Abstract
In the present study, the cottonseed peptides (CPs) were prepared from cottonseed meal fermented by Bacillus subtilis BJ-1. Chemical composition and antioxidant activities of the CP were investigated. The results showed that the CP contained a balanced ratio of amino acids which dominantly composed of glutamic acid (211.7 g kg−1), aspartic acid (81.4 g kg−1), and arginine (97.5 g kg−1). The molecular weight distribution of CP was lower than 1000 Da. The CP presented a concentration-dependent effect on the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity, hydroxyl radical activity, metal-chelating ability, and reducing power. The half maximal effective concentration values for the DPPH and hydroxyl radicals’ scavenging activities, metal-chelating activity, and reducing power were 3.41, 3.28, 1.80, and 1.66 mg mL−1, respectively. Moreover, CP at the lowest concentration of 0.01 mg mL−1 protected the H2O2-induced oxidant death in Raw 264.7 cells. These data suggest that CP could be used as a potential antioxidant for applications in food ingredients.
Con el objetivo de investigar la composición química y las actividades antioxidantes de los péptidos de semilla de algodón (CPs), éstos fueron preparados a partir de harina de semilla de algodón fermentada por Bacillus subtilis bj-1. Los resultados obtenidos mostraron que los CP contienen una proporción equilibrada de aminoácidos compuesta principalmente por ácido glutámico (2117 g kg−1), ácido aspártico (81,4 g kg−1) y arginina (97,5 g kg−1). Asimismo, se constató que la distribución del peso molecular de los CP fue menor a 1000 Da. Además, se evidenció que los CP presentaron un efecto sobre la actividad de eliminación del radical 1.1-difenil-2-picrilhidrazil (DPPH), la actividad del radical hidroxilo, la capacidad quelante de metales y en el poder reductor, vinculado a su concentración. Los valores medios de la máxima concentración que resulta efectiva para las actividades de eliminación de los radicales DPPH e hidroxilo, así como para la actividad quelante de metales y el poder reductor fueron 3,41, 3,28, 1,80, y 1,66 mg mL−1, respectivamente. Por otra parte, en una concentración mínima de 0,01 mg mL−1, los CP evitaron la muerte del oxidante inducida por H2O2 en células Raw 264,7. Estos datos sugieren que los CP podrían ser utilizados como un antioxidante potencial para aplicaciones en los ingredientes alimentarios.
Introduction
A wide range of reactive oxygen species is generated in the living organisms and the food system, including non-free-radical species such as singlet oxygen and hydrogen, as well as free radicals such as hydroxyl radical. The overproduction of these radicals not only causes the deterioration of the lipid-containing foods, but also induces oxidative stress that plays a critical role in many lifestyle-related diseases (Wanasundara, Shahidi, & Shukla, Citation1997). To provide protection against oxidative damage of the human body, several synthetic antioxidants are broadly used as additives, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole. However, the safety of these antioxidants is being questioned (Botterweck, Verhagen, Goldbohm, Kleinjans, & Brandt, Citation2000) and the identification of antioxidant properties from natural sources increasingly attracts more attention (Pan, Jiang, & Pan, Citation2011; Zhang et al., Citation2011).
Cottonseed meal (CSM) is the main by-product of the cottonseed oil extraction process. The protein fractions of CSM have long been considered as an attractive and promising protein source, consisting of approximately 22–56% crude protein by weight (Nagalakshmi, Rao, Panda, & Sastry, Citation2007). However, the use of CSM as a protein supplement in food and feed industry is limited due to the presence of anti-nutritional compounds such as gossypol. Several studies have developed methods to remove gossypol and recover proteins from CSM for human consumption (Gerasimidis, Fillou, Babatzimcpoulou, Tassou, & Katsikas, Citation2007; Tsaliki, Pegiadou, & Doxastakis, Citation2004).
Solid-state fermentation of CSM by dietary microorganisms has been reported as an effective way to reduce free gossypol and improve the in vitro digestibility of cottonseed proteins (Tang et al., Citation2012). Recent studies using peanut meal or soybeans as a substrate have demonstrated that Bacillus subtilis could improve the antioxidant property of water extracts from these plant-protein sources by increasing the concentrations of beneficial components, such as small-size peptides and free amino acids (Lee, Yang, & Mau, Citation2008; Zhang et al., Citation2011; Zhu, Fan, Cheng, & Li, Citation2008). However, to the best of our knowledge, there are very few studies examining CSM as source of bioactive peptides and no antioxidant activity has been reported in the cottonseed peptides (CPs) derived from Bacillus-fermented CSM.
Therefore, the objective of the present study was to examine the chemical and antioxidant properties of CP produced by solid-state fermentation. The chemical composition and molecular weight distribution were determined. The antioxidant activities were evaluated in terms of the scavenging ability on 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical and hydroxyl radical, reducing power, chelating ability on ferrous ions, as well as the protective effects on H2O2-induced Raw 264.7 cell death.
Materials and methods
Materials and chemicals
Defatted CSM was purchased from China Cotton-Unis Co., Ltd (Beijing, China). Dimethyl sulfoxide (DMSO), DPPH, BHT, Folin-Ciocalteu’s reagent, penicillin, streptomycin, papain (from papaya latex), and H2O2 were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Potato dextrose broth and potato dextrose agar were purchased from Difco (Sparks, MD, USA). Raw264.7 cell line was purchased from Jiancheng Biological Engineering Institute (Nanjing, China). Dulbecco’s modified Eagle’s medium (DMEM), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and fetal bovine serum were from Gibco (Grand Island, NY, USA). All other reagents and chemicals used were of analytical grade and were obtained from Sangon Biotech Co. (Shanghai, China).
Microorganism and culture condition
Bacillus subtilis BJ-1 was purchased from China General Microbiological Culture Collection (Beijing, China). The B. subtilis strain was grown and maintained on potato dextrose agar slopes at 4°C. The culture of strain was prepared by transferring a loop of bacterial cells from the slant to 50 mL of potato dextrose broth. Then, the broth was incubated at 37°C for 14 h in an air bath shaker. Cells were harvested by centrifuge at 1500 × g for 10 min at 4°C and re-suspended in sterile 0.9% (w/v) peptone to a final concentration of approximately 106 colony-forming units (CFU) mL−1. The resulting cell suspension was served as the inoculum for the CSM fermentation.
Solid-state fermentation of CSM
The solid-state fermentation of CSM was prepared by the method of Sun, Tang et al. (Citation2012) with minor modifications. Briefly, CSM (100 g) was mixed thoroughly with 100 mL of distilled water and transferred into a screw-capped glass bottle (500 mL). The soaked CSM was cooked at 121°C for 15 min in an autoclave and cooled to room temperature to prevent bacterial contamination during the fermentation process (Zhang, Xu, Zhao, Sun, & Yang, Citation2007). The cooled CSM was inoculated with 1% (v/w) of diluted culture of B. subtilis BJ-1 and 1.0 g kg−1 of papain (EC 3.4.22.2). After mixing thoroughly, the CSM was incubated in a bed-packed incubator at 30°C and 70% relative humidity for 12, 24, 36, and 48 h, respectively. After fermentation, fresh samples of fermented CSM were used to determine the B. subtilis BJ-1 numbers and then dried at 45°C in a hot-air oven for 24 h. The dried samples were measured for peptide content and stored in plastic bags at −20°C for CP extraction.
Preparation of CP
The extraction was performed according to the method of He et al. (Citation2012). Briefly, the dried meals (5 g) were extracted three times with 100 mL of distilled water and homogenized for 20 min at room temperature. The extract was combined and centrifuged at 4000 × g for 20 min. The supernatant was filtered through an ultra-filtration membrane with molecular weight cut-off of 3000 Da (Millipore, Billerica, MA, USA). The resulting filtrate was pooled, concentrated by an evaporator at 30°C, and freeze dried. The peptide extracts were stored at −20°C until chemical analysis. The extraction yield was calculated by determining the weight of freeze-dried extracts as a percentage of total weight of meal used. The CP was dissolved in the distilled water before used for the antioxidant activity assays.
Chemical analysis
The fat (method 920.39) and ash (method 942.05) contents of freeze-dried CP were determined by Association of Official Analytical Chemists (AOAC, Citation1999) procedures. The protein content was measured by the Kjeldahl method multiplying with a conversion factor of 6.25. The fiber content was determined by the filter bag technique after digesting with H2SO4 and NaOH (American Oil Chemists Society [AOCS], Citation2009). Free gossypol content (method Ba 7 b-96) was determined according to the official method (AOCS, Citation2009). Total phenol content was determined according to the method described by Lee et al. (Citation2008). The total polyphenolic content was expressed as milligram gallic acid equivalents per gram of crude extract. The peptide content in fermented CSM was determined according to the method of Niu, Jiang, and Pan (Citation2013). The number of B. subtilis BJ-1 was determined using the dilution-plate method according to our previous study (Tang et al., Citation2012).
Amino acid composition and molecular weight distribution
The total amino acid profile of the lyophilized CP was determined after hydrolysis with 6 mol L−1 HCl at 110°C for 24 h using an amino acid analyzer (Sykam 433D, Eresing, Germany) (Pan et al., Citation2011). Molecular weight distribution of CP was determined by a Waters e2695 system, with a ProteinPak 60 column (7.8 × 300 mm, 200–20,000 Da, Waters, Milford, MA, USA), in combination with UV detector (2489, Waters). Elution was at 35°C using the buffer (0.2 mol L−1 phosphate buffered saline, pH 8.0) at a flow rate of 0.5 mL min−1. The CP was monitored at 220 nm. A molecular weight calibration curve was obtained from the following standards from Sigma: cytochrome C (12,700 Da), aprotinin (6511 Da), bacitracin (1450 Da), tetrapeptide Gly-Gly-Tyr-Arg (451 Da), and tripeptide Gly-Gly-Gly (189 Da). The elution curve was integrated using the Empower 2 software (v 2.1, Waters, Milford, MA, USA).
Measurement of antioxidant activities
DPPH radical-scavenging assay
The DPPH radical-scavenging capacity was determined according to the method described by Zhang et al. (Citation2011) with a slight modification. Briefly, a water solution (100 μL) of CP at different concentrations was mixed with 2.9 mL of 0.1 mM DPPH solution (in methanol). The mixture was incubated in the dark for 30 min at room temperature. The absorbance at 517 nm was recorded against a reference sample (100% methanol). BHT was used as a positive control. The scavenging effect on DPPH radical was expressed as given below:
Hydroxyl radical-scavenging assay
The assay of hydroxyl radical scavenging was performed according to a method described by Ajibola, Fashakin, Fagbemi, and Aluko (Citation2011). An aliquot (50 μL) of CP at different concentration was first combined with 50 μL of ferrous sulfate and 50 μL of 1,10-phenanthroline. Then, 50 μL of 0.01% hydrogen peroxide solution was added to initiate the reaction. The mixture was incubated at 37°C for 1 h, and the absorbance at 536 nm was measured by a spectrophotometer. BHT was used as a positive control. The hydroxyl radical activity was calculated as follows:
Metal-chelating assay
A portion of 1 mL aliquot of the lyophilized CP (in distilled water) at different concentrations was added to 3.7 mL of distilled water and mixed thoroughly. Then, it was combined with 0.1 mL of ferrous chloride (2 mM) and 0.2 mL of ferrozine. The mixture was homogenized vigorously and left at room temperature for 10 min. The absorbance at 562 nm was recorded and distilled water replacing protein samples was served as a control. The chelating effect was calculated by the following equation:
Reducing power assay
The reducing power of CP was measured according to the method of Ajibola et al. (Citation2011) with modifications. Peptide extracts of CSM before and after fermentation (0.25 mL in distilled water) was mixed with 0.25 mL of sodium phosphate buffer (0.2 M, pH 6.6) and 0.25 mL of 1.0% (w/v, dissolved in distilled water) potassium ferricyanide. The resulting mixture was incubated at 50°C for 20 min. After cooling to the room temperature, the solution was combined with 0.25 mL of 10% (w/v) trichloroacetic acid, 0.2 mL of distilled water, and 50 μL of 0.1% (w/v) ferric chloride. After 10 min of reaction, the mixture was centrifuged at 2000 × g for 5 min. The resulting supernatant was collected and the absorbance of mixture was recorded at 700 nm.
Cell culture and cell viability assay
The Raw 264.7 cells were grown and maintained in DMEM supplemented with 10% (v/v) fetal calf serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. Cells were cultured at 37°C with 5% CO2. Cell viability was measured using a colorimetric assay with MTT (Xu et al., Citation2011). Cells were seeded at 1.0 × 104 cells per well on 96-well plates and incubated 12 h before treatment. The culture medium of each well was discarded and replaced with fresh complete medium with different concentrations (0.01–5 mg mL−1) of CPs. Control cells were replaced with DMEM only. After another 24 h of incubation, 20 μL of MTT (10 mg mL−1) solution was added to each well. The plates were incubated in dark at 37°C for 4 h and the media were aspirated and replaced by 200 μL of DMSO to dissolve the formazan. The absorbance in each well was measured at 570 nm after 10-min incubation. Cell assays were performed in quadruplet wells for each sample. The cell viability was calculated by the equation as follows:
Protective effect of the water extract against H2O2-induced cell death
To study the protective effects of CP on cell death induced by H2O2, Raw 264.7 cells were treated with DMEM containing various concentrations (0.01, 0.1, 0.5, 1, and 2.5 mg mL−1) of CP for 24 h as described above. Then, the media were aspirated and the oxidant stress was induced by exposing cells to 500 μM H2O2 (dissolved in DMEM) for 12 h. Finally, 20 μL of MTT was added to each well and the relative cell viability was measured at 540 nm by a SpectraMax M5 microplate reader (Molecular Device Co., Sunnyvale, CA, USA). The cells treated in the same procedure without CP and H2O2 were used as the positive control.
Statistical analysis
The results were analyzed by one-way analysis of variance using the SPSS 15.0 (SPSS Inc., Chicago, IL, USA). The significance of the differences between means was calculated by Duncan’s multiple-range test. The results were presented as mean ± standard deviation. The half maximal effective concentration value (EC50) was calculated by Excel 2007 software using quadratic equations. P value of < 0.05 was considered statistically significant.
Results and discussion
Solid-state fermentation
In our study, the concentrations of peptides and B. subtilis BJ-1 population increased with the increasing fermentation time (Supplemental Figure 1). After 48 h of fermentation, both the peptide content and microbial number reached their maximum values of 128.6 mg g−1 and 1.25 × 107 CFU g−1, respectively. The results suggest that the cottonseed proteins could be used as a nitrogen source for microbial growth and effectively hydrolyzed to peptides during fermentation. Similarly, He et al. (Citation2012) demonstrated a significant increase in soluble peptide content within the first 2 days of rapeseed meal fermentation. Another study by Niu et al. (Citation2013) also reported a maximum level of peptides at 48 h of fermentation when B. subtilis was used to produce wheat germ peptides. Thus, the 48-h ferments were selected to extract CP and determine its proximate composition and antioxidant activities. On the other hand, papain was used in the present study for increasing the production of low molecular weight compounds with better functional and nutritional characteristics (Amadou, Le, & Shi, Citation2013). The addition of papain significantly increased the peptide content of fermented CSM (243.3 mg g−1) after 48 h of fermentation, compared with those without the addition of papain (169.4 mg g−1). The result is in good agreement with our previous study, which showed a significant increase in soluble protein content after the addition of papain in the fermentation of CSM (H. Sun et al., Citation2012).
Extraction yield and chemical composition of CP
The average yield of lyophilized CP reached 19.2% after fermentation and ultrafiltration (). Similar extraction yield has been reported based on fermented soybean substrate (Lee et al., Citation2008) and fermented foxtail millet extracts (Amadou et al., Citation2013). As expected, the CP had higher protein content, compared with that of the CSM, making them a high-quality source of protein. This result is in good agreement with previous study, which reported protein fraction (84.04 g kg−1) as the major element of peptide extracts from fermented rapeseed meal (He et al., Citation2012). The increase in protein content of CP can be due to the higher solubilization of protein fractions in CSM after fermentation. Proteolytic enzymes released by Bacillus strains may lead to the accumulation of both free amino acids and peptides in the cottonseed extracts as reported in studies dealing with fermented soybean products (Zhu et al., Citation2008). On the other hand, the devoid of water-insoluble non-protein compounds such as fiber during the extraction process may also account for the increased protein content of CP. This view is supported by the reduced contents of ash, fat, and fiber in the CP, compared with those of CSM (). Other reasons for the increase in protein content includes the hydrolysis of protein fractions during the autoclaving process of CSM, although no significant differences were observed in peptide contents after sterilization in our preliminary experiment (data not shown). Moreover, more than 80% of the total phenolic compounds were eliminated, compared with the total phenolic content of 1.34 mg gallic acid equivalents per gram of CSM. The result is consistent with the findings of Chabanon, Chevalot, Framboisier, Chenu, and Marc (Citation2007), who reported a 75% reduction in the content of phenolic compounds after a water–ethanol treatment in the extraction of rapeseed proteins. Oskoueian, Abdullah, Hendra, and Karimi (Citation2011) also reported that phenolic compounds in CSM could be effectively extracted by using methanol solution. Thus, the low yield of phenols may be attributable to the fact that only the water-soluble phenol was extracted. Interestingly, the content of anti-nutritional component cannot be detected in the CP after fermentation, ultrafiltration, and lyophilization. The reduction of free gossypol content may be partly due to the binding of proteins, and/or special proteins secreted by bacteria during fermentation (H. Sun et al., Citation2012; Yang, Sun, Guo, & Weng, Citation2012). Similarly, He et al. (Citation2012) also reported a significant decrease in the contents of anti-nutritional components, especially glucosinolate in the peptide extracts of fermented rapeseed meal.
Table 1. Average yield and proximate composition of cottonseed meal (CSM) and cottonseed peptides (CPs).
Tabla 1. Rendimiento promedio y composición proximal de la harina de semilla de algodón (CSM) y de los péptidos de semilla de algodón (CPs).
DPPH radical-scavenging activity
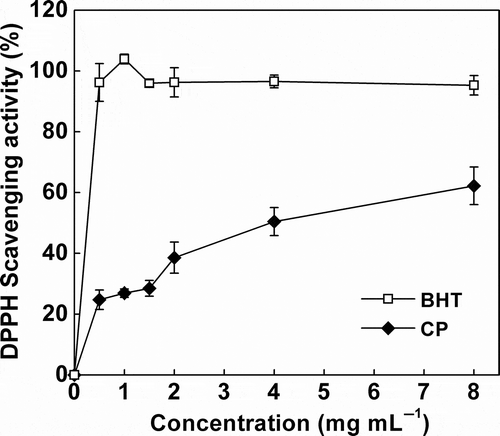
The DPPH is a stable lipophilic free radical that has been widely used to test the efficacy of antioxidants. As shown in the dose–response curve (), the DPPH radical-scavenging effect of the CP increased with the increasing concentration up to 8 mg mL−1. To further evaluate the antiradical efficiency of CP against DPPH, we calculated the EC50 value of CP and BHT (positive control), which is defined as the amount of antioxidant required to eliminate the radical content by 50%. The EC50 value for CP was 3.41 mg mL−1, higher than that of BHT (0.04 mg mL−1) and the 165 μg mL−1 reported by He et al. (Citation2012) for rapeseed peptides produced by solid-state fermentation. However, similar value of 3.16 mg mL−1 has been reported for the peptides isolated from fermented wheat germ (Niu et al., Citation2013). Our results indicate that CP probably possessed electron donors which convert DPPH radicals into harmless products. This finding is in good agreement with that observed in peanut peptides (Zhang et al., Citation2011) and soybean peptides (Zhu et al., Citation2008), which showed a significant increase in the DPPH scavenging effect after Bacillus fermentation. The scavenging effect of CP can be attributed to the function of proteolysis products that are formed during the fermentation process (Yin, Tong, & Jiang, Citation2005). Many active peptides that can scavenge the free radicals exist in the CSM protein hydrolysates (Gao, Cao, & Li, Citation2010). Besides, some bacterial metabolites produced by Bacillus strains, such as polysaccharide, may also account for the increase in scavenging activity of DPPH radicals (Kumar, Joo, Choi, Koo, & Chang, Citation2004; Zhang et al., Citation2011). Readers also should be noticed that our finding does not exclude the possibility that the gossypol may be partly account for the antioxidant activity of CPs, given that gossypol could bind to protein fraction during the fermentation process.
Figure 1. 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activity of cottonseed peptides (CPs). Each value is expressed as mean ± SD (n = 3). Butylated hydroxytoluene (BHT) was used as a positive control.
Figura 1. Actividad de los péptidos de semilla de algodón (CP) en la eliminación del radical 1,1-difenil-2-picrilhidrazyl. Cada valor se expresa como una media ± DE (n = 3). Se utilizó butilhidroxitolueno (BHT) como control positivo.
Hydroxyl radical-scavenging activity
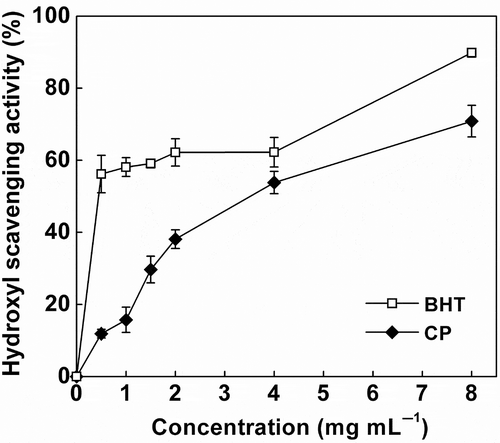
The hydroxyl radicals are the most reactive species among the oxygen radicals and severely induce damage to almost any adjacent biomolecules (Lee, Koo, & Min, Citation2004). The hydroxyl radical-scavenging effect of CP increased steadily in the concentration range of 0–8 mg mL−1 (), suggesting a concentration-dependent trend. The EC50 values for CP and BHT were 3.28 and 1.33 mg mL−1, respectively. No data comparison could be made because of the shortage of available literatures concerning the hydroxyl scavenging activity of CP. Although the EC50 value of CP was much higher than that of BHT, the value is lower than the 6.04 mg mL−1 for fermented wheat germ peptides (Niu et al., Citation2013) and 4.92 mg mL−1 for rapeseed protein hydrolysates (Pan et al., Citation2011). Recently, Lee et al. (Citation2008) reported a high hydroxyl scavenging activity of water extracts derived from Monascus fermented soybeans and ascribed the effects to phenolic compounds. In the present study, the peptide extracts of fermented CSM contained high contents of protein fractions and very few phenols. Therefore, the active peptides and/or free amino acids could also attribute to the hydroxyl radical activity of CP. Our findings indicate that CP is a good hydroxyl radical scavenger and could be used as a promising antioxidant-rich food ingredient.
Figure 2. Hydroxyl radical-scavenging activities of cottonseed peptides (CPs). Each value is expressed as mean ± SD (n = 3). Butylated hydroxytoluene (BHT) was used as a positive control.
Figura 2. Actividades de los péptidos de semilla de algodón (CPs) en la eliminación del radical hidroxilo. Cada valor se expresa como una media ± DE (n = 3). Se utilizó butilhidroxitolueno (BHT) como control positivo.
Metal-chelating activity
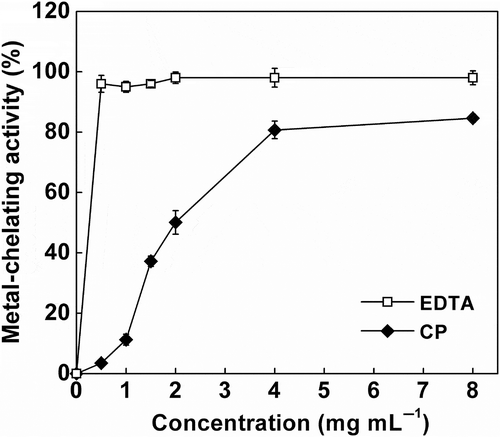
Transition metal ions, such as Cu2+ and Fe2+ are involved in generation of reactive oxygen species in vivo (Lee et al., Citation2004). Thus, the chelation of these metal ions may help to retard the oxidation process. The metal-chelating activity of CP increased with the increasing concentrations (). The EC50 value of CP was 1.80 mg mL−1 calculated from nonlinear regression, which is much lower than that of rapeseed peptides with an EC50 value of 7 mg mL−1 (He et al., Citation2012). However, EC50 value in the present study is still acceptable compared to the results for peanut peptides (Zhang et al., Citation2011), which demonstrated 76.32% metal-chelating activity at a concentration of 2 mg mL−1. Our results indicate that CP could be used as effective antioxidants. Similar result has been reported in peptides from Bacillus-fermented peanut meals (Zhang et al., Citation2011). However, Yin et al. (Citation2005) reported no significant increase in metal-chelating activity of mackerel minces after 48 h of fermentation by lactic acid bacteria. This discrepancy may be attributed to the different starter organisms used in fermentation procedure. Additionally, the metal-chelating activity of CP was lower than that of ethylene diamine tetraacetic acid (EDTA) at concentrations ranged from 0 to 8 mg mL−1. However, CP could be applied in food at a much higher concentration than EDTA, due to the toxicological restrictions on synthetic antioxidants.
Figure 3. Metal-chelating activity of cottonseed peptides (CPs). Each value is expressed as mean ± SD (n = 3). Ethylene diamine tetraacetic acid (EDTA) was used as a positive control.
Figura 3. Actividad quelante de metales de los péptidos de semilla de algodón (CPs). Cada valor se expresa como media ± DE (n = 3). Se utilizó el ácido etilendiaminotetraacético (EDTA) como control positivo.
Reducing power
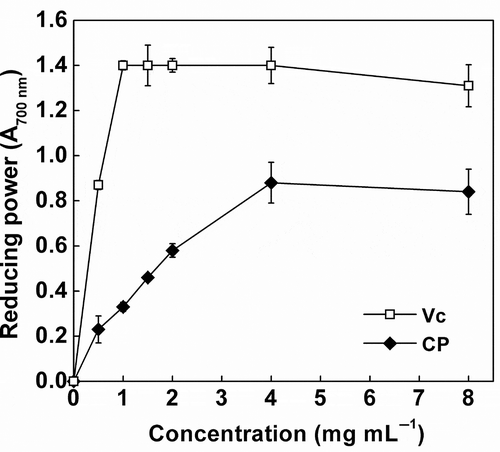
Reducing power of a compound is closely related to its ability to donate electrons. Therefore, the measurement of reducing power is widely used to assess the potential antioxidant ability of natural antioxidants. At the concentration from 0 to 8 mg mL−1, the reducing power of CP was lower than that of ascorbic acid, which is a well-recognized reducing agent. The reducing power of CP increased gradually with the increasing concentration, reaching a peak value at 8 mg mL−1 (). The EC50 value of CP was 1.66 mg mL−1 for reducing power, much higher than that of commercial antioxidants (0.01 mg mL−1). The result is in agreement with the results reported by Zhang, Wang, and Xu (Citation2008) on rapeseed peptides. Since the relationship between reducing power and antioxidant activity, our results obviously suggest a good ability of CP to donate electrons. Similar findings of the reducing power have been previously reported in fermented soybean products (Zhu et al., Citation2008) and peanut meal extracts (Zhang et al., Citation2011).
Figure 4. Reducing power of cottonseed peptides (CPs). Each value is expressed as mean ± SD (n = 3). Ascorbic acid (Vc) was used as a positive control.
Figura 4. Poder reductor de los péptidos de semilla de algodón (CPs). Cada valor se expresa como una media ± DE (n = 3). Se utilizó el ácido ascórbico (Vc) como control positivo.
Cytotoxicity and protection of the H2O2-induced cell death
The cell viability assay was performed to test the cytotoxic effects of CP on Raw 264.7 cells (). The CP could increase (P < 0.05) the cell viability when the concentration was 0.01 or 0.1 mg mL−1. However, the cell viability of Raw 264.7 cells reduced (P < 0.05) to less than 80% if the concentration of CP was 5 mg mL−1. These results are in agreement with previous studies on the cytotoxicity of protein hydrolysates from fermented mackerel (Yin et al., Citation2005) and fermented black soybean broth (Lin, Wu, Liang, Kwan, & Chen, Citation2012). The decrease in cell viability may be due to the high concentration of CP that brings high ions or solutes in cell culture medium and influences the osmotic pressure of the cell environment. The data indicate that CP is safe for Raw 264.7 cells at the concentrations below 5 mg mL−1. Therefore, 0.01, 0.1, 0.5, 1, and 2.5 mg mL−1 of CP were used in the following cell assay.
Figure 5. Cytotoxicity of cottonseed peptides (CPs) (A) and its protective effects against H2O2-induced decrease of Raw 264.7 cell viability (B). Each value is expressed as mean ± SD (n = 3). Bars with different alphabets have significantly different mean values at P < 0.05. Cells treated with complete medium only were used as a positive control (C). Cells treated with H2O2 only were used as a negative control (H2O2).
Figura 5. Citotoxicidad de los péptidos de semilla de algodón (CPs) (A) y sus efectos protectores contra la reducción de la viabilidad de células Raw 264,7 inducida por H2O2 (B). Cada valor se expresa como una media ± DE (n = 3). Las barras con letras diferentes tienen valor medio significativamente diferente en P < 0,05. Las células tratadas solo con un medio completo fueron utilizadas como un control positivo (C). Las células tratadas solo con H2O2 fueron utilizadas como un control negativo (H2O2).
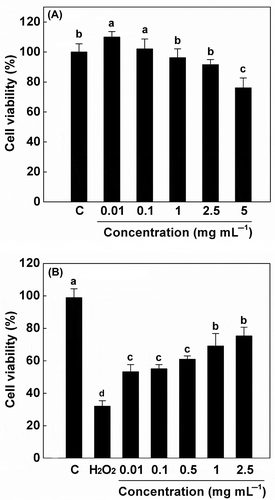
To further investigate the biological antioxidant activity of CP, the protective effects of these fractions against H2O2-induced oxidant death of Raw 264.7 cells were accessed. The H2O2 plays an important role in radical forming after it penetrates into the cytoplasm, and could induce cell apoptosis (L. Sun et al., Citation2012). As shown in , the viability of Raw 264.7 cells decreased (P < 0.05) to approximately 36% compared with that of the negative control. Pre-treatment with CP was able to alleviate the cell death in a clear dose-dependent manner at concentration of 0.01–2.5 mg mL−1. The cell viability reached the peak level to 79.98 ± 3.79% when the cells were pretreated with 2.5 mg mL−1 of CP. This effect may be attributed to the radical-scavenging activity of CP as discussed above. Similarly, Zhong, Ma, Lin, and Luo (Citation2011) reported that peptide fractions derived from silver carp processing by-product protein hydrolysates exhibited cyto-protective effects against H2O2-induced cell damage of human intestinal epithelial cells. Katayama, Xu, Fan, and Mine (Citation2006) also reported a protective effect of oligo-peptides from egg yolk hydrolysates on cell death induced by oxidative stress. Our results suggest that CP may act as an antioxidant in H2O2-treated cell models.
Amino acid composition
To determine the possible effects of amino acids profile on the antioxidant activity, CP was subjected to amino acid composition analysis. The total amino acid content of CP is 854.1 g kg−1 of sample (), which is similar to that of rapeseed peptides (He et al., Citation2012), but is less than the value of 960.7 g kg−1 reported by Zhang et al. (Citation2011) in peanut peptides. In general, glutamic acid, aspartic acid, and arginine were the major amino acids of the CP, which is consistent with the amino acid profile of CSM (Tang et al., Citation2012). The total hydrophobic amino acid (valine, leucine, proline, methionine, phenylalanine, and isoleucine) content was 283.1 g kg−1. In accordance with the present results, protein hydrolysate fractions from rapeseed (Zhang et al., Citation2008) and yam bean seed (Ajibola et al., Citation2011) that exhibited the strong reducing power contained a high amount of hydrophobic amino acids. Gao et al. (Citation2010) also reported a strong hydroxyl radical-scavenging activity of peptide fraction from cottonseed protein hydrolysates that were rich in hydrophobic amino acids. Therefore, the antioxidant activity of CP might be due to the hydrophobic amino acids and antioxidant peptides, although the sequence of peptides may also be a contributing factor. Moreover, previous study has ascribed the higher antioxidant activity of protein hydrolysates to an increase in the concentration of the acidic (aspartic acid and glutamic acid) and basic (lysine, arginine, and histidine) amino acids (Zhang et al., Citation2011). Especially, histidine can neutralize radical species to form stable oxidation products due to its imidazole ring (Pan et al., Citation2011). The total contents of basic and acid amino acids were 166.2 and 283.1 g kg−1, respectively (), suggesting that these amino acids could also account for the antioxidant activity of CP. Furthermore, the essential amino acids (isoleucine, leucine, methionine, lysine, phenylalanine, threonine, tryptophan, valine, and histidine) made up 296.2 g kg−1 and were close to those of amino acid requirements for human adult nutrition (Food and Agriculture Organization [FAO], Citation2007). Thus, the CP could be considered as a nutritional food source with well-balanced amino acid composition.
Table 2. Total amino acid composition of cottonseed peptides (g kg–1 dry weight of extract sample).
Tabla 2. Composición de los aminoácidos totales de los péptidos de semilla de algodón (g kg−1 peso seco de la muestra extraída).
Molecular weight distribution
The molecular weight distribution profile of CP was analyzed by gel filtration chromatography. CP was mainly composed of the low molecular weight substances ranged from below 158 Da to 3000 Da () with 56.52% of the fractions less than 1000 Da. This result indicates that CP contained a large amount of peptides with 10 or less amino acid residuals, which is consistent with previous study by Lee, Rho, Kim, Lee, and Lee (Citation2013), who reported that about 46% of the peptides in fermented soybean extracts had a molecular weight less than 1000 Da. However, the proportion of low molecular weight peptides was much lower than that reported for peanut peptides (86.89%) (Zhang et al., Citation2011). The discrepancy is probably due to the differences in strains and fermentation substrates, suggesting that peanut meal protein were more susceptible to degradation with microbes than cottonseed proteins. Moreover, several studies have related the antioxidant activity of hydrolyzed proteins to the contents of small molecular weight peptides and amino acids produced during the fermentation of raw protein materials (Amadou et al., Citation2013; Niu et al., Citation2013). Thus, the high content of low molecular weight peptides ranging from 158 to 1000 Da probably associated with the high antioxidant activity of CP, although these bioactive peptides still needs to be identified in the future study.
Table 3. Molecular weight distribution of cottonseed peptides.
Tabla 3. Distribución del peso molecular de los péptidos de semilla de algodón.
Conclusions
In conclusion, the CP prepared by fermentation displayed a dose-dependent effect on the free radical-scavenging activity, hydroxyl radical activity, metal-chelating ability, and reducing power. Moreover, CP protected the Raw 264.7 cells from H2O2-induced cell damage. The CPs were also of high nutritional value due to its balanced ratio of essential amino acids. This study suggests that the CP may be used as a good source of natural antioxidants for food ingredients. Further studies are still needed to isolate antioxidant peptides from CP.
Supplementary material
The supplementary material for this article is available at http://dx.doi.org/10.1080/19476337.2014.948072
Supplemental Figure 1. The concentrations of B. subtilis BJ-1 and peptides in the fermented cottonseed meal during the solid-state fermentation. Each value is expressed as mean ± SD (n = 3).
Download TIFF Image (961.8 KB)Acknowledgments
This research is supported by the “San-Nong-Liu-Fang” Sci-Tech Support Program of Zhejiang Province and the General Research Program of Zhoushan, China (2012C33015).
References
- Ajibola, C. F., Fashakin, J. B., Fagbemi, T. N., & Aluko, R. E. (2011). Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. International Journal of Molecular Sciences, 12, 6685–6702. doi:10.3390/ijms12106685
- Amadou, I., Le, G. W., & Shi, Y. H. (2013). Evaluation of antimicrobial, antioxidant activities, and nutritional values of fermented foxtail millet extracts by Lactobacillus paracasei FN032. International Journal of Food Properties, 16, 1179–1190. doi:10.1080/10942912.2011.579673
- AOAC. (1999). Official methods of analysis (16th ed.). Washington, DC: Association of Official Analytical Chemists.
- AOCS. (2009). Official methods and recommended practices of the AOCS (6th ed.). Chicago, IL: American Oil Chemists Society.
- Botterweck, A. A., Verhagen, H., Goldbohm, R. A., Kleinjans, J., & Brandt, P. A. (2000). Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in the Netherlands cohort study. Food and Chemical Toxicology, 38, 599–605. doi:10.1016/S0278-6915(00)00042-9
- Chabanon, G., Chevalot, I., Framboisier, X., Chenu, S., & Marc, I. (2007). Hydrolysis of rapeseed protein isolates: Kinetics, characterization and functional properties of hydrolysates. Process Biochemistry, 42, 1419–1428. doi:10.1016/j.procbio.2007.07.009
- FAO. (2007). Protein and amino acid requirements in human nutrition (WHO technical report series, No. 935). Geneva, Switzerland: World Health Organization (WHO) Press. Retrieved from whqlibdoc.who.int/trs/who_trs_935_eng.pdf
- Gao, D., Cao, Y., & Li, H. (2010). Antioxidant activity of peptide fractions derived from cottonseed protein hydrolysate. Journal of the Science of Food Agriculture, 90, 1855–1860.
- Gerasimidis, K., Fillou, D. T., Babatzimcpoulou, M., Tassou, K., & Katsikas, H. (2007). Preparation of an edible cottonseed protein concentrate and evaluation of its functional properties. International Journal of Food Sciences and Nutrition, 58, 486–490. doi:10.1080/09637480701288488
- He, R., Ju, X. R., Yuan, J., Wang, L. F., Girgih, A. T., & Aluko, R. E. (2012). Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Research International, 49, 432–438. doi:10.1016/j.foodres.2012.08.023
- Katayama, S., Xu, X. M., Fan, M. Z., & Mine, Y. (2006). Antioxidative stress activity of oligophosphopeptides derived from hen egg yolk phosvitin in Caco-2 cells. Journal of Agricultural and Food Chemistry, 54, 773–778. doi:10.1021/jf052280d
- Kumar, C. G., Joo, H. S., Choi, J. W., Koo, Y. M., & Chang, C. S. (2004). Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme and Microbial Technology, 34, 673–681. doi:10.1016/j.enzmictec.2004.03.001
- Lee, J., Koo, N., & Min, D. B. (2004). Reactive oxygen species, aging, and antioxidative nutraceuticals. Comprehensive Reviews in Food Science and Food Safety, 3, 21–33. doi:10.1111/j.1541-4337.2004.tb00058.x
- Lee, J. S., Rho, S. J., Kim, Y. W., Lee, K. W., & Lee, H. G. (2013). Evaluation of biological activities of the short-term fermented soybean extract. Food Science and Biotechnology, 22, 973–978. doi:10.1007/s10068-013-0172-z
- Lee, Y. L., Yang, J. H., & Mau, J. L. (2008). Antioxidant properties of water extracts from Monascus fermented soybeans. Food Chemistry, 106, 1128–1137. doi:10.1016/j.foodchem.2007.07.047
- Lin, C. C., Wu, P. S., Liang, D. W. M., Kwan, C. C., & Chen, Y. S. (2012). Quality, antioxidative ability, and cell proliferation-enhancing activity of fermented black soybean broths with various supplemental culture medium. Journal of Food Science, 77, C95–C101. doi:10.1111/j.1750-3841.2011.02443.x
- Nagalakshmi, D., Rao, S. V. R., Panda, A. K., & Sastry, V. R. B. (2007). Cottonseed meal in poultry diets: A review. The Journal of Poultry Science, 44, 119–134. doi:10.2141/jpsa.44.119
- Niu, L. Y., Jiang, S. T., & Pan, L. J. (2013). Preparation and evaluation of antioxidant activities of peptides obtained from defatted wheat germ by fermentation. Journal of Food Science and Technology, 50, 53–61. doi:10.1007/s13197-011-0318-z
- Oskoueian, E., Abdullah, N., Hendra, R., & Karimi, E. (2011). Bioactive compounds, antioxidant, xanthine oxidase inhibitory, tyrosinase inhibitory and anti-inflammatory activities of selected agro-industrial by-products. International Journal of Molecular Sciences, 12, 8610–8625. doi:10.3390/ijms12128610
- Pan, M., Jiang, T. S., & Pan, J. L. (2011). Antioxidant activities of rapeseed protein hydrolysates. Food and Bioprocess Technology, 4, 1144–1152. doi:10.1007/s11947-009-0206-y
- Sun, H., Tang, J. W., Yao, X. H., Wu, Y. F., Wang, X., & Feng, J. (2012). Improvement of the nutritional quality of cottonseed meal by Bacillus subtilis and the addition of papain. International Journal of Agriculture and Biology, 14, 563–568.
- Sun, L., Yau, H. Y., Wong, W. Y., Li, R. A., Huang, Y., & Yao, X. Q. (2012). Role of TRPM2 in H2O2-induced cell apoptosis in endothelial cells. Plos One, 7, e43186. doi:10.1371/journal.pone.0043186
- Tang, J. W., Sun, H., Yao, X. H., Wu, Y. F., Wang, X., & Feng, J. (2012). Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian-Australasian Journal of Animal Sciences, 25, 393–400. doi:10.5713/ajas.2011.11381
- Tsaliki, E., Pegiadou, S., & Doxastakis, G. (2004). Evaluation of the emulsifying properties of cottonseed protein isolates. Food Hydrocolloids, 18, 631–637. doi:10.1016/j.foodhyd.2003.11.001
- Wanasundara, P. K. J. P. D., Shahidi, F., & Shukla, V. K. S. (1997). Endogenous antioxidants from oilseeds and edible oils. Food Reviews International, 13, 225–292. doi:10.1080/87559129709541106
- Xu, Y., Zhao, H., Zhang, M., Li, C. J., Lin, X. Z., Sheng, J., & Shi, W. (2011). Variations of antioxidant properties and NO scavenging abilities during fermentation of tea. International Journal of Molecular Sciences, 12, 4574–4590. doi:10.3390/ijms12074574
- Yang, X., Sun, J. Y., Guo, J. L., & Weng, X. Y. (2012). Identification and proteomic analysis of a novel gossypol-degrading fungal strain. Journal of the Science of Food and Agriculture, 92, 943–951. doi:10.1002/jsfa.4675
- Yin, L. J., Tong, Y. L., & Jiang, S. T. (2005). Improvement of the functionality of minced mackerel by hydrolysis and subsequent lactic acid bacterial fermentation. Journal of Food Science, 70, M172–M178. doi:10.1111/j.1365-2621.2005.tb07146.x
- Zhang, S. B., Wang, Z., & Xu, S. Y. (2008). Antioxidant and antithrombotic activities of rapeseed peptides. Journal of the American Oil Chemists’ Society, 85, 521–527. doi:10.1007/s11746-008-1217-y
- Zhang, W. J., Xu, Z. R., Zhao, S. H., Sun, J. Y., & Yang, X. (2007). Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Animal Feed Science and Technology, 135, 176–186. doi:10.1016/j.anifeedsci.2006.06.003
- Zhang, Y., Zhang, H., Wang, L., Guo, X., Qi, X., & Qian, H. (2011). Influence of the degree of hydrolysis (DH) on antioxidant properties and radical-scavenging activities of peanut peptides prepared from fermented peanut meal. European Food Research and Technology, 232, 941–950. doi:10.1007/s00217-011-1466-0
- Zhong, S., Ma, C., Lin, Y. C., & Luo, Y. (2011). Antioxidant properties of peptide fractions from silver carp (Hypophthalmichthys molitrix) processing by-product protein hydrolysates evaluated by electron spin resonance spectrometry. Food Chemistry, 126, 1636–1642. doi:10.1016/j.foodchem.2010.12.046
- Zhu, Y. P., Fan, J. F., Cheng, Y. Q., & Li, L. T. (2008). Improvement of the antioxidant activity of Chinese traditional fermented Okara (Meitauza) using Bacillus subtilis B2. Food Control, 19, 654–661. doi:10.1016/j.foodcont.2007.07.009
