid profile of BG and SG is presented in . As expected, the amino acids present in higher proportion were Gly, Pro, and Hyp due to the repeating pattern of gelatin mentioned previously. Also evident is the higher amount of the imino acids Pro and Hyp in BG compared to SG as widely reported in the literature (Gómez-Estaca et al., Citation2009; Haug et al., Citation2004; Joly-Duhamel et al., Citation2002). Joly-Duhamel et al. (Citation2002) reported Pro content of 130.3 g/kg of protein and Hyp content of 122.3 g/kg of protein in BG, whereas Arnesen and Gildberg (Citation2007) reported 106.0 g/kg of protein of Pro and 60.0 g/kg of protein of Hyp in gelatin extracted from Atlantic salmon (S. salar). These authors obtained higher concentration of imino acids than the values reported in this work probably due to differences in the gelatin extraction process, based on differences in concentration of acidic or alkaline solutions, temperature, and time used. Chiou et al. (Citation2006) and Gómez-Guillén et al. (Citation2009) also reported higher levels of Pro and Hyp in porcine and BGs (imino acid content 20% and 50% higher, respectively) compared to gelatins from marine sources. In our study, the content of Pro and Hyp was ~10% and ~53% higher in BG compared to SG, respectively. Also there is a fraction of charged amino acids (e.g. arginine, glutamic acid, aspartic acid, and lysine) in both gelatins, where the concentration of these amino acids was higher in BG than SG (~27% and ~21% of total amino acids, respectively). The differences observed in imino acids content and charged residues content between the two gelatin types was compensated by higher glycine content in SG (~340 g/kg). Interestingly, the amino acids, tryptophan, asparagine, and glutamine, were not detected in this study, suggesting that their concentrations were below the detection limit of the HPLC unit.
Table 2. Amino acid profile in gelatin of different origin. In all samples the variation coefficient (cv) was lower than 5%.
Tabla 2. Perfil de aminoácido en gelatina de distinto origen. El coeficiente de variación (cv) fue menos de 5% en todas las muestras.
3.2. Thermal properties
Thermal properties of gelatin–chitosan films determined by DSC analysis are presented in . The statistical significance of gelatin type, chitosan concentration and their correspondent interactions (gelatin and chitosan) on the thermal properties are presented in . The first temperature scan shows a semi-crystalline material, with significant differences (p < 0.10) in the parameters Tg, ΔCp, and ΔH between BG and SG (). The differences in these parameters have been explained in terms of the molecular weight of the biopolymer. Indeed, previous works have shown that higher molecular weight of BG produced higher values of Tg and Tm (Rahman et al., Citation2008). The lower Tg of BG compared to the Tg value of SG was related to the differences in MC between the two samples (Arnesen & Gildberg, Citation2007; Rahman et al., Citation2008). The results also show significant differences in ΔCp values (p < 10%) () at similar MC (p > 10%), which would indicate differences in the structural configuration of both materials. In fact, lower ΔCp value at Tg has been attributed to higher chain rigidity due to limited number of conformations and molecular weight (McGonigle, Cowie, Arrighi, & Pethrick, Citation2005).
Table 3. Thermal properties determined by DSC in films of BG and SG as a function of chitosan content. Values in bracket correspond to standard deviation between replicates.
Tabla 3. Propiedades térmicas determinadas por DSC en películas de BG y de SG, como función del contenido de quitosano. Los valores en corchetes corresponden a la desviación estándar entre réplicas.
Table 4. p-Value calculated by using a two-way ANOVA test.
Tabla 4. Valor-p calculado mediante una prueba ANOVA de clasificación doble.
These experimental data from thermal analysis can be correlated to the well-known differences in mechanical properties and gelling temperature of mammalian gelatins compared to those obtained from cold water fish, which has been explained by differences in triple-helix formation kinetics (Joly-Duhamel et al., Citation2002). The latter is also relevant to the differences in ΔH between both gelatins observed in this study ( and ). Joly-Duhamel et al. (Citation2002) using optical rotation, determined that triple-helix formation kinetics was dependent on gelatin molecular weight. They observed differences of ~40% in triple-helix content for gelatins with molecular weights varying from 147.7 × 103 g/mol (BG) to 74.6 × 103 g/mol (cod skin gelatin) at the same reference temperature. Therefore, a higher content of helical structure would require greater denaturation energy, which would yield greater values of ΔH reported by DSC (Bigi et al., Citation2004; Joly-Duhamel et al., Citation2002).
Regarding the presence of chitosan in the films, our results show it had a significant effect on Tg, ∆Cp, Tm, and ∆H values ( and ). The difference (p < 0.10) observed in Tg values (1st scan) could be related to the hygroscopic nature of chitosan, which increased the MC in the films. As discussed previously, the differences in ∆Cp values were related to changes in structural configuration attributed to restrictive effects of chitosan during the refolding of gelatin chains (Arvanitoyannis et al., Citation1998; Gómez-Estaca et al., Citation2011). Our results also showed that the effect of chitosan on ∆Cp values (p < 0.10) was dependent on gelatin type, suggesting differences in how this biopolymer interacts with each gelatin, likely based in the differences observed in the amino acid profile of BG and SG (Section 3.1.2).
Variation of Tm of the films by the presence of chitosan was statistically significant (p < 0.10). Tm in BG films increased when chitosan concentration increase, while in SG the addition of chitosan decreased the Tm of the material. Similarly to the variations reported for Tg, this behavior can be explained by differences in MC. Indeed, in BG the presence of chitosan reduced the MC of the films but in in the case of SG, chitosan increased the MC with the consequent plasticizing effect of water (). Although statistical differences in melting ∆H were nonsignificant (p > 0.10) between both gelatins when chitosan was present, the values of ∆H were higher in BG–chitosan films compared to SG–chitosan films. Differences in ∆H would suggest differences in crystalline fraction and crystal morphology in the samples, which in our case could be closely related to the temperature of casting at which films were made (Staroszczyk, Pielichowska, Sztuka, Stangret, & Kołodziejska, Citation2012). Indeed, the casting temperature used (5 ± 1°C) was significantly lower than the gelation temperature of mammal gelatin but close to the gelation temperature of the SG used in this work (Joly-Duhamel et al., Citation2002). Casting of films carried out at temperatures above the gelation temperature would encourage a random coil configuration in gelatin (Staroszczyk et al., Citation2012) favoring lower crystallinity content in the polymeric matrix.
As discussed previously, the variations in the ΔCp values at Tg related to the energy transfer through the chitosan–gelatin network may suggest differences related to the complexity of the structure and possible differences in how chitosan interacts with each type of gelatin.
also presents the combined effect of the factors gelatin (independent of its origin) and chitosan on the thermal properties of films. As expected, if gelatin or chitosan have an effect on the values of ∆Cp and Tg, the combined effect of both also should produce significant effects on these thermal parameters. Interestingly in the case of ∆H values, this statement was not followed as the combined effect of both biopolymers did not produce differences in the value of ∆H (p > 10%), suggesting some sort of compensation effect between gelatin and chitosan.
Although differences observed in ∆Cp, Tg, and ∆H have been related to MC in hydrophilic biopolymers, two-way ANOVA analysis showed that neither gelatin and chitosan by themselves nor the combined effect of the two produced significant differences in MC of the films (). This behavior might suggest possible limitations of moisture determination methods (e.g. oven at 105°C for 24 h). This point is particularly important as small changes in MC in hydrophilic polymers can generate important variations in thermal properties of system as has been widely reported previously in the literature (Slade & Levine, Citation1995a, Citation1995b).
3.3. Effect of amino acid profile on gelatin–chitosan interaction
Interactions between specific amino acids from both gelatins and chitosan were studied using a chemometric analysis. X variables represent interaction between amino acids and chitosan and Y variables represent variations in the thermal properties by the addition of chitosan (see further description in Section 2.5). Results of this analysis are shown in .
Figure 1. Chemometric analysis of the gelatin–chitosan interaction. (A) Significant coefficients (p < 0.10) of the B matrix calculated by PLSR (coefficients are shown normalized to be visualized in the heat-map). (B) VIP-plot of the X variables (amino acidic interaction with chitosan) shown as heat-map.
Figura 1. Análisis quimiométrico de la interacción gelatina-quitosano. (A) Coeficientes significativos (p < 0,10) de la matriz B calculados por PLSR (los coeficientes aparecen normalizados, a fin de visualizarlos en el mapa de calor). (B) Ploteo VIP de las variables X (interacción de aminoácidos con quitosano) mostrado como mapa de calor.
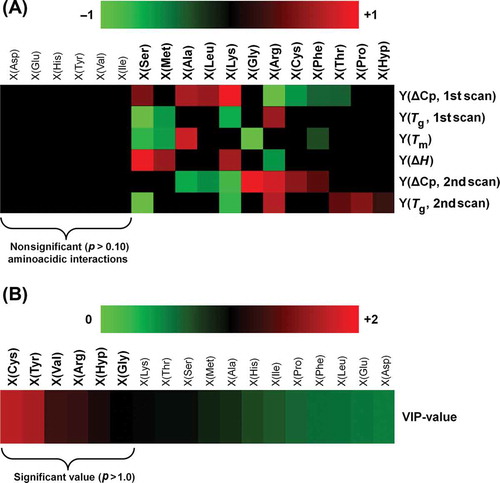
) depicts a heat-map showing the coefficients of the PLSR-based analysis (see further description in Section 2.5). Each coefficient represents the influence of X variables on Y variables. There are 12 amino acidic interactions having significant effect (p < 0.10) on the thermal properties. Interestingly, the interaction between chitosan and Gly had a significant effect on Tm and ΔCp (2nd scan), while the other interaction promoted by Pro and Hyp had an effect on Tg of the fully amorphous material (2nd scan). ) shows a VIP-plot, which indicates the importance of each X variables. The main VIP-values (larger than 1) correspond to amino acidic interaction produced by Cys, Tyr, Val, Arg, Hyp, and Gly.
This result is interesting because the analysis showed that amino acidic residues would interact with chitosan during the gelation process and casting stage. The analysis showed the importance of the residues Gly and Hyp, which was expected due to their role in the primary structure of protein and the high concentration present in both gelatins (). Hyp has an –OH group on the side chain of the molecule to interact with water (Bella et al., Citation1995; Brodsky & Ramshaw, Citation1997), whereas Gly due to its low molecular weight and high concentration is important in the orientation of polar or charged lateral groups of amino acids, which is important for linkages with specific zones of the chitosan molecule. Other residues such as Cys, Tyr, and Arg, all charged positively at pH ~ 7, participated in the interaction between the polymers despite their low concentration in the films (). However, other amino acids with ionizable groups on their side chain should also have had an important role in gelatin–chitosan interactions. For example acidic amino acids Asp and Glu, which were detected in high concentration () and are negatively charged at pH ~ 7 due to the pK of side chain 3.65 and 4.25, respectively, were not relevant in the interaction between the two polymers. This result is interesting considering that chitosan with a pKa of 6.5 on the amine groups is insoluble at neutral pH (Bowman & Leong, Citation2006), and normally the process of chitosan sample preparation includes the acidification of medium in order to promote its solubilization in water. Hence, under our sample preparation conditions the interaction gelatin–chitosan should have been promoted by Asp and Glu, but the chemometric analysis did not show that behavior.
Therefore, these results suggest that rather than concentration of certain amino acids in the protein chain, the interaction between gelatin and chitosan would be determined by how the side chain of the amino acidic residue is orientated to the external medium, the physicochemical properties of the molecule (e.g. charge density distribution across the molecule and isoelectric point) and conditioning pH.
Also at this point, the role of aliphatic residues (such as Val) could be important in encouraging the orientation of certain side chains by taking place in specifically positions along the chains of the polymer. On the other hand, in the case of the gelatin, the high amount of Asp and Glu could be relevant in the process of intramolecular renaturation and aggregation during the gelation and casting stage. Therefore, it is possible that these residues do not interact with chitosan during film formation, not having specific role in facilitating gelatin–chitosan interactions.
4. Conclusions
Chitosan significantly affected the microstructure of the films as indicated by thermal analysis, particularly the melting and glass transition temperatures (2nd scan) that were independent of the type of gelatin. Changes in thermal parameters of BG suggest an increase in thermal stability when chitosan is included, which could be related to variations in molecular mobility. The different trend observed for SG would indicate major interference by chitosan during protein refolding during cooling due to differences in molecular weight and amino acid profiling. In case of enthalpies associated to gelatin melting enthalpies, the decreasing was related to changes in crystal morphology.
The chemometric analysis, PLSR, used in this study allowed to us pinpoint specific amino acid residues from both gelatins that would interact preferentially with chitosan, generating a different microstructure in the film when the carbohydrate was present. In particular, the analysis showed that residues of Gly and Hyp influenced the interaction between gelatin and chitosan as was expected, due to the high concentration of Gly typical in gelatin and the polar characteristic of Hyp. Interestingly, PLSR also showed that the interaction between both the polymers was also driven by other amino acidic residues such as Cys, Tyr, Val, and Arg, which would encourage the orientation of certain side chains to the external medium. Additionally, the effect of the amino acids Asp and Glu would be limited to gelatin–gelatin interaction rather than in the gelatin–chitosan interaction.
Acknowledgements
Financial support given by PAI [grant number 79130039], Fondecyt [grant number1110607] and Fondecyt [grant Number1120166] from CONICYT-Chile is gratefully acknowledged.
References
- Acevedo, C., Somoza, R., Weinstein-Oppenheimer, C., Silva, S., Moreno, M., Sánchez, E., … Enrione, J. (2013). Improvement of human skin cell growth by radiation-induced modifications of a Ge/Ch/Ha scaffold. Bioprocess and Biosystems Engineering, 36, 317–324. doi:10.1007/s00449-012-0786-1
- Ahmad, M., & Benjakul, S. (2011). Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocolloids, 25, 381–388. doi:10.1016/j.foodhyd.2010.07.004
- AOAC. (1995). Official methods of analysis. Washington, DC: AOAC International.
- Arnesen, J. A., & Gildberg, A. (2007). Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresource Technology, 98, 53–57. doi:10.1016/j.biortech.2005.11.021
- Arvanitoyannis, I. S., Nakayama, A., & Aiba, S.-I. (1998). Chitosan and gelatin based edible films: State diagrams, mechanical and permeation properties. Carbohydrate Polymers, 37, 371–382. doi:10.1016/S0144-8617(98)00083-6
- Baruch, L., & Machluf, M. (2006). Alginate–chitosan complex coacervation for cell encapsulation: Effect on mechanical properties and on long-term viability. Biopolymers, 82, 570–579. doi:10.1002/bip.20509
- Bella, J., Brodsky, B., & Berman, H. M. (1995). Hydration structure of a collagen peptide. Structure, 3, 893–906. doi:10.1016/S0969-2126(01)00224-6
- Berman, H. M., Kramer, R. Z., Bella, J., Mayville, P., & Brodsky, B. (1999). Sequence dependent conformational variations of collagen triple-helical structure. Nature Structural Biology, 6, 454–457. doi:10.1038/8259
- Bigi, A., Panzavolta, S., & Rubini, K. (2004). Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials, 25, 5675–5680. doi:10.1016/j.biomaterials.2004.01.033
- Bowman, K., & Leong, K. W. (2006). Chitosan nanoparticles for oral drug and gene delivery. International Journal of Nanomedicine, 1, 117–128. doi:10.2147/nano.2006.1.2.117
- Brodsky, B., & Ramshaw, J. A. M. (1997). The collagen triple-helix structure. Matrix Biology, 15, 545–554. doi:10.1016/S0945-053X(97)90030-5
- Chen, T., Embree, H. D., Brown, E. M., Taylor, M. M., & Payne, G. F. (2003). Enzyme-catalyzed gel formation of gelatin and chitosan: Potential for in situ applications. Biomaterials, 24, 2831–2841. doi:10.1016/S0142-9612(03)00096-6
- Chiou, B.-S., Avena-Bustillos, R. J., Bechtel, P. J., Jafri, H., Narayan, R., Imam, S. H. … Orts, W. J. (2008). Cold water fish gelatin films: Effects of cross-linking on thermal, mechanical, barrier, and biodegradation properties. European Polymer Journal, 44, 3748–3753. doi:10.1016/j.eurpolymj.2008.08.011
- Chiou, B.-S., Avena-Bustillos, R. J., Shey, J., Yee, E., Bechtel, P. J., Imam, S. H. … Orts, W. J. (2006). Rheological and mechanical properties of cross-linked fish gelatins. Polymer, 47, 6379–6386. doi:10.1016/j.polymer.2006.07.004
- de Moura, M. R., Aouada, F. A., Avena-Bustillos, R. J., McHugh, T. H., Krochta, J. M., & Mattoso, L. H. C. (2009). Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. Journal of Food Engineering, 92, 448–453. doi:10.1016/j.jfoodeng.2008.12.015
- Díaz, P., López, D., Matiacevich, S., Osorio, F., & Enrione, J. (2011). State diagram of salmon (Salmo salar) gelatin films. Journal of the Science of Food and Agriculture, 91, 2558–2565. doi:10.1002/jsfa.4451
- Enrione, J., Díaz-Calderón, P., Weinstein-Oppenheimer, C., Sánchez, E., Fuentes, M., Brown, D., … Acevedo, C. (2013). Designing a gelatin/chitosan/hyaluronic acid biopolymer using a thermophysical approach for use in tissue engineering. Bioprocess and Biosystems Engineering, 36, 1947–1956. doi:10.1007/s00449-013-0971-x
- Eriksson, L., Johansson, E., Kettaneh-Wold, N., Trygg, J., Wikstrom, C., & Wold, S. (2006). Multi and megavariate data analysis. Part I: Basic principles and applications. Umea: Umetrics Academy.
- Eysturskarð, J., Haug, I. J., Ulset, A.-S., & Draget, K. I. (2009). Mechanical properties of mammalian and fish gelatins based on their weight average molecular weight and molecular weight distribution. Food Hydrocolloids, 23, 2315–2321. doi:10.1016/j.foodhyd.2009.06.007
- Gómez-Estaca, J., Gómez-Guillén, M. C., Fernández-Martín, F., & Montero, P. (2011). Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocolloids, 25, 1461–1469. doi:10.1016/j.foodhyd.2011.01.007
- Gómez-Estaca, J., Montero, P., Fernández-Martín, F., & Gómez-Guillén, M. C. (2009). Physico-chemical and film-forming properties of bovine-hide and tuna-skin gelatin: A comparative study. Journal of Food Engineering, 90, 480–486. doi:10.1016/j.jfoodeng.2008.07.022
- Gómez-Guillén, M. C., Pérez-Mateos, M., Gómez-Estaca, J., López-Caballero, E., Giménez, B., & Montero, P. (2009). Fish gelatin: A renewable material for developing active biodegradable films. Trends in Food Science & Technology, 20, 3–16. doi:10.1016/j.tifs.2008.10.002
- Goy, R. C., de Britto, D., & Assis, O. B. G. (2009). A review of the antimicrobial activity of chitosan. Polímeros: Ciencia E Tecnologia, 19, 241–247. doi:10.1590/S0104-14282009000300013
- Haug, I. J., Draget, K. I., & Smidsrød, O. (2004). Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocolloids, 18, 203–213. doi:10.1016/S0268-005X(03)00065-1
- Janssen, P. S. L., Nispen, J. W., Melgers, P. A. T. A., Bogaart, H. W. M., Hamelinck, R. L. A. E., & Goverde, B. C. (1986). HPLC analysis of phenylthiocarbamyl (PTC) amino acids. I. Evaluation and optimization of the procedure. Chromatographia, 22, 345–350. doi:10.1007/BF02268788
- Jayakumar, R., Nwe, N., Tokura, S., & Tamura, H. (2007). Sulfated chitin and chitosan as novel biomaterials. International Journal of Biological Macromolecules, 40, 175–181. doi:10.1016/j.ijbiomac.2006.06.021
- Joly-Duhamel, C., Hellio, D., & Djabourov, M. (2002). All gelatin networks: 1. biodiversity and physical chemistry. Langmuir, 18, 7208–7217. doi:10.1021/la020189n
- Karim, A. A., & Bhat, R. (2008). Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends in Food Science & Technology, 19, 644–656. doi:10.1016/j.tifs.2008.08.001
- Karim, A. A., & Bhat, R. (2009). Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids, 23, 563–576. doi:10.1016/j.foodhyd.2008.07.002
- Ledward, D. A. (1986). Gelation of gelatin. In J. R. Mitchell & D. A. Ledward (Eds.), Functional properties of food macromolecules (pp. 171–201). London: Elsevier Applied Science.
- Leuenberger, B. H. (1991). Investigation of viscosity and gelation properties of different mammalian and fish gelatins. Food Hydrocolloids, 5, 353–361. doi:10.1016/S0268-005X(09)80047-7
- McGonigle, E. A., Cowie, J. M. G., Arrighi, V., & Pethrick, R. A. (2005). Enthalpy relaxation and free volume changes in aged styrene copolymers containing a hydrogen bonding co-monomer. Journal of Materials Science, 40, 1869–1881. doi:10.1007/s10853-005-1206-6
- Nada, A. M. A., El-Sakhawy, M., Kamel, S., Eid, M. A. M., & Adel, A. M. (2006). Mechanical and electrical properties of paper sheets treated with chitosan and its derivatives. Carbohydrate Polymers, 63, 113–121. doi:10.1016/j.carbpol.2005.08.028
- No, H. K., Meyers, S. P., Prinyawiwatkul, W., & Xu, Z. (2007). Applications of chitosan for improvement of quality and shelf life of foods: A review. Journal of Food Science, 72, R87–R100. doi:10.1111/j.1750-3841.2007.00383.x
- Park, S. Y., Marsh, K. S., & Rhim, J. W. (2002). Characteristics of different molecular weight chitosan films affected by the type of organic solvents. Journal of Food Science, 67, 194–197. doi:10.1111/j.1365-2621.2002.tb11382.x
- Persikov, A. V., Ramshaw, J. A. M., Kirkpatrick, A., & Brodsky, B. (2000). Amino acid propensities for the collagen triple-helix. Biochemistry-US, 39, 14960–14967. doi:10.1021/bi001560d
- Rabea, E. I., Badawy, M. E. T., Stevens, C. V., Smagghe, G., & Steurbaut, W. (2003). Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules, 4, 1457–1465. doi:10.1021/bm034130m
- Rahman, M. S., Al-Saidi, G. S., & Guizani, N. (2008). Thermal characterisation of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin. Food Chemistry, 108, 472–481. doi:10.1016/j.foodchem.2007.10.079
- Rivero, S., García, M. A., & Pinotti, A. (2009). Composite and bi-layer films based on gelatin and chitosan. Journal of Food Engineering, 90, 531–539. doi:10.1016/j.jfoodeng.2008.07.021
- Sionkowska, A., Wisniewski, M., Skopinska, J., Kennedy, C. J., & Wess, T. J. (2004). Molecular interactions in collagen and chitosan blends. Biomaterials, 25, 795–801. doi:10.1016/S0142-9612(03)00595-7
- Slade, L., & Levine, H. (1995a). Glass transition and water-food structure interactions. In J. E. Kinsella (Ed.), Advances in food and nutrition research (Vol. 38, pp. 103–269). Academic Press.
- Slade, L., & Levine, H. (1995b). Water and the glass transition-dependence of the glass transition on composition and chemical structure: Special implications for flour functionality in cookie baking. Journal of Food Engineering, 24, 431–509. doi:10.1016/0260-8774(95)90766-5
- Staroszczyk, H., Pielichowska, J., Sztuka, K., Stangret, J., & Kołodziejska, I. (2012). Molecular and structural characteristics of cod gelatin films modified with EDC and tgase. Food Chemistry, 130, 335–343. doi:10.1016/j.foodchem.2011.07.047
- Taravel, M. N., & Domard, A. (1993). Relation between the physicochemical characteristics of collagen and its interactions with chitosan: I. Biomaterials, 14, 930–938. doi:10.1016/0142-9612(93)90135-O
- Taravel, M. N., & Domard, A. (1995). Collagen and its interaction with chitosan: II. Influence of the physicochemical characteristics of collagen. Biomaterials, 16, 865–871. doi:10.1016/0142-9612(95)94149-F
- Tharanathan, R. N., & Kittur, F. S. (2003). Chitin – the undisputed biomolecule of great potential. Critical Reviews in Food Science & Nutrition, 43, 61–87. doi:10.1080/10408690390826455
- Vishu Kumar, A. B., Varadaraj, M. C., Gowda, L. R., & Tharanathan, R. N. (2005). Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochemical Journal, 391, 167–175. doi:10.1042/BJ20050093
- Zhou, P., & Regenstein, J. M. (2005). Effects of alkaline and acid pretreatments on Alaska Pollock skin gelatin extraction. Journal of Food Science, 70, c392–c396. doi:10.1111/j.1365-2621.2005.tb11435.x
