Abstract
Extraction and characterization of collagen were carried out in rabbit skins as a new alternative for collagen type I. Acetic acid and pepsin were for the extraction of soluble and insoluble collagen, respectively. The enzymatic treatment yielded higher amount of collagen (71%). The average pH value was 6.3, no matter what the method of extraction was. Denaturation temperature of collagen was found at 36°C approximately in two different techniques: Rheometer and Differential Scanning Calorimetry (DSC). Despite the amount of collagen in solution was low, its viscosity was high because of the hydrodynamic behaviour of collagen molecules. Sodium dodecyl sulphate polyacrylamide gel electrophoresis results showed three different bands that reflected two alpha-chains and one beta-chain with molecular weights of 102, 118 and 220 kDa, respectively. Determination of hydroxyproline gave evidence that the extracted material was collagen. It was concluded that rabbit skin could be an alternative source for the extraction of collagen.
Se realizó la extracción y caracterización de colágeno en pieles de conejo como una nueva alternativa para colágeno tipo I. Se usaron ácido acético y pepsina para la extracción de colágeno soluble e insoluble respectivamente. El tratamiento enzimático fue el que rindió alta cantidad de colágeno (71%). El pH promedio fue de 6,3 sin importar cuál fue el método de extracción. La temperatura de desnaturalización del colágeno se encontró a 36°C aproximadamente mediante dos diferentes técnicas: Reometría y DSC. A pesar de que la cantidad de colágeno en la solución fue bajo, su viscosidad fue alta debido a el comportamiento hidrodinámico de las moléculas. Los resultados de SDS–PAGE mostraron tres diferentes bandas en las que se reflejaron dos cadenas alfa y una cadena beta con pesos moleculares de 102, 118 and 220 kDa respectivamente. La determinación del contenido de hidroxiprolina dio evidencia de que el material extraído fue colágeno. Se concluyó que la piel de conejo podría ser una alternativa de materia prima para la extracción de colágeno.
Palabras claves:
Introduction
Collagen is a product highly demanded and used in the cosmetic, pharmaceutics and food industry due to its molecular properties. This fibrous protein can be obtained from different sources and it is the main component of skins, bones and tendons (Ahmad & Benjakul, Citation2010; Delvin, Citation2004; Huang, Shiau, Chen, & Huang, Citation2011; Jongjareonrak, Benjakul, Visessanguan, Nagai, & Tanaka, Citation2005; Yan, Li, Zhao, & Qin, Citation2012). At least 29 different types of collagen with a singular amino acid sequence, structure and function have been identified (Li et al., Citation2013; Liu, Liang, Regenstein, & Zhou, Citation2012). Collagen is a biopolymer composed of two alpha-1 chains and one alpha-2 chain. It is composed of a third part of glycine, and it does not contain tryptophan and lower amounts of tyrosine and histidine (Huang et al., Citation2011; Kittiphattanabawon, Benjakul, Visessanguan, Nagai, & Tanaka, Citation2005; Muyonga, Cole, & Duodu, Citation2004; Schrieber & Gareis, Citation2007).
Collagen is a unique triple helix structure conformed for three polypeptide chains, and it is conformed in quaternary structures. In this structure, it can be found ordered macroscopic ultra structures. Its molecular characteristics obey to the constant repetition of glycine followed by proline and hydroxyproline. The primary structure of collagen type I has a single chain with 1014 amino acids approximately with a molecular weight around 100,000 g mol−1 (Brinckmann, Notbohm, & Müler, Citation2005; Chien, Citation1975; Fathima, Madhan, Rao, Nair, & Ramasami, Citation2004; Nalinanon, Benjakul, Kishimura, & Osako, Citation2011).
The main sources of collagen come from skins and bones of bovine and porcine animals. However, some diseases such as bovine spongiform encephalopathy and foot and mouth disease as well as religious issues had lead the researchers and collagen users to find different sources of collagen (Jongjareonrak et al., Citation2005; Nalinanon, Benjakul, Visessanguan, & Kishimura, Citation2007). On the other side, Mexican producers of rabbit meat face a problem related to the proper disposal of by-products such as rabbit skins. The main aim of this project was to use this organic residue as a raw material for the extraction of collagen.
Materials
New Zealand rabbit skins with 40–50% of water content were used in this experiment. They were obtained from Tulancingo, Hidalgo. Acetic acid reactive grade (99% purity), sodium chloride reactive grade, porcine digestive protease (Pepsin), dialysis membrane tubing with 6–8 kDa molecular weight cut-off, phosphorous pentoxide (98.5% purity) from Sigma-Aldrich, hydrochloric acid solution at 50% (v/v), cooper sulphate 0.05 M, sodium hydroxide 1.25 M, hydrogen peroxide at 6%, sulphuric acid 0.9 M and 4-dimethylaminobenzaldehyde at 5%.
Methods
Conditioning of the skin before collagen extraction
The rabbit skins were soaked to recover its amount of water up to 70% followed by a fleshing process to remove flesh, connective tissue and fat. Then, the skins were shaved to remove most of the hair.
Extraction of collagen
The extractions were executed according to previous works (Kittiphattanabawon et al., Citation2005) with some modifications. Pre-treated skins were cut into small squares of 1 cm2 approximately and suspended in 0.5 M acetic acid solution at a solid-to-solvent ratio of 1:10 (w/v). The sample was in a shaker machine (Bellco biotechnology) at 140 rpm. for 24 hours at room temperature (20 ± 2°C). After this time, the sample was filtered and the liquid portion was called ‘acid extraction 1’. Then, the residues were treated in the same conditions as mentioned before in order to obtain a second and third acid extractions referred to as acid 1, acid 2 and acid 3.
After the three acid extractions, the non-dissolved residue was used for pepsin soluble collagen extraction as follows: the residues were resuspended in a new solution of 0.5 M acetic acid at a concentration of 1:10 (w/v) and pepsin at a concentration of 1 g/L. This solution was placed in a shaker machine at the same speed as described above for 48 hours, this process is referred to as ‘enzymatic treatment’.
All the samples were filtered and stored at 4°C at the end of every single extraction. Acid and enzymatic collagens were precipitated with a solution of 2.6 M sodium chloride. The precipitated material was centrifuged for 15 minutes at a relative centrifugal force of 3380xg in a centrifuge model Z36HK (HERMLE Labortechnik GmbH, Wehingen, Germany). The samples were resuspended in five volumes of 0.5 M acetic acid and dialysed for 48 hours in distilled water and dialysis membrane tubing with 6–8 kDa molecular weight cut-off. The resulted dialysed material was freeze-dried.
Viscosity measurements
Viscosity was measured in extracted samples by using a calibrated AR200 rheometer of TA instruments and stainless steel concentric cylinders. Temperature was swept from 10°C to 50°C at a heating speed of 5°C per minute.
Measurement of pH
Measurement of pH was performed according to the methodology described in literature (NMX-F-317-S, Citation1978). A pH meter of HANNA model HL2211 previously calibrated with buffer solutions at pH 4 and pH 7 was used. Dialysed collagen samples at 20°C were placed in a beaker with gently stirring for homogenization. Then, the glass electrode was dipped into the solution. After each measurement, the reading was registered. The probe was rinsed with distilled water and wiped to absorb any remaining water.
Determination of collagen
According to the methodology described in the literature (Lollar, Citation1958), 0.05 g of dried collagen powder was digested in 3 mL of 50% hydrochloric acid for 16 hours at 100°C. The hydrolysed sample was cooled and transferred to a 25-mL volumetric flask with 10 mL of distilled water. After mixing, the flask was made up to the mark. From this sample, 2 mL was taken and diluted in 100 mL distilled water. For the determination of hydroxyproline, 2 mL of this solution was added to a 25-mL volumetric flask, followed by 2 mL of 0.05 M copper sulphate solution, 2 mL of 1.25 M sodium hydroxide solution and 2 mL of 6% hydrogen peroxide solution. Three replicates were considered in this experiment and a blank. Samples were gently shaken for 5 minutes and were placed in a water bath at 40°C for 15 minutes to remove excess hydrogen peroxide. The flasks were cooled in water, and then 10 mL of 0.9 M sulphuric acid and 5 mL of 5% dimethylaminobenzaldehyde solution were added. The flasks were placed in a water bath at 70°C for 30 minutes to develop the colour. After cooling at room temperature, the samples were diluted to the mark using distilled water. The absorbance of each solution was measured against the blank at 555 nm in a UV–visible Jenway Genova spectrophotometer.
Molecular weight determination
The sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE) determination was performed according to Laemmli and Quittner (Citation1974) method with some modifications. One millilitre of dialysed collagen was dissolved in 0.5 M Tris–HCl buffer pH 6.8 (1% SDS, 10% glycerol, and 0.01% bromophenol blue) and boiled for 5 minutes. Then, 13 μL of the denatured sample and 8 μL of marker with a molecular weight from 10 kDa to 220 kDa (BenchMark Protein Ladder) were loaded into wells at the top of the polyacrylamide gel. This gel contained a 4% stacking gel on top of 10% resolving gel. A voltage of 50 Volts was applied for 30 minutes, and once the mobility of proteins reached the resolving layer, the voltage was increased to 120 Volts for 3 hours in order to see the separation of proteins according to size. As electric current ran through the buffer, the negatively charged proteins migrated towards the anode and lower molecular weight proteins reached the bottom of the gel. After migration of proteins, the gel was stained with 0.2% Coomassie brilliant blue for 30 minutes in 50% methanol and 10% acetic acid (v/v), then it was destained with 20% methanol and 5% acetic acid (v/v) and distilled water.
Thermal properties
Thermal properties of collagen extractions were measured in a TA instrument DSC Q 2000. It was calibrated with indium (Tm, onset = 156.6°C, ΔH = 28.45 J/g) and cyclohexane (Tm, onset = 6.5°C). An average of 45 mg of collagen solution was packed and sealed in a 50-μL aluminium sample pan. An empty pan with the same characteristics was utilized as a reference. One heating scan was performed from 10°C to 50°C at 5°C per minute.
Statistics
A randomized design experiment and an analysis of variance (ANOVA) were carried out with the statistical program SPSS statistics v17. Tukey’s test at a level p ≤ 0.05 was applied to experimental data. Three replicates per treatment were considered in this experiment.
Results and discussions
Yielding of extractions
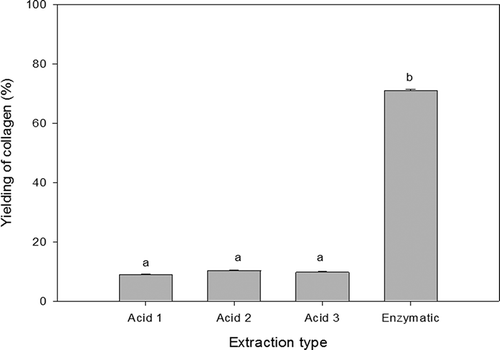
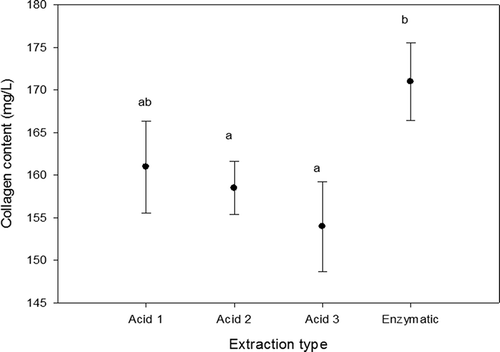
The yielding of the three acid extractions as well as the enzymatic extraction of rabbit collagen is shown in . There were found significant statistical differences (p < 0.05) in the enzymatic extraction compared with the others. However, no significant statistical differences (p > 0.05) were observed between the acid extractions reporting values around 9% each one. The yielding of enzymatic treatment was 71%. These results suggest that most of the collagen in rabbit skin is in the insoluble form. They agree with the literature where acetic acid was the best solvent for collagen extraction (Skierka & Sadowska, Citation2007). Previous works carried out in fish skin (Liu et al., Citation2012; Matmaroh, Benjakul, Prodpran, Encarnacion, & Kishimura, Citation2011; Nalinanon et al., Citation2007; Skierka & Sadowska, Citation2007; Zhang et al., Citation2007) showed that acid extractions yield lower amounts of collagen in comparison with the enzymatic method (Huang et al., Citation2011; Matmaroh et al., Citation2011; Singh, Benjakul, Maqsood, & Kishimura, Citation2011). Some pre-treatment parameters such as mechanical effect, pH, temperature and time could affect the yielding of collagen during extraction process.
Solid content
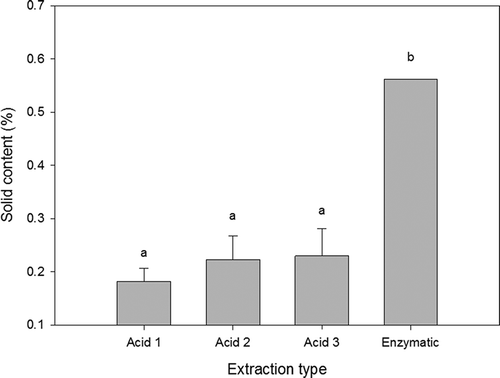
The solid content data were calculated by deleting the water content results from the dialysed sample weight. The percentage of solids in the enzymatic sample resulted higher than 0.5% as can be seen in . This sample showed a very viscous consistency despite its small amount of solids when compared to the acid extracted samples. This phenomenon can be due to the hydrodynamic volume of collagen as they expand in solution and provoke an increment on its viscosity (Wu & Koiwa, Citation2012).
pH
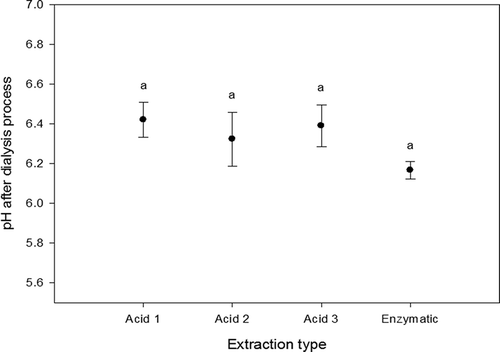
Measurements of pH were carried out according to the literature (NMX-F-317-S, Citation1978). From , it can be seen that the pH values of dialysed samples of collagen were stabilized in the range of 6.2–6.4, and there were no significant statistical differences between extractions (p > 0.05). This means that the dialysis stage removed salts and acids until the samples achieve an average pH value of 6.3, no matter what the extraction method was. At this pH value, the collagen molecules tend to form little fibres (Harris & Reiber, Citation2007; Harris, Soliakov, & Lewis, Citation2013; Li, Asadi, Monroe, & Douglas, Citation2009). These results suggest that collagen obtained from rabbit skins could be used in the cosmetology industry as the obtained pH value is closer to the neutral pH values.
Viscosity
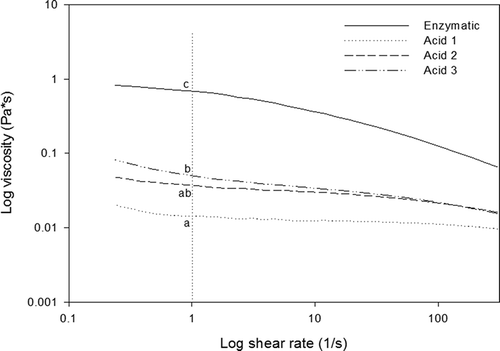
From , it can be seen that viscosity of collagen samples resulted higher in enzymatic treatment compared with the acid extractions. However, the viscosity in acid extractions appeared to increase as the number of extractions increased. Looking at the viscosity around log of shear rate 1 (1/s), there were found significant statistical differences (p < 0.05) between the samples. This behaviour could be due to the solid content in samples as illustrated in . Also, it was observed that the viscosity increases as the shear rate decreases, which means at higher speed the collagen molecules in solution get aligned turning easy the deformation of the sample which is expressed in a reduction of viscosity (Lai, Li, & Li, Citation2008).
Figure 4. Viscosity of rabbit skin collagen extracts as a function of log shear rate. Different letters represent significant differences at log shear rate 1 (1/s).
Figura 4. Viscosidad del colágeno de piel de conejo en función de log velocidad de cizalla. Las letras diferentes representan diferencias significativas a una log velocidad de cizalla de 1 (1/s).
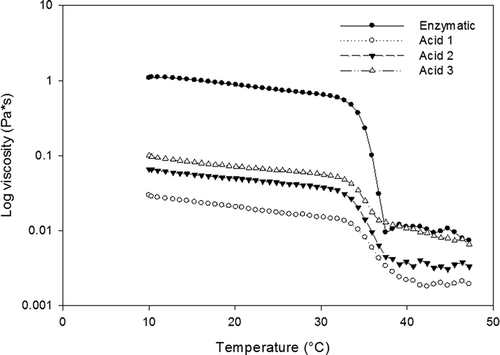
Thermal properties of collagen are important because this biological molecule is sensible to temperature above 40°C due to denaturation issues (Gustavson, Citation1956; Ramachandran, Citation1967). shows the viscosity behaviour of dialysed collagen as a function of temperature. Temperature was swept from 10°C to 50°C. The viscosity decreased considerably in the range of 32.7°C to 38.3°C due to the denaturation of collagen, and the average denaturation temperature was 36.0°C. But after 38°C, the samples were equilibrated, showing viscosities around zero values. Previous works carried out with different species such as fish showed the same behaviour; however, the range of temperature was observed between 25°C and 32°C. (Huang et al., Citation2011; Nalinanon et al., Citation2007; Safandowska & Pietrucha, Citation2013; Yan et al., Citation2008; Zhang et al., Citation2007). The thermal stability of collagen obeys to the matrix of hydrogen bonds. These bonds connect the hydroxyl group of the hydroxyproline with the acid group of collagen. Therefore, differences in the amount of hydroxyproline are related to the differences in denaturation temperature of collagen (Nalinanon et al., Citation2007; Safandowska & Pietrucha, Citation2013; Selvakumar, Ling, Covington, & Lyddiatt, Citation2012). It is well known that the higher the amount of hydroxyproline, the higher the denaturation temperature of collagen (Gustavson, Citation1956). This statement suggests that fish skins have lower amount of this amino acid compared with rabbit skins.
Collagen content
Collagen is a singular animal protein because it is the only one that contains high amounts of hydroxyproline as well as glycine and proline (Laemmli & Quittner, Citation1974; Ramachandran, Citation1967). The presence of these amino acids gives evidence that the extracted material relates to collagen. illustrates the results obtained in the determination of hydroxyproline and transformed as an amount of collagen. It was observed that the enzymatic extraction showed higher values compared with the acid extractions. However, between the acid treatments, lower values were found as the number of extractions increased. From these results, it can be seen that the higher amount of collagen in skin is insoluble.
Molecular weight of collagen
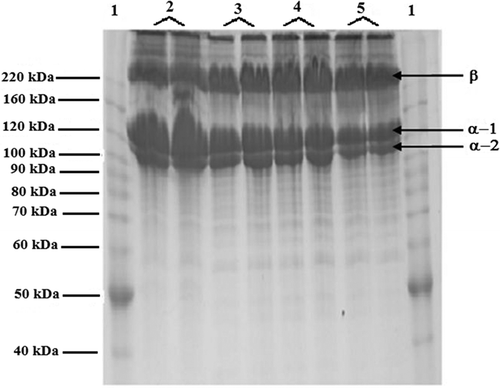
shows the two main collagen chains: alpha-1 and alpha-2 as a band form. They showed molecular weights of 118 and 102 kDa, respectively. A third band was observed at 220 kDa, and it was assumed that this band is a beta-chain from cross-linking between two chains. Similar results were observed from previous works with different species showing alpha-1, alpha-2 and beta-chain conformations (Cheng, Hsu, Chang, Lin, & Sakata, Citation2009; Li et al., Citation2013; Lin, Lin, & Su, Citation2011; Santos et al., Citation2013; Tamilmozhi, Veeruraj, & Arumugam, Citation2013). There were no differences of molecular weight between the acid and enzymatic treatments, which means that the enzymes did not alter the structure of the collagen molecule (Skierka & Sadowska, Citation2007).
Figure 7. Molecular weight of rabbit skin collagen from different extraction methods. Keywords: 1. Marker; 2. Enzymatic; 3. Acid 1: 4. Acid 2; 5. Acid 3. There were used two replicates per treatment.
Figura 7. Peso molecular de colágeno de piel de conejo proveniente de diferentes métodos de extracción. Palabras clave: 1. Marcador; 2. Enzimático; 3. Acido 1: 4.- Acido 2; 5. Acido 3. Se realizaron dos repeticiones por cada tratamiento.
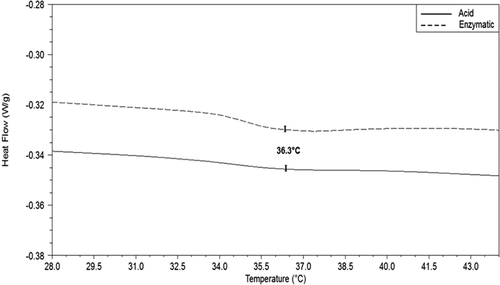
Thermal properties
Thermograms from dialysed rabbit skin collagen samples showed the behaviour of the collagen when treated under thermal conditions. The thermal stability of collagen is one of the main properties of interest of this biological material as this parameter refers to the stability of the triple helix structure. shows only two curves because thermograms from acid 1, acid 2 and acid 3 treatments overlapped each other. However, the average temperature of denaturation resulted around 36.3°C for all treatments. This value agrees with those obtained in the measurement of viscosity of collagen as a function of temperature described above (see ). Thermal stability of collagen is associated with the restriction of secondary structure of the polypeptide chain which is governed for proline and hydroxyproline rings and their hydrogen bonds (Nalinanon et al., Citation2011). The denaturation temperature of collagen is an irreversible process where the triple helix structure is lost due to the rupture of hydrogen bonds (Rochdi, Foucat, & Renou, Citation2000). The typical denaturation temperature of collagen is around 40°C; however, this temperature value varies from species to species, and the denaturation temperature of fish collagen is lower than that of collagen extracted from mammals (Huang et al., Citation2011; Yan et al., Citation2008; Zhang et al., Citation2007).
Conclusion
The extraction of collagen from rabbit skins was satisfactory with significant yielding with the use of enzymes. Rabbit skins could be an alternative source for the extraction of collagen type I and at the same time to give an additional value to this agro industrial by-product.
References
- Ahmad, M., & Benjakul, S. (2010). Extraction and characterisation of pepsin-solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chemistry, 120(3), 817–824. doi:10.1016/j.foodchem.2009.11.019
- Brinckmann, J., Notbohm, H., & Müler, P. K. (2005). Collagen. Primer in structure, processing and assembly. The Netherlands: Springer.
- Cheng, F.-Y., Hsu, F.-W., Chang, H.-S., Lin, L.-C., & Sakata, R. (2009). Effect of different acids on the extraction of pepsin-solubilised collagen containing melanin from silky fowl feet. Food Chemistry, 113(2), 563–567. doi:10.1016/j.foodchem.2008.08.043
- Chien, J. C. W. (1975). Solid-State Characterization of the Structure and Property of Collagen. Journal of Macromolecular Science, Part C, 12(1), 1–80. doi:10.1080/15321797508076103
- Delvin, T. M. (2004). Bioquímica (4th ed.). Barcelona: Glicsman Martin.
- Fathima, N. N., Madhan, B., Rao, J. R., Nair, B. U., & Ramasami, T. (2004). Interaction of aldehydes with collagen: Effect on thermal, enzymatic and conformational stability. International Journal of Biological Macromolecules, 34(4), 241–247. doi:10.1016/j.ijbiomac.2004.05.004
- Gustavson, H. (1956). The chemistry and reactivity of collagen. New York, NY: Academic Press.
- Harris, J. R., & Reiber, A. (2007). Influence of saline and pH on collagen type I fibrillogenesis in vitro: Fibril polymorphism and colloidal gold labelling. Micron, 38(5), 513–521. doi:10.1016/j.micron.2006.07.026
- Harris, J. R., Soliakov, A., & Lewis, R. J. (2013). In vitro fibrillogenesis of collagen type I in varying ionic and pH conditions. Micron, 49(0), 60–68. doi:10.1016/j.micron.2013.03.004
- Huang, Y.-R., Shiau, C.-Y., Chen, H.-H., & Huang, B.-C. (2011). Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish (Diodon holocanthus). Food Hydrocolloids, 25(6), 1507–1513. doi:10.1016/j.foodhyd.2011.02.011
- Jongjareonrak, A., Benjakul, S., Visessanguan, W., Nagai, T., & Tanaka, M. (2005). Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chemistry, 93(3), 475–484. doi:10.1016/j.foodchem.2004.10.026
- Kittiphattanabawon, P., Benjakul, S., Visessanguan, W., Nagai, T., & Tanaka, M. (2005). Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chemistry, 89(3), 363–372. doi:10.1016/j.foodchem.2004.02.042
- Laemmli, U. K., & Quittner, S. F. (1974). Maturation of the head of bacteriophage T4: IV. The proteins of the core of the tubular polyheads and in vitro cleavage of the head proteins. Virology, 62(2), 483–499. doi:10.1016/0042-6822(74)90409-7
- Lai, G., Li, Y., & Li, G. (2008). Effect of concentration and temperature on the rheological behavior of collagen solution. International Journal of Biological Macromolecules, 42(3), 285–291. doi:10.1016/j.ijbiomac.2007.12.010
- Li, Y., Asadi, A., Monroe, M. R., & Douglas, E. P. (2009). pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Materials Science and Engineering: C, 29(5), 1643–1649. doi:10.1016/j.msec.2009.01.001
- Li, Z.-R., Wang, B., Chi, C.-F., Zhang, Q.-H., Gong, Y.-D., Tang, J.-J., … Ding, G.-F. (2013). Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocolloids, 31(1), 103–113. doi:10.1016/j.foodhyd.2012.10.001
- Lin, Y.-K., Lin, T.-Y., & Su, H.-P. (2011). Extraction and characterisation of telopeptide-poor collagen from porcine lung. Food Chemistry, 124(4), 1583–1588. doi:10.1016/j.foodchem.2010.08.018
- Liu, D., Liang, L., Regenstein, J. M., & Zhou, P. (2012). Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chemistry, 133(4), 1441–1448. doi:10.1016/j.foodchem.2012.02.032
- Lollar, R. M. (1958). Hydroxyproline analysis as a tool for tannery chemists. Journal of the American Leather Chemists Association, 53(3), 162.
- Matmaroh, K., Benjakul, S., Prodpran, T., Encarnacion, A. B., & Kishimura, H. (2011). Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chemistry, 129(3), 1179–1186. doi:10.1016/j.foodchem.2011.05.099
- Muyonga, J. H., Cole, C. G. B., & Duodu, K. G. (2004). Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chemistry, 85(1), 81–89. doi:10.1016/j.foodchem.2003.06.006
- Nalinanon, S., Benjakul, S., Kishimura, H., & Osako, K. (2011). Type I collagen from the skin of ornate threadfin bream (Nemipterus hexodon): Characteristics and effect of pepsin hydrolysis. Food Chemistry, 125(2), 500–507. doi:10.1016/j.foodchem.2010.09.040
- Nalinanon, S., Benjakul, S., Visessanguan, W., & Kishimura, H. (2007). Use of pepsin for collagen extraction from the skin of bigeye snapper (Priacanthus tayenus). Food Chemistry, 104(2), 593–601. doi:10.1016/j.foodchem.2006.12.035
- NMX-F-317-S. (1978). Determinación de pH en alimentos. Determination of pH in foods. Normas Mexicanas. Dirección General de Normas. Retrieved 15 May, 2014, from http://www.colpos.mx/bancodenormas/nmexicanas/NMX-F-317-S-1978.PDF
- Ramachandran, G. (1967). Treatise on collagen (Vol. 1). London: Academic Press.
- Rochdi, A., Foucat, L. C., & Renou, J.-P. (2000). NMR and DSC studies during thermal denaturation of collagen. Food Chemistry, 69(3), 295–299. doi:10.1016/S0308-8146(99)00267-8
- Safandowska, M., & Pietrucha, K. (2013). Effect of fish collagen modification on its thermal and rheological properties. International Journal of Biological Macromolecules, 53(0), 32–37. doi:10.1016/j.ijbiomac.2012.10.026
- Santos, M. H., Silva, R. M., Dumont, V. C., Neves, J. S., Mansur, H. S., & Heneine, L. G. D. (2013). Extraction and characterization of highly purified collagen from bovine pericardium for potential bioengineering applications. Materials Science and Engineering: C, 33(2), 790–800. doi:10.1016/j.msec.2012.11.003
- Schrieber, R., & Gareis, H. (2007). Gelatine handbook. Theory and industrial practice. Weinheim, Germany: Wiley-VCH.
- Selvakumar, P., Ling, T. C., Covington, A. D., & Lyddiatt, A. (2012). Enzymatic hydrolysis of bovine hide and recovery of collagen hydrolysate in aqueous two-phase systems. Separation and Purification Technology, 89(0), 282–287. doi:10.1016/j.seppur.2012.01.046
- Singh, P., Benjakul, S., Maqsood, S., & Kishimura, H. (2011). Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chemistry, 124(1), 97–105. doi:10.1016/j.foodchem.2010.05.111
- Skierka, E., & Sadowska, M. (2007). The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua). Food Chemistry, 105(3), 1302–1306. doi:10.1016/j.foodchem.2007.04.030
- Tamilmozhi, S., Veeruraj, A., & Arumugam, M. (2013). Isolation and characterization of acid and pepsin-solubilized collagen from the skin of sailfish (Istiophorus platypterus). Food Research International, 54(2), 1499–1505. doi:10.1016/j.foodres.2013.10.002
- Wu, X., & Koiwa, H. (2012). One-step casting of laemmli discontinued sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. Analytical Biochemistry, 421(1), 347–349. doi:10.1016/j.ab.2011.10.004
- Yan, M., Li, B., Zhao, X., & Qin, S. (2012). Effect of concentration, pH and ionic strength on the kinetic self-assembly of acid-soluble collagen from walleye pollock (Theragra chalcogramma) skin. Food Hydrocolloids, 29(1), 199–204. doi:10.1016/j.foodhyd.2012.02.014
- Yan, M., Li, B., Zhao, X., Ren, G., Zhuang, Y., Hou, H., & Fan, Y. (2008). Characterization of acid-soluble collagen from the skin of walleye pollock (Theragra chalcogramma). Food Chemistry, 107(4), 1581–1586. doi:10.1016/j.foodchem.2007.10.027
- Zhang, Y., Liu, W., Li, G., Shi, B., Miao, Y., & Wu, X. (2007). Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chemistry, 103(3), 906–912. doi:10.1016/j.foodchem.2006.09.053