Abstract
Starches’ rheological properties can be improved in association with other hydrocolloids in order to expand their industrial applications. Xyloglucan (XG) was mixed with starches and heated until complete starch gelatinization. Using the rheological tan delta (G”/G’) measurement and iodine-XG competition assay, an ideal proportion of 5:1 for interaction between high-amylose cornstarch and the XG was determined. The starch-amylose synergism was found to be related to the amount of amylose. For yam and high-amylose cornstarch, the G”/G’ declined from 0.075 to 0.073 (p < 0.05) and from 0.138 to 0.101 (p > 0.05), respectively, for the starch and hydrocolloid–starch mixture. For amylopectin-rich samples, cassava, normal, and waxy cornstarches, where the reaction is antagonistic, the G”/G increased from 0.390 to 0.865 (p < 0.05), 0.100 to 0.210 (p > 0.05), and 0.446 to 6.740 (p < 0.05), respectively, for starch and hydrocolloid–starch mixture. These synergistic interactions can be used by industries to manufacture products with the desired rheological properties.
Las propriedades reológicas del almidón se pueden mejorar mediante modificaciones químicas o asociación con otros hidrocoloides con el fin de ampliar sus aplicaciones industriales. Xiloglucano (XG) se mezcló y se caliento hasta la gelatinización completa del almidón con almidones. Uso de la tangente delta reológico (G “/G’) y el ensayo de competición de yodo -XG, se determinó una proporción ideal de 5:1 para la interacción entre el alto contenido de amilosa del almidón de maíz y la mezcla de hidrocoloides. El sinergismo se encontró estar relacionada con la cantidad de amilosa en el almidón. Para ñame y almidón de maíz de alta amilosa, el G”/G’ disminuyó 0,075-0,073 (p < 0,05), y desde 0,138 hasta 0,101 (p > 0,05), respectivamente, para la mezcla de almidón y almidón – hidrocoloide. Para muestras ricas de amilopectina, mandioca, almidones normales y de maíz cerosos, en donde la reacción es antagónico, el G”/G‘ aumentaron 0,390-0,865 (p < 0,05), 0,100 a 0,210 (p > 0,05) y desde 0,446 hasta 6,740 (p < 0,05), respectivamente, para el almidón y la mezcla de hidrocoloide – almidón. Estas interacciones sinérgicas pueden ser utilizados por las industrias para la fabricación de productos con las propiedades reológicas deseadas.
Keywords:
Palabras claves:
Introduction
Starches are the reserve carbohydrate present in plants and provide 70–80% of calories consumed worldwide by humans. They are deposited in the form of granules ranging from 1 up to 100 μm. Starches have an enormous number of uses in the food industry and in other areas, including adhesion, binding, film formation, gelling, and thickening (Pérez & Bertoft, Citation2010; Zobel & Stephen, Citation1995).
Some starch properties, such as the high viscosity and retrogradation of pastes and syneresis of gels, can be undesirable depending on the application. These can be controlled by chemical modifications or mixture with hydrocolloids to improve the product’s texture/rheology (for a complete review about hydrocolloid and starch mixtures, see BeMiller, Citation2011). However, there are very few reports about the interaction between starches and xyloglucan (XG).
Prabhanjan and Ali (Citation1995) found that mixing solutions of tamarind XG and normal cornstarch resulted in a high-viscosity paste, with increased pseudoplasticity and decreased apparent pasting temperature, and that carboxymethylation and hydroxypropylation of the XG diminished this interaction. However, Yoshimura, Takaya, and Nishinari (Citation1999) showed by rheological analysis the absence of synergistic interaction between tamarind XG and normal cornstarch, although the hydrocolloid decreased the retrogradation of the paste and syneresis of the gel.
Freitas, Gorin, Neves, and Sierakowski (Citation2003) studied XG interaction with normal cornstarch and found that apparently the interaction is dependent on the amylose content, since waxy starch presented an antagonistic interaction with XG, with phase separation in XG and amylopectin. Another important conclusion was that the absence of the interaction observed by Yoshimura et al. (Citation1999) could be related to the addition of normal cornstarch, with 270 g.kg−1 amylose and high amylopectin content, to hydrocolloid solutions.
Regarding the interaction between starch and XG, Onyechi, Judd, and Ellis (Citation1998) observed that the presence of flour made from Detarium senegalense, which is rich in an XG with high molecular weight, was the primary factor responsible for the reduction in the postprandial level of glucose and insulin in healthy human volunteers consuming a bread and stew meal. The mechanism is not clear, but at least two possibilities can be considered: either the starch interacts with XG, reducing the starch’s digestibility, or the XG causes the viscosity in the intestines to increase, reducing the enzyme diffusion and the postprandial level of glucose.
Our group has performed several studies with XGs, mainly those from the seeds of Hymenaea courbaril (Jatobá), obtained from different Brazilian locations, whose structure and properties are described in Freitas et al. (Citation2003), Freitas et al. (Citation2005), Lima et al. (Citation2003), Martin, Freitas, Obayashi, and Sierakowski (Citation2003), and Freitas, Busato, Mitchell, and Silveira (Citation2011).
We previously investigated the interactions between XG on the one hand and either high-amylose starch or waxy starch on the other and observed a synergistic interaction with the first and antagonistic interaction with the second (Freitas et al., Citation2003). The present study investigated (1) the effect of the amylose content in starches and mixtures of starches with XG, using the free amylose content in the mixtures as an indication of interaction; (2) whether XG interacts with amylose or amylopectin, determined by dynamic oscillatory rheological analysis; and (3) the nature of any interactions, determined by using atomic force microscopy (AFM).
Material and methods
Plant material
The XG, extracted from H. courbaril seeds, was obtained as described by Freitas et al. (Citation2005). The samples were selected in function of the level of amylose. To represent high-amylose starch samples, we used high-amylose cornstarch (Sigma S-4180, St. Louis, MO, USA) and yam starch (Dioscorea alata), donated by Dr. Maria Vitória E. Grossmann, Londrina State University, Paraná, Brazil, extracted and purified as described by Alves, Grossmann, and Silva (Citation1999). To represent high-amylopectin starches, we used common cornstarch (Sigma S-4126), waxy cornstarch (Sigma S-9679), and cassava starch (Manihot utilissima), obtained using the procedures of Willinger (Citation1964) and donated by Dr. José Domingos Fontana of Paraná Federal University.
Chemical analysis
Total carbohydrate was assayed by the phenol-H2SO4 method, as described by Dubois, Gilles, Hamilton, Rebers, and Smith (Citation1956), using a mixture of xylose, galactose, and glucose, in the molar ratio of 3:1:4, as standard for XG, determined in our previous studies as the usual molar ratio of carbohydrates from XG (Freitas et al., Citation2005), and only glucose for starches. Protein content was determined by the method of Hartree (Citation1972). Ash was measured by placing 2 g of each sample into a porcelain crucible and then placing it in a controlled furnace preheated to 600°C, keeping it at this temperature for 2 h, after which the sample was transferred to desiccator, cooled, and weighed immediately. Moisture was measured by placing 2 g of each sample and dried to constant weight at 100°C under pressure ≤100 mm Hg. Finally, total lipid contents were determined after diethyl ether extraction in a Soxhlet device. Ash, moisture, and total lipid were determined according to the Association of Official Analytical Chemistry (AOAC, Citation1995), methods 942.05, 925.01, and 920.39, respectively.
Amylose and amylopectin samples were prepared from the high-amylose cornstarch and waxy cornstarch, after autoclaving at 121°C/1 atm for 60 and 30 min, respectively. The samples were defatted, which was carried out by refluxing in petroleum ether for 5 h followed by drying at 100°C. Then, the amylose content was determined by the method based on Chrastil (Citation1987). Amylose and waxy amylopectin, after defatting and drying, were used as standards. For this, mixtures of gelatinized high-amylose cornstarch and high-amylopectin cornstarch were prepared containing 700, 500, 400, 200, and 0 g.kg−1 amylose. A straight line was obtained, and the free amylose content in the samples was determined.
The monosaccharide contents of XG were determined as described by Freitas et al. (Citation2005). Briefly, the monosaccharide contents of XGs were determined after partial acid hydrolysis with 720 g.kg−1 H2SO4 for 1 h on ice, and complete hydrolysis effected by dilution to 80 g.kg−1 and heating at 100°C for 4 h (Selvendran, March, & Ring, Citation1979). The solutions were neutralized with BaCO3 and filtered and the filtrate from each hydrolysis was reduced with NaBH4, followed by acetylation with pyridine–Ac2O (1:1 v/v) for 12 h at 25°C (Wolfrom & Thompson, Citation1963). The resulting alditol acetates were analyzed by GC-MS in a Varian Saturn 2000R mass spectrometer(Sugarland, TX, USA), using a DB-225 capillary column at 220°C with nitrogen as the carrier gas.
Free amylose content in the XG and starch mixture
The amylose interaction with tri-iodide ions was used to quantify the amylose content in mixtures with XG. To avoid interference of the XG–iodine interaction, for each proportional mixture of amylose and XG, we used a XG/iodide solution in the blank tube. Furthermore, the amylose cornstarch concentration was kept constant (50 g.L−1) and different XG concentrations were used (2, 4, 5, 10, 15, 20, and 25 g.L−1). The data from this experiment were used to calculate the mean and standard deviation of independent samples and analyzed in triplicate. We used XG in the blank tube, in concentrations from 2 to 25 g.L−1, to discount any interaction of XG with .
Average molar mass (Mw) and radius of giration (Rgw)
The dn/dc of the XG was determined with a Waters 2410 differential refractometer (Milford, MA, USA) at a wavelength of 546 nm, using concentrations of 1.0, 0.5, 0.25, and 0.125 g.L−1, after filtration through Millipore filter with 0.45 μm pore size.
Aqueous solutions of XG (0.5 g.L−1) were filtered through a Millipore filter (0.22 μm) and injected into a high pressure size exclusion chromatography (HPSEC) with 2000, 500, 250, and 120 Waters ultrahydrogel columns. Detection was carried out with a Waters 2410 differential refractometer and a light scattering multiangle at 632.8 nm DAWN DSP-F Wyatt technology model (Santa Barbara, CA, USA). The eluent used in this system was 0.1 mol.L−1 of sodium nitrite, containing 200 mg.L−1 of azide, at a flow rate of 0.6 mL.min−1.
The amylose and amylopectin were obtained after gelatinization using an autoclave and dried using freeze–drying process. After that, the samples were dispersed in dimethylsulfoxide (DMSO) to reduce chain aggregation, using concentrations from 0.1 to 1.0 g.L−1, in batch mode of light scattering, in order to obtain the Zimm plot (Equation (1)):
where K is defined as the optical constant, c is the concentration of the polymer, Rθ is the differential intensity of the scattering of polarized light by the intensity of the solution and solvent, and q is the scattering vector. The result was a double extrapolation to angle and concentration near zero, giving molecular mass (Mw), root mean square radius of gyration <S2>1/2 or Rg and second virial coefficient A2.
Oscillatory rheological analysis
For the high-amylose cornstarch sample and mixtures, the gels were obtained at 50 g.L−1 after heating at 121°C/0.1 MPa/60 min. For the other starch samples and mixtures, the parameters were 121°C/1 atm/30 min, until the complete gelatinization process, monitored using an optical microscope. After this, the samples were refrigerated at 5°C for 24 h before analysis. All experiments were carried out in triplicate. Rheometry was performed using an HAAKE oscillatory rheometer, model RS 75 (Karlsruhe, Baden-Württemberg, Germany), sensor C 60/2°, with a Peltier system to control the temperature. The strain used in the frequency sweep experiments (0.02–10 Hz) was appropriate for the viscoelastic-linear region in which the gel structure was preserved.
Using the above approach, it was possible to determine the dynamic storage shear modulus (G’) and the loss shear modulus (G”), and from this the G”/G’ ratio, called tan δ, was used here to describe the interactions. The synergistic interaction dependence between XG and amylose is presented here as tan δ at 1 Hz.
Adsorption of XG onto amylose or amylopectin films using AFM
A mixture of DMSO and water (60:40) was used and the high-amylose cornstarch or waxy cornstarch was dispersed at 1 mg.mL−1, with heating at 80°C during 2 h until complete gelatinization of the starch. Then, 50 μL of the sample was deposited onto gold crystals, using a spin-coating apparatus at 1500 rpm, 25°C, during 1 min, after which the crystals were washed three times with ultrapure water. The XG at 1 mg.mL−1 was added to the surface, using a flow cell, until constant signal of quartz microbalance (QCM), using water as solvent. Then, the gold crystals were washed with ultrapure water flow until constant mass, measured also using QCM. The thin film was dried in a desiccator for 24 h, using silica and under vacuum before AFM analysis.
AFM analyses were performed in air using an Agilent microscope (Agilent Technologies, Santa Clara, CA, USA) and Pico Image software (Agilent Technologies). Tapping mode images were obtained with Vistaprobes® (Nanoscience Instruments, Inc., Phoenix, AZ, USA) silicon tips (nominal spring constant of 48 N.m−1 and resonance frequency of 180 kHz) by scanning an area of 4.0 µm × 4.0 µm. The data were treated with the Gwyddion software (Czech Metrology Institute, Okružní, Brno, Czech Republic).
Statistical analysis
Analysis of variance (ANOVA) followed by the Tukey test to distinguish the amylose content and tan δ values were performed on Prism 6 software (Irvine, CA, USA), with significance of 0.05.
Results and discussion
Chemical analysis
The chemical composition of the polysaccharide samples investigated is shown in , where the XG sample had 850 g.kg−1 of carbohydrate, 29 g.kg−1 of protein, and 108 g.kg−1 of moisture. Its monosaccharide composition presented a molar ratio of 43:36:16:5 for Glc:Xyl:Gal:Ara, respectively, confirming the observation of Freitas et al. (Citation2005).
Table 1. Chemical composition of starches and XG samples.
Tabla 1. Composición química de los almidones y las muestras XG.
The determination of the amylose content in each sample was based on Chrastil (Citation1987), using a straight-line equation obtained by different proportions of defatted high-amylose cornstarch (700 g.kg−1 of amylose in the starch and 300 g.kg−1 of amylopectin in the starch) and high-amylopectin cornstarch mixtures (1000 g.kg−1 of amylopectin in the starch). The contents were 362 g.kg−1 of amylose in the starch and 230 g.kg−1 of amylose in the starch for the yam and cassava starch, respectively, and 275 g.kg−1 of amylose in the starch for the normal cornstarch, used as a control.
Molar mass determination
The XG weight average molar mass (Mw) and root mean square radius of gyration, determined by HPSEC–multiangle laser light scattering (MALLS) analysis, were 8.7 × 105 g.mol−1 and 60.2 nm, respectively. The intrinsic viscosity was determined by rheological analysis as 590 mL.g−1, using 0.1 mol.L−1 sodium nitrite containing 200 mg.L−1 of azide as solvent.
Zimm plot light scattering was used to determine the molar mass of starch samples, in a batch model, using DMSO as the solvent. The molar masses obtained were 1.29 × 106 g.mol−1 (high-amylose cornstarch) and 1.35 × 107 g.mol−1 (waxy cornstarch). The results with cornstarch were similar to those previously published by Han, Lim, and Lim (Citation2005), who found 1.50 × 106 g.mol−1 for amylose. Radosta, Haberer, and Vorwerg (Citation2001) found for potato amylose starch an average molar mass from 1 × 105 to 1 × 106 g.mol−1 and for potato starches, including the amylopectin, from 2.3 to 3.7 × 107 g.mol−1. The authors also used DMSO as solvent because it partially dissolves the crystalline structure of starch and ensures high solution stability, especially in the case of amylose, even after a long storage time.
The lower content of amylopectin in the samples rich in amylose is responsible for the lower molar mass value compared with samples rich in amylopectin. In all Mw determinations, the A2 values were positive, on the order of ~10−5 mL.mol.g−2, indicating that DMSO is a good solvent system for starch samples.
Interaction between XG and starches
Autoclaving was used to gelatinize the starches, according to García-Alonso, Jimenez-Escrig, Martin-Carron, Bravo, and Sauro-Calixto (Citation1999). The authors observed that gelatinization at 100°C at atmospheric pressure compared with gelatinization using autoclaving at 120°C and 1.97 atm did not influence starch gelatinization and the degree of polymerization, suggesting that both methods of gelatinization lead to similar final products. Additionally, the autoclaving was more easily monitored and reproducible than heating at atmospheric pressure.
Different gelatinization times were used due to differences in the gelatinization process of high-amylose starch and the other starches, measured by microscopic analysis to determine the time necessary for complete gelatinization of the samples.
The ion has the capacity to form a colored complex with free amylose, whose absorbance was read at 620 nm. Consequently, we used the amylose quantitation method to determine the free amylose content in mixtures of amylose with XG. For this purpose, the capacity of binding between iodine and amylose was measured in the presence or absence of different concentrations of XG.
Evaluations of the mixtures’ rheological properties using different proportions of XG and high-amylose cornstarch were performed. In these experiments, the amylose concentration was kept constant (50 g.L−1) and the XG concentration varied from 2 to 20 g.L−1, in a similar way to the iodine-free amylose starch determination during the interaction studies ().
Figure 1. Comparison between the competition assay between iodine and xyloglucan for high-amylose cornstarch and tan delta (δ) of the interactions between xyloglucan and high-amylose cornstarch.
Figura 1. Comparacion entre el ensayo de competicion entre el yodo y xiloglucano con almidon de maiz de alta amilosa y tan delta (δ) de las interacciones entre xiloglucano y almidon de maiz de alto contenido de amilosa.
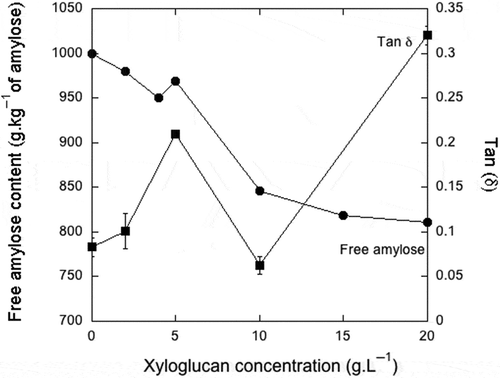
It can be observed in that by keeping the high-amylose cornstarch content constant (50 g.L−1) and increasing the XG concentration (2, 4, 5, 10, 15, 20, and 25 g.L−1), a maximum interaction was observed, with a proportional reduction of tri-iodine–amylose interaction, suggesting a reduction (p < 0.05) in the free amylose when at XG:high-amylose cornstarch ratio of 10:50 g.L−1.
These results clearly demonstrate that XG binds to amylose, reducing the free amylose content in our experiments.
Comparing the high-amylose cornstarch to the mixture 2:50 g.L−1 of XG:high-amylose cornstarch, it was possible to observe almost the same rheological behavior of the high-amylose cornstarch alone at 50 g.L−1 (p > 0.05). Increasing the concentration of XG to 5 g.L−1 the tan δ increased (p < 0.05). A solid-like behavior can be observed due to low tan δ value at 10 g.L−1 of XG and 50 g.L−1 of high-amylose cornstarch ( and ). In the ratio 20:50 g.L−1 of XG:amylose cornstarch, due to excess of the hydrocolloid (XG) in the mixture, an increase in the loss modulus (G”), with reduction in the storage modulus (G’), was observed, which also increased the tan δ ().
Figure 2. Tan δ values for xyloglucan–high-amylose cornstarch interactions.
Figura 2. Valores de tan δ para las interacciones xiloglucano-almidon de maiz de alta amilosa.
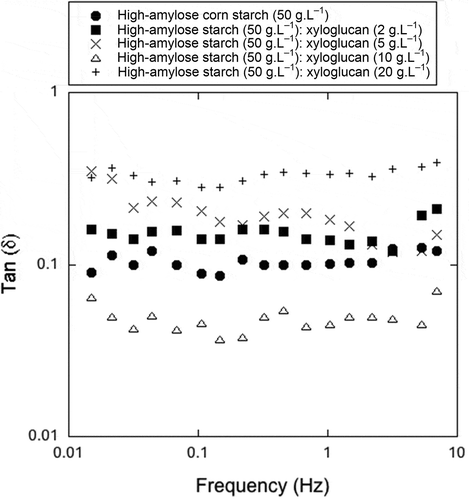
Table 2. G, G”, η*, tan δ of the high-amylose cornstarch, normal cornstarch, waxy cornstarch, yam starch, and cassava starch and the interactions with xyloglucan (XG) at 1 Hz.
Tabla 2. G, G”, η*, tan δ del almidón de alta amilosa de maíz, almidón de maíz normal, almidón de maíz ceroso, almidón de ñame y almidón de yuca y las interacciones con xiloglucano (XG) a 1 Hz.
The interaction between XG and high-amylose cornstarch was studied using a mixture of XG (10 g.L−1) and high-amylose cornstarch (50 g.L−1). Gels containing only high-amylose cornstarch at 50 and 60 g.L−1 were used for rheological control. A stress of 0.5 Pa was used for the frequency ramps, corresponding to viscoelastic linear behavior in the samples and generating the lowest deformation values (<10%) in all the samples (data not shown).
In order to evaluate the strength of a gelatinized system, it is necessary to analyze the relation between the viscous or loss modulus (G”) and the storage or dynamic storage modulus (G’) rather than the absolute isolated modulus values. The G”/G’ ratio (tan δ) is a good indicator of the solid- or liquid-like behavior of samples. shows the G’, G”, η*, and tan δ of amylose cornstarch at 50 g.L−1, high-amylose cornstarch at 60 g.L−1, and mixture of XG (10 g.L−1) and high-amylose cornstarch (50 g.L−1) at the frequency of 1 Hz. The results suggest that the presence of the XG reduced the tan δ significantly in comparison with the mixture with the high-amylose cornstarch at 50 g.L−1. The contribution of XG is purely viscous in the mixture system, so the reduced tan δ could be attributed to the XG–high-amylose cornstarch interaction.
The effects of mixtures of XG (10 g.L−1) and normal cornstarch (50 g.L−1) are reported in and compared with the normal cornstarch at 50 and 60 g.L−1, but without statistical significance. The results indicate that the absence of the hydrocolloid in the mixture generated a more solid-like structure at 50 and 60 g.L−1, confirming the results previously published by Yoshimura et al. (Citation1999).
Tan δ values for waxy cornstarch at 50 and 60 g.L−1 and for the mixture at 10:50 g.L−1 of XG:waxy cornstarch, respectively, in , show an increase in tan δ and G” and reduction in the G’ when XG was added to the mixture with waxy cornstarch (p < 0.05). Apparently, the starch with almost exclusive amylopectin content interacts antagonistically with XG. This effect was related to the exclusion of XG, due to the presence of amylopectin. This exclusion of the hydrocolloid was also observed by Freitas et al. (Citation2003), who reported a phase separation after centrifugation of a XG:waxy cornstarch mixture.
To confirm the influence of the amylose content on the XG:starch interaction, we studied the mixtures of yam and cassava starch, with amylose contents of 360 and 210 g.kg−1 of amylose in starch, respectively. Mali, Grossmann, Garcia, Martino, and Zaritzky (Citation2002) found a ratio of 300 g.kg−1 of amylose in yam starch. The difference with our results can be attributed to the methods used to quantify amylose. Also, we considered the dry base, removing the moisture, to calculate our amylose level, which can explain the higher results than those previously published.
As can be seen in , the mixture of XG (10 g.L−1) with yam starch (50 g.L−1) and the pure yam starch at concentrations of 60 g.L−1 gave practically the same G’, G”, η*, and tan δ values, expressed as the mean and standard deviation of three independent analyses.
Yam starch has higher amylose content than waxy and normal cornstarch and also contains amylopectin with fewer branch points (Freitas, Paula, Feitosa, Rocha, & Sierakowski, Citation2004; Mali et al., Citation2002). It resulted in a gel with solid-like behavior. The presence of XG significantly reduced the tan δ compared with the sample of yam starch at 50 g.L−1 (p < 0.05).
In experiments with XG and cassava starch mixtures, the stress in viscoelastic linear behavior was 0.1 Pa. shows the effect of frequency on the G’, G”, η*, and tan δ for cassava starch at concentrations of 50 and 60 g.L−1 and for the mixture of XG (10 g.L−1) and cassava starch (50 g.L−1). As can be seen, the tan δ for the cassava starch at 50 and 60 g.L−1 shows a more characteristic solid-like behavior when compared with the mixture of XG (10 g.L−1) and cassava starch (50 g.L−1) (p < 0.05). This effect occurs due to the presence of a high content of amylopectin in this starch, which promotes an antagonistic interaction, generating the exclusion of XG.
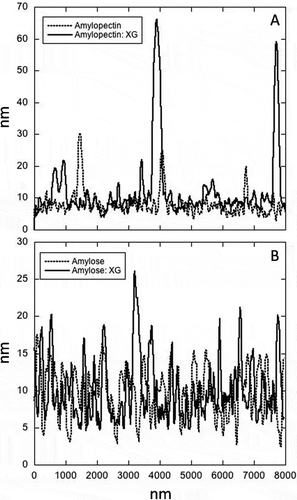
Using AFM, it was possible to observe that in the waxy starch there was less deposition of globular XG () when compared to waxy starch alone (). The presence of amylose, both in the absence () and in the presence of XG (), promoted an increase of the surface roughness ( and ) when compared with amylopectin, suggesting that the hydrocolloid was deposited onto all the film extension due to the interactions between XG and amylose.
Figure 3. AFM analysis of a deposit of waxy cornstarch at 1 mg.mL−1 (A), high-amylose cornstarch at 1 mg.mL−1 (B), waxy cornstarch with xyloglucan at 1 mg.mL−1 (C), and high-amylose cornstarch with xyloglucan at 1 mg.mL−1 (D).
Figura 3. Análisis por MFA de un depósito de almidón ceroso de maíz a 1 mg.mL−1 (A), almidón de maíz de alta amilosa 1 mg.mL−1 (B), almidón de maíz ceroso con xiloglucano a 1 mg.mL−1 (C) y almidón de alta amilosa de maíz con xiloglucano a 1 mg.mL−1 (D).
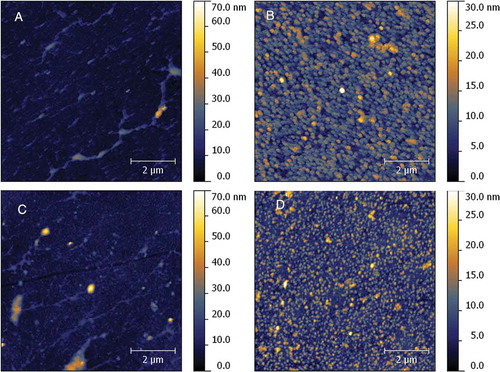
Figure 4. AFM comparative topographies of the films.
Figura 4. MFA topografías comparativos de las películas.
Temsiripong, Pongsawatmanit, Ikeda, and Nishinari (Citation2005) observed that the tan δ value equal to unity (G” = G’) shifts to a lower frequency with an increase of XG content in tapioca starch with 220 g.kg−1 of amylose. Apparently, XG forms a continuous phase in a tapioca starch–XG mixture and confines the starch into a discontinuously dispersed phase.
In the dispersions of cornstarch with 250 g.kg−1 of amylose and XG mixtures, G” was larger than G’ (high tan δ) with an increase of XG content (Yoshimura et al., Citation1999). A similar tendency was found for other polysaccharides with cornstarch (Alloncle & Doublier, Citation1991).
By evaluating the influence of tamarind seed XG on the rheological properties and thermal stability of tapioca starch, Pongsawatmanit, Temsiripong, Ikeda, and Nishinari (Citation2006) observed that at a total polysaccharide concentration of 50 g.kg−1 of mixture, the presence of XG increased the liquid-like properties of the mixtures in a dose-dependent way, as shown by the increase of tan δ.
By using dynamic rheological analysis, Kim, Patel, and BeMiller (Citation2013) observed that rice and all rice starch–hydrocolloid combinations, except starch–xanthan combinations, became more solid-like as the amylose contents of the starches increased. For the controls and the rice starch–sodium alginate, starch–hydroxypropylmethylcellulose and starch–carrageenan combinations, tan δ values decreased steadily as the amylose content increased. The tan δ for rice starch–guar gum composite gels all increased as the amylose content of the starch increased.
BeMiller (Citation2011) observed that the hydrocolloid–starch interaction is dependent on amylose content and, in general, is due to differences in hydrocolloid characteristics such as the degree of branching, molar mass and molecular flexibility, and the presence of charge. In XG, the high molar mass and worm-like conformation of the hemicellulose could be a determinant in the interaction with amylose.
Conclusion
This work demonstrated that the XG has positive synergistic interaction only with starches containing high-amylose content. In starches with low-amylose content, an antagonistic reaction occurs, probably due to XG exclusion by the amylopectin fraction of the starch. The ideal mass proportion for the interaction between amylose and XG was determined by iodine/XG competition and rheological analysis. AFM also confirmed a more pronounced interaction with amylose than with amylopectin cornstarch.
Additional information
Funding
References
- Alloncle, M., & Doublier, J. L. (1991). Viscoelastic properties of maize starch/hydrocolloid pastes and gels. Food Hydrocolloids, 5, 455–467. doi:10.1016/S0268-005X(09)80104-5
- Alves, R. M. L., Grossmann, M. V. E., & Silva, R. S. S. F. (1999). Gelling properties of extruded yam (Dioscorea alata) starch. Food Chemistry, 67(2), 123–127. doi:10.1016/S0308-8146(99)00064-3
- AOAC. (1995). Official methods of analysis of AOAC international (16th ed.). Arlington, TX: Association of Official Analytical Chemistry.
- BeMiller, J. N. (2011). Pasting, paste, and gel properties of starch–hydrocolloid combinations. Carbohydrate Polymers, 86, 386–423. doi:10.1016/j.carbpol.2011.05.064
- Chrastil, J. (1987). Improved colorimetric determination of amylose in starches or flours. Carbohydrate Research, 159, 154–158. doi:10.1016/S0008-6215(00)90013-2
- Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356. doi:10.1021/ac60111a017
- Freitas, R. A., Busato, A. P., Mitchell, D. A., & Silveira, J. L. M. (2011). Degalatosylation of xyloglucan: Effect on aggregation and conformation, as determined by time dependent static light scattering HPSEC–MALLS and viscosimetry. Carbohydrate Polymers, 83, 1636–1642. doi:10.1016/j.carbpol.2010.10.021
- Freitas, R. A., Gorin, P. A. J., Neves, J., & Sierakowski, M. R. (2003). A rheological description of mixtures of a galactoxyloglucan with high amylose and waxy corn starches. Carbohydrate Polymers, 51, 25–32. doi:10.1016/S0144-8617(02)00095-4
- Freitas, R. A., Martin, S., Santos, G. L., Valenga, F., Buckeridge, M. S., Reicher, F., & Sierakowski, M. R. (2005). Physico-chemical properties of seed xyloglucans from different sources. Carbohydrate Polymers, 60, 507–514. doi:10.1016/j.carbpol.2005.03.003
- Freitas, R. A., Paula, R. C., Feitosa, J. P. A., Rocha, S., & Sierakowski, M. R. (2004). Amylose contents, rheological properties and gelatinization kinetics of yam (Dioscorea alata) and cassava (Manihot utilissima) starches. Carbohydrate Polymers, 55, 3–8. doi:10.1016/S0144-8617(03)00142-5
- García-Alonso, A., Jimenez-Escrig, A., Martin-Carron, N., Bravo, L., & Sauro-Calixto, F. (1999). Assessment of some parameters involved in the gelatinization and retrogradation of starch. Food Chemistry, 66, 181–187. doi:10.1016/S0308-8146(98)00261-1
- Han, J., Lim, H., & Lim, S. T. (2005). Comparison between size exclusion chromatography and micro-batch analyses of corn starches in DMSO using light scattering detector. Starch-Stärke, 57, 262–267. doi:10.1002/star.200400365
- Hartree, E. F. (1972). Determination of protein: A modification of the Lowry method that gives a linear photometric response. Analytical Biochemistry, 48, 422–427. doi:10.1016/0003-2697(72)90094-2
- Kim, H. S., Patel, B., & BeMiller, J. (2013). Effects of the amylose-amylopectin ratio on starch-hydrocolloid interactions. Carbohydrate Polymers, 98, 1438–1448. doi:10.1016/j.carbpol.2013.07.035
- Lima, N. N., Quoirin, M., Naddaf, Y. G., Wilhelm, H. M., Ribas, L. L. F., & Sierakowski, M. R. (2003). A xyloglucan from seeds of the native Brazilian species Hymenaea courbaril for micropropagation of Marubakaido and Jonagored apples. Plant Cell Report, 21, 402–407.
- Mali, S., Grossmann, M. V. E., Garcia, M. A., Martino, M. N., & Zaritzky, N. A. (2002). Microstructural characterization of yam starch films. Carbohydrate Polymers, 50, 379–386. doi:10.1016/S0144-8617(02)00058-9
- Martin, S., Freitas, R. A., Obayashi, E., & Sierakowski, M. R. (2003). Physico–chemical aspects of galactoxyloglucan from the seeds of Hymenaea courbaril and its tetraborate complex. Carbohydrate Polymers, 54, 287–295. doi:10.1016/S0144-8617(03)00073-0
- Onyechi, U. A., Judd, P. A., & Ellis, P. R. (1998). African plant foods rich in non-starch polysaccharides reduce postprandial blood glucose and insulin concentrations in healthy human subjects. British Journal of Nutrition, 80, 419–428.
- Pérez, S., & Bertoft, E. (2010). The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch-Stärke, 62, 389–420. doi:10.1002/star.201000013
- Pongsawatmanit, R., Temsiripong, T., Ikeda, S., & Nishinari, K. (2006). Influence of tamarind seed xyloglucan on rheological properties and thermal stability of tapioca starch. Journal of Food Engineering, 77, 41–50. doi:10.1016/j.jfoodeng.2005.06.017
- Prabhanjan, H., & Ali, S. Z. (1995). Studies on rheological properties of tamarind kernel powder, its derivatives and their blends with maize starch. Carbohydrate Polymers, 28, 245–253. doi:10.1016/0144-8617(95)00106-9
- Radosta, S., Haberer, M., & Vorwerg, W. (2001). Molecular characteristics of amylose and starch in dimethyl sulfoxide. Biomacromolecules, 2, 970–978. doi:10.1021/bm0100662
- Selvendran, R. R., March, J. F., & Ring, S. G. (1979). Determination of aldoses and uronic acid content of vegetable fiber. Analytical Biochemistry, 96, 282–292. doi:10.1016/0003-2697(79)90583-9
- Temsiripong, T., Pongsawatmanit, R., Ikeda, S., & Nishinari, K. (2005). Influence of xyloglucan on gelatinization and retrogradation of tapioca starch. Food Hydrocolloids, 19, 1054–1063. doi:10.1016/j.foodhyd.2005.02.005
- Willinger, A. H. A. (1964). Potato starch. In R. R. L. Wistler (Ed.), Method in carbohydrate chemistry. New York, NY: Academic Press.
- Wolfrom, M. L., & Thompson, A. (1963). Acetylation methods. Methods in Carbohydrate Chemistry, 2, 211–215.
- Yoshimura, M., Takaya, T., & Nishinari, K. (1999). Effects of xyloglucan on the gelatinization and retrogradation of corn starch as studied by rheology and differential scanning calorimetry. Food Hydrocolloids, 13, 101–111. doi:10.1016/S0268-005X(98)00075-7
- Zobel, H. F., & Stephen, A. M. (1995). Starch: Structure, analysis, and application. In A. M. Stephen (Ed.), Food polysaccharides and their applications (19-65). New York, NY: Taylor & Francis.
