Abstract
Broccoli (Brassica oleracea var. italica) is an excellent source of bioactive compounds. Frequently, it is commercialized frozen, though it has to be thawed before consumption. However, defrosting methods can affect the nutritional and sensory properties of broccoli. Therefore, the effect of defrosting (microwaving and boiling) for serving broccoli either cold or hot on the content of bioactive compounds (vitamin C, carotenoids, phenolic compounds, and glucosinolates) and sensory acceptability of frozen broccoli was studied. Marked losses of hydrosoluble compounds were observed after boiling. Carotenoids increased after short-time boiling (~20%) but dramatically decreased after microwave-defrosting (between 30% and 40% less). Nevertheless, short defrosting using microwave showed the less overall losses. Moreover, microwave-based methods were preferred by consumers. Microwave-defrosting of broccoli for a short period of time may be the method of choice for better retention of bioactive compounds and organoleptic properties.
El brócoli (Brassica oleracea var. italica) es una excelente fuente de compuestos bioactivos. Frecuentemente, es comercializado congelado, aunque debe ser descongelado antes de ser consumido. No obstante, los métodos de descongelación pueden afectar las propiedades nutritivas y sensoriales del brócoli congelado. Por consiguiente, el efecto del descongelamiento (microondas y hervido) para servir brócoli frío o caliente en el contenido de compuestos bioactivos (vitamina C, carotenoides, compuestos fenólicos y glucosinolatos) y la aceptabilidad sensorial del brócoli congelado fue estudiado. Pérdidas marcadas de compuestos hidrosolubles fueron observadas después de hervir. Los carotenoides incrementaron después de hervir por tiempo corto (~20%) pero decrecieron después de la descongelación por microondas (entre 30% y 40% menos). Sin embargo, el descogelamiento corto utilizando microondas mostró las menores pérdidas globales. Además, los métodos por microondas fueron preferidos por los consumidores. El descongelamiento corto por microondas puede ser el método de elección para una mejor retención de nutracéuticos y propiedades organolépticas del brócoli congelado.
Introduction
Broccoli (Brassica oleracea var. italica) is a green plant that belongs to the cruciferous group of vegetables. Broccoli has been widely studied mainly due to its rich content of bioactive molecules such as vitamin C, glucosinolates, phenolic compounds, and carotenoids (Mahn & Reyes, Citation2012; Moreno, Carvajal, López-Berenguer, & García-Viguera, Citation2006; Vallejo, Tomás-Barberán, & García-Viguera, Citation2002). Therefore, it is considered to play an important role in the prevention of chronic diseases. Moreover, several studies have shown its potential against cancer, cardiovascular diseases, diabetes, and neurodegenerative diseases (Armah et al., Citation2013; Bahadoran et al., Citation2012; Fahey, Zhang, & Talalay, Citation1997; James et al., Citation2012; Jeffery & Araya, Citation2009; Johnson, Citation2002; Murashima, Watanabe, Zhuo, Uehara, & Kurashige, Citation2004; Riso, Martini, Visioli, Martinetti, & Porrini, Citation2009; Wu et al., Citation2004).
Nevertheless, broccoli is often commercialized as frozen florets and has to be thawed before consumption. In order to thaw frozen broccoli, consumers generally use microwaving or boiling water for serving broccoli either cold, to simply defrost broccoli, or hot, to obtain the characteristics of cooked broccoli. Defrosting can negatively affect the chemical composition of broccoli (Bernhardt & Schlich, Citation2006; Nicoli, Anese, & Parpinel, Citation1999; Podsędek, Citation2007), as well as its organoleptic properties (Bongoni, Verkerk, Steenbekkers, Dekker, & Stieger, Citation2014). Thus, the objective of the present study was to evaluate the effect of different defrosting methods (using boiling water or microwave) for serving broccoli either cold or hot, on the content of bioactive compounds (vitamin C, phenolics, carotenoids, and glucosinolates) and sensory acceptability of frozen broccoli so as to give consumers a better recommendation based on the method that best retains its nutritional and sensory properties.
Materials and methods
Chemicals
Sulfatase (from Helix pomatia), Folin-Ciocalteu reagent (2 N), sodium carbonate (Na2CO3), sephadex A-25, acetonitrile (HPLC grade), methanol (HPLC grade), tert-Butyl methyl ether (tMBE; HPLC grade), sodium acetate, sinigrin hydrate, ascorbic acid (AA), and gallic acid (GA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Acetic acid, acetone (HPLC grade), and isopropanol (HPLC grade) were purchased from Desarrollo de Especialidades Químicas (San Nicolás de los Garza, NL, México). Desulfoglucoraphanin was obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
Plant material, processing and defrosting studies
Frozen broccoli florets (Brassica oleracea cv. Tlaloc®) were provided by Frigorizados La Huerta (San Francisco de los Romo, Aguascalientes, Mexico). To obtain frozen samples, broccoli was cut to produce florets of 1.9–2.5 cms length. Thereafter, the florets were sanitized with a solution containing 45 ppm of peracetic acid, and blanched by steaming (90°C for 100 s). Finally, the blanched broccoli florets (equilibrated at 11°C) were frozen by subjecting the samples to −26°C for 8 min in an Individual Quick Freezing (IQF) fluidised tunnel system, obtaining a final temperature of −16°C in the broccoli florets. One day after processing, frozen broccoli samples were transported to the FEMSA-Biotechnology Center of Tecnológico de Monterrey-Campus Monterrey (Monterrey, N.L., México) for the corresponding studies. Frozen broccoli was rapidly subjected to phytochemical analysis after defrosting treatments.
Four defrosting methods were tested. To serve broccoli either cold or hot, samples were defrosted using a stove (Whirlpool, Benton Harbor, MI, USA) or a microwave oven (General Electric, Fairfield, CT, USA) at full power (1000 W). To serve cold, ~320 gr of frozen broccoli were either introduced for 40 s in a stove containing 1 L boiling water (Stove Method 1, SM1) or in a microwave at full power (1000 W) for 90 s with an additional 60 s after mixing (Microwave Method 1, MM1). To serve hot, for the stove method, samples were left for an additional 120 s in the hot water after the initial 40 s (SM2), and for the microwave method, frozen broccoli was introduced for 180 s with an additional 120 s after mixing (MM2). These methods were chosen based on the recommendations of the label of the commercialized product. Untreated frozen florets were used as controls.
Extraction and quantification of total phenolic compounds and vitamin C
The total phenolic content (PC) and vitamin C content were determined simultaneously by Folin-Ciocalteu method as described by Sánchez-Rangel, Benavides, Heredia, Cisneros-Zevallos, and Jacobo-Velázquez (Citation2013) with some modifications. Broccoli samples (5 g) were homogenized with methanol (20 mL) using a tissuemizer (Advanced homogenizing system, VWR). Samples were then centrifuged (4000 rpm, 1 h, 4°C) and the supernatant was recovered. Fifteen μL of each broccoli extract was diluted with 240 μL of ultrapure water in a 96-well microplate and the Folin-Ciocalteu reagent (0.25 N, 15 μL) was added. After incubation for 3 min, absorbance values were determined at 765 nm using a plate reader (BioTek, Winooski, VT, USA). These values were compared against a standard curve prepared of ascorbic acid (AA) solutions in order to quantify total vitamin C in the extracts. Afterwards, the same samples were used to determine PC. Thirty μL of Na2CO3 (1 N) was added to each mixture and the plate was incubated for 2 h at room temperature in the dark. Absorbance was read at 765 nm and values were compared against a gallic acid (GA) standard curve. The concentration of phenolic compounds and vitamin C was expressed, respectively, as mg of GA equivalents and mg of AA equivalents per kg of broccoli dry weight (DW). To calculate the concentration of phenolic compounds and AA in dry weight (DW), the moisture content of the samples was determined by the air-oven method. Briefly, broccoli (approximately 5 g) were placed in a metal dish and dried for 48 h at 60°C. Five repetitions were done for each sample.
Extraction, identification, and quantification of total glucosinolates
For the chromatographic determination of glucosinolates, extraction was done as described by Gallaher, Gallaher, and Peterson (Citation2012) with some modifications. Broccoli samples were lyophilized prior to extraction. For myrosinase inactivation, broccoli powder (0.2 g) was placed in a tube (15 mL) and preheated at 80°C for 10 min in a water bath. Hot methanol (4.5 mL) was added to the samples and the tubes were capped loosely and heated at 80°C for 20 min. Afterwards, 1 mL of boiling water was added, the tubes were capped tightly and heated for 30 min at 80°C. Before myrosinase inactivation, a 3 mM solution of sinigrin (50 μL) was added as internal standard to each sample. Samples were removed from the water bath, cooled to room temperature, homogenized, and centrifuged (18000 g, 20 min, 4°C). The clear supernatant was transferred to another tube and the pellet was re-suspended in 2.5 mL of 90% methanol. Samples were centrifuged again at the same conditions and the supernatant was pooled with the first one. The pellet was resuspended in 2.5 mL of 90% methanol and spun again; the supernatant was pooled with the other two supernatants.
Afterwards, glucosinolates were desulphated and purified using mini-columns (Thermo Fisher Scientific, Waltham, MA, USA) as described by Saha et al. (Citation2012). Clear supernatant (3 mL) was added into a prepared column and was allowed to drip through slowly. Columns were washed with 2 × 0.5 mL of water followed by 2 × 0.5 mL of 0.02 M sodium acetate. Purified sulfatase (75 μL) was added to each sample and was left at room temperature overnight (12 h). Collection vials were placed under needles and desulfoglucosinolates were eluted with a total of 1.25 mL of water (0.5 mL + 0.5 mL + 0.25 mL).
The HPLC system used was composed of a quaternary pump, an autosampler, and a diode array detector (DAD) (1260 Infinity, Agilent Technologies, Santa Clara, CA, USA). Desulfoglucosinolates were separated on a 4.6 mm × 250 mm, 5 μm, C18 reverse phase column (Luna, Phenomenex, Torrace, CA, USA). Separation of desulfoglucosinolates was performed as reported by Vallejo, Tomás-Barberán, and García-Viguera (Citation2003a). Mobile phases consisted of water (Phase A) and acetonitrile (Phase B) with a flow rate of 1.5 mL min−1 and a gradient of 0/100, 28/80, 30/100 (min/% phase A). For the quantification of glucosinolates, a standard curve of desulfoglucoraphanin was prepared in the range of 0–700 μM. The concentration of total glucosinolates was expressed as μmol of desulfoglucoraphanin equivalents per g of broccoli DW.
The tentative identification of glucosinolates was performed by three different methods: (a) identification by comparison with retention time and UV/Visible spectra characteristics of commercial standards, (b) identification by interpretation of UV/Visible spectra and comparison with previous reports, and (c) identification by order of elution reported in the literature.
Extraction, identification, and quantification of total carotenoids
Extraction and chromatographic separation of carotenoids was done as described by Becerra-Moreno, Alanís-Garza, Mora-Nieves, Mora-Mora, and Jacobo-Velázquez (Citation2014). Broccoli (1 g) was homogenized with 0.1% BHT acetone solution (10 mL) using a tissuemizer. Homogenates were filtered using Whatman No. 1 filter papers (Piscataway, NJ, USA) and the remaining solids were resuspended in 10 mL of 0.1% BHT acetone solution and filtered again. Acetone extracts were concentrated in a rotary evaporator (BÜCHI Labortechnik, AG, Flawil, Switzerland) at 35°C, 60 rpm and 250 mbar. Dry samples were re-dissolved in isopropanol (1 mL), filtered through nylon membranes (0.45 μm) and injected into the HPLC system.
Carotenoids were separated on a 4.6 × 150 mm, 3 μm, C30 reverse phase column (Waters Corp, Mildford, MA, USA). The mobile phases consisted of methanol/water (96:4, v/v, phase A) and tMBE (phase B) with a flow rate of 0.75 mL/min and a gradient of 0/95, 10/90, 40/55, 45/25, 50/0, and 57/95 (min/% phase A). For the quantification of individual carotenoids, standard curves of β-carotene and lutein were prepared at a range of 0.4–6.0 ppm (mg/L). Carotene and xantophyll concentrations were expressed as mg of β-carotene and lutein equivalents per kg of broccoli DW, respectively. To calculate the concentration of individual carotenoids in DW, the moisture content of the samples was determined by the air-oven method. The tentative identification of carotenoids was performed similarly to that of glucosinolates.
Consumers acceptability test
Consumers were students and workers of Tecnológico de Monterrey – Campus Monterrey with ages between 18 and 55 years who consumed broccoli at least once a week. Two consumer panels composed of 120 persons each (52% men and 48% women) were used. In the first study, consumers were provided with two samples of cold-served broccoli (SM1 and MM1), while in the second panel, 120 other people were given two hot-served samples (SM2 and MM2). The samples were presented at random to the consumers. Water and crackers (with neutral flavor) were available for consumers to clean palate between samples. Respondents were asked to evaluate the acceptability of the samples using a hedonic scale (1–9, 1 = dislike very much, 5 = indifferent, 9 = like very much). Finally, consumers were asked to choose between both given samples.
Statistical analysis
Statistical analyses of chemical analyses were performed using three treatment repetitions. Data represent the mean values of samples and their standard error. Analyses of variance (ANOVA) were conducted using JMP software version 5.0 (SAS Institute Inc., Cary, NC, USA) and mean separations performed using the LSD test (p < 0.05).
Results and discussion
Effect of defrosting methods on total phenolics compounds and vitamin C content
The total PC and vitamin C content of frozen and defrost broccoli are shown in . With the exception of MM1, the total PC content dramatically decreased due to the different defrosting methods (). SM1 and SM2 caused the greatest losses (~45% and ~73%, respectively), while MM2 showed ~34% less concentration of phenolic compounds than the control (frozen broccoli). These results are in agreement with previous reports. Studies by Zhang and Hamauzu (Citation2004) showed that total PC of fresh broccoli decreased from ~31% to ~71% in a time-dependent manner when boiled from 30 to 300 s, while Miglio, Chiavaro, Visconti, Fogliano, and Pellegrini (Citation2008) reported a decrease of ~72% in total PC of boiled broccoli in moderate flame as compared with raw broccoli. Meanwhile, Francisco, Velasco, Moreno, Garcia-Viguera, and Cartea (Citation2010) reported a dramatic loss on total PC content of turnip, another vegetable from the Brassica genus, after domestic cooking with boiling water for 15 min. Broccoli contains mainly flavonoids and hydroxycinnamic acids (Vallejo, Tomás-Barberán, & García-Viguera, Citation2003b). Due to the high polarity of these groups of phenolic compounds, one of the most common solvents for their extraction is hot water (Chen, Inbaraj, & Chen, Citation2012; Robbins, Citation2003). Moreover, Vallejo et al. (Citation2003b) reported that ~13% of the total phenolics were lost into the water used for the cooking of raw broccoli. Meanwhile, results by Martínez-Hernández et al. (Citation2013) showed that 36% of total phenolics were leached into water after boiling for 210 s. Thus, phenolics present in broccoli are more susceptible to leaching into the surrounding hot water used for defrosting.
Figure 1. Total phenolic compounds (A) and vitamin C (B) of frozen and thawed broccoli. Different letters in the same chart indicate statistical difference by the LSD test (p < 0.05). Abbreviations: Stove Method 1 (SM1, broccoli boiled for 40 s); Stove Method 2 (SM2, broccoli boiled until second boiling was observed); Microwave Method 1 (MM1, broccoli microwaved for 150 s); Microwave Method 2 (MM2, broccoli microwaved for 300 s).
Figura 1. Compuestos fenólicos totales (A) y vitamina C (B) del brócoli congelado y descongelado. Diferentes letras en la misma gráfica indican diferencia estadísticamente significativa por la prueba LSD (p < 0,05). Abreviaciones: Método por estufa 1 (SM1, brócoli hervido por 40 s); Método por estufa 2 (SM2, brócoli hervido hasta que se observó un segundo hervor); Método por microondas 1 (MM1, brócoli descongelado en microondas por 150 s); Método por microondas 2 (MM2, brócoli descongelado en microondas por 300 s).
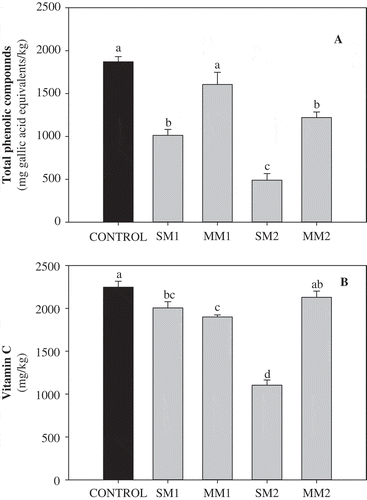
Regarding the microwave defrosting methods (MM1 and MM2), results showed that microwaving frozen broccoli for prolonged time has a negative effect on its total PC. While MM1 showed no significant effect, MM2 had a negative effect on total PC on frozen broccoli. This may be due to thermal degradation, which can possibly be caused by more prolonged cooking times (Porter, Citation2012). This may be supported by results presented by Zhang and Hamauzu (Citation2004), in which PC decrease in a time-dependent manner when cooked by microwave at a power of 600 W. Meanwhile, Wachtel-Galor, Wong, and Benzie (Citation2008) reported a dramatic decrease on total PC in broccoli (>90%), when microwaved with 100 mL of water for 5 min at 750 W of power. Nonetheless, their microwave cooking method involved the use of water, which may also be responsible for phenolic losses.
Every defrosting method, except MM2, had a negative effect on vitamin C content (). The greatest loss of vitamin C was observed in SM2 (51%), whereas SM1 and MM1 caused minor losses of ~10% and ~15%, respectively. Vitamin C content was mainly affected by boiling water methods. Previous reports have shown that ascorbic acid content dramatically decreases during boiling of broccoli florets (Borges-Marques, Von Atzingen, Pinto, & Silva, Citation2004; Gliszczyńska-Świglo et al., Citation2006; Miglio et al., Citation2008; Vallejo et al., Citation2002; Yuan, Sun, Yuan, & Wang, Citation2009). Zhang and Hamauzu (Citation2004) reported that this loss is due to a reduced retention of vitamin C in the tissues. Furthermore, Lee and Kader (Citation2000) described that an important part of vitamin C losses in water-based cooking methods was caused by leaching in surrounding water in a similar way as with phenolic compounds. Analysis of water used for conventional cooking of broccoli (dipped in boiling water for 5 min, 1 gr of broccoli/mL) showed that ~8% of total vitamin C was lost into water (Vallejo et al., Citation2002). On the contrary, microwaving methods had minimal effects on vitamin C content of frozen broccoli. Interestingly, MM2 had no significant effects on vitamin C content, while MM1 did show a minimal but significant decrease on total vitamin C. Likewise, Howard, Wong, Perry, and Klein (Citation1999) reported slight losses of ascorbic acid (~10%) in frozen broccoli microwaved at a power of 700 W for 9 min with 30 g of water. Similarly, Yuan et al. (Citation2009) reported a ~16% decrease of ascorbic acid in broccoli when microwaved for 5 min at full power (1000 W) and 10 mL of water, compared to raw samples. The highest degradation of vitamin C observed in MM1 as compared with MM2 may be explained in terms of degradation attributed to temperature or increases attributed to higher extractability. It is well known that temperature induce cell disruption producing higher extractability of bioactive compounds, however thermal-induced degradation of molecules can also occur. In the particular case of MM1 it is likely that cell disruption is not significantly occurring, whereas vitamin C exposed to temperature is being degraded. On the other hand, for MM2 the extractability is increased to levels that overcome the effect of temperature-induced degradation of vitamin C.
Effect of defrosting methods on total and individual glucosinolates
Total and individual glucosinolates were determined in frozen and defrost broccoli (, ). A typical HPLC-DAD glucosinolate chromatogram of frozen broccoli is shown in . Individual glucosinolates were identified as indicated in . The glucosinolate profile obtained herein was similar to those reported in the literature (Barbieri, Pernice, Maggio, De Pascale, & Fogliano, Citation2008; Miglio et al., Citation2008), nevertheless, the major glucosinolate detected in the present study was glucobrassicin, while Miglio et al. (Citation2008) reported neoglucobrassicin as the major one and reported the presence of 4-methoxyglucobrassicin but not 4-hydroxyglucobrassicin. Barbieri et al. (Citation2008) also reported glucobrassicin to be the major glucosinolate on broccoli, nevertheless, other glucosinolates not identified in the present study such as glucoalyssin and 4-methoxyglucobrassicin were also quantified. These differences in the qualitative and quantitative profiles may be due to differences in the cultivars, growing conditions, and postharvest treatments applied in each study. One of the main differences between the plant material used herein and previous studies is that florets used in the present study were previously subjected to an industrial freezing process (cutting, blanching, and rapid freezing), which may also affect the chemical profile and the bioavailability of bioactive compounds of broccoli (Podsędek, Citation2007).
Figure 2. Typical HPLC-DAD glucosinolate chromatogram (shown at 227 nm) obtained from methanol/water extracts of frozen broccoli. Tentative identification of peaks was performed as indicated in . Peak assignment: (1) Glucoraphanin; (2) 4-hydroxyglucobrassicin; (3) Glucobrassicin; (4) Neoglucobrassicin; (IS) Sinigrin (internal standard).
Figura 2. Perfil cromatográfico típico obtenido mediante HPLC-PDA de glucosinolatos (mostrado a 227 nm) presentes en extracto metanólico/acuoso de brócoli congelado. La identificación tentativa de los picos cromatográficos se realizó como se indica en la . Asignación de picos: (1) Glucorafanina; (2) 4-hidroxiglucobrasicina; (3) Glucobrasicina; (4) Neoglucobrasicina; (IS) Sinigrina (estándar interno).
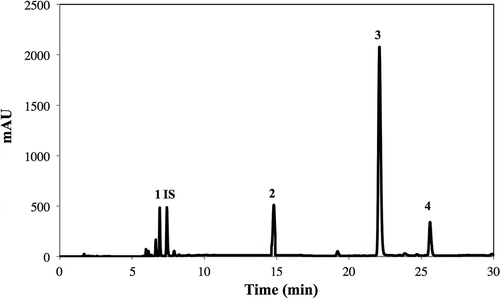
Table 1. Tentative identification of broccoli (Brassica oleracea var. Italica) glucosinolate chromatographic profile obtained by HPLC-PDA.
Tabla 1. Identificación tentativa del perfil cromatográfico de glucosinolatos de brócoli (Brassica oleracea var. Italica) obtenido por HPLC-PDA.
The total and individual glucosinolate content of frozen and defrost broccoli is shown in . Defrosting methods SM1, SM2, and MM2 showed lower total glucosinolates levels (~16%, ~60%, and ~20%, respectively) as compared with the control, whereas MM1 showed no significant difference (p > 0.05). Similar results were reported by other authors regarding boiling of raw broccoli (Cieslik, Leszczyńska, Filipiak-Florkiewicz, Sikora, & Pisulewski, Citation2007; Gliszczyńska-Świglo et al., Citation2006; Miglio et al., Citation2008; Vallejo et al., Citation2002; Yuan et al., Citation2009). Analysis of water used for cooking broccoli suggested that glucosinolates were lost mainly due to leaching into water, while ~10% of the losses were attributable to degradation (Ciska & Kozlowska, Citation2001).
Table 2. Concentrations of individual glucosinolates present in frozen and thawed broccoli.
Tabla 2. Concentraciones de glucosinolatos individuales presentes en brócoli congelado y descongelado.
Similar to what happened with phenolic compounds, glucosinolate content was also decreased by more prolonged microwave defrosting time (MM2), while moderate microwave defrosting showed (MM1) no significant changes. Vallejo et al. (Citation2002) reported that a 5 min microwave-cooking at full power (1000 W) of fresh broccoli lead to a dramatic loss of ~74% of total glucosinolates. Meanwhile, Yuan et al. (Citation2009) reported losses of ~58% in total glucosinolates after 5 min of microwave-cooking at full power (1000 W). According to Verkerk and Dekker (Citation2004), red cabbage myrosinase is inactivated after microwaving for 4.8 min at a full power of 900 W. Moreover, this caused red cabbage to reach temperatures of over 100°C after 2.8 min. Furthermore, Oerlemans, Barrett, Bosch-Suades, Verkerk, and Dekker (Citation2006) reported that red cabbage glucosinolates showed significant degradation at temperatures of ≥110°C. These results may explain why glucosinolates are not degraded with moderate time microwaving (MM1, 150 s) but they suffer from degradation when samples are microwaved for 5 min (MM2).
Regarding individual glucosinolates, glucoraphanin and 4-hydroxyglucobrassicin showed the highest stability, particularly when microwaved (MM1 and MM2) or with moderate-time boiling (SM2, ). Moreover, both glucosinolates showed an increase in their concentration with MM1 (~49% and ~43%, respectively). In these cases, short thermal treatment can lead to cell disruption, which may lead to a major extractability of both glucosinolates (Ciska & Kozlowska, Citation2001). In agreement with Vallejo et al. (Citation2002), glucobrassicin and neoglucobrassicin are lost in a very similar rate and in a higher rate than glucoraphanin when broccoli is subjected to boiling water. This may be due to the polarity of the latter, which is lower than that of glucobrassicin and neoglucobrassicin, the most polar glucosinolates detected in the samples. Furthermore, according to Oerlemans et al. (Citation2006), indole glucosinolates are more suceptible to thermal degradation than aliphatic glucosinolates. According to Rungapamestry, Duncan, Fuller, and Ratcliffe (Citation2007), glucobrassicin and neoglucobrassicin are less stable than other glucosinolates like glucoraphanin. Nevertheless, neoglucobrassicin showed higher stability for microwave-based methods than glucobrassicin, which showed similar decreases for MM1 and MM2.
Effect of defrosting methods on total and individual carotenoids
A typical HPLC-DAD carotenoid chromatogram of frozen broccoli is shown in . The identification of individual carotenoids was performed as indicated in . The carotenoid profile of frozen broccoli is similar than those reported in literature (Müller, Citation1997; Zhang & Hamauzu, Citation2004), where lutein was the major carotenoid. Nevertheless, Zhang and Hamauzu (Citation2004) did not report the presence of neoxanthin and β-cryptoxanthin. Again, this difference in carotenoid profiles may be due to a difference in the cultivars used as well the growing conditions and postharvest treatments. Compared with the control, total carotenoid content was higher by ~20% in SM1, but ~22%, ~30%, and ~39% lower in the SM2, MM1, and MM2 samples, respectively.
Figure 3. Typical HPLC-DAD carotenoid chromatogram (shown at 450 nm) from acetone (0.1% BHT) extracts of frozen broccoli. Tentative identification of peaks was performed as indicated in . Peak assignment: (1) Neoxanthin; (2) Violaxanthin; (3) Chlorophyll b; (4) Lutein; (5) Chlorophyll a; (6) β-cryptoxanthin; (7) β-carotene.
Figura 3. Perfil cromatográfico típico obtenido mediante HPLC-PDA de carotenoides (mostrado a 227 nm) presentes en extracto acetónico (0.1% BHT) de brócoli congelado. La identificación tentativa de los picos cromatográficos se realizó como se indica en la . Asignación de picos: (1) Neoxantina; (2) Violaxantina; (3) Clorofila b; (4) Luteína; (5) Clorofila a; (6) β-criptoxantina; (7) β-caroteno.
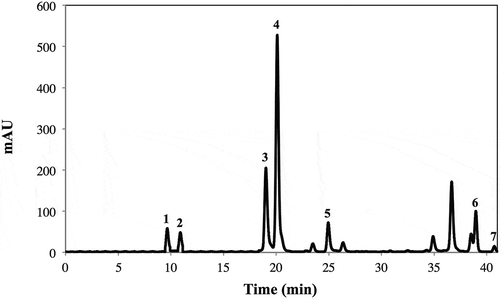
Table 3. Tentative identification of broccoli (Brassica oleracea var. Italica) carotenoid and chloropyll chromatographic profile obtained by HPLC-PDA.
Tabla 3. Identificación tentativa del perfil cromatográfico de carotenoides y clorofilas en brócoli (Brassica oleracea var. Italica) obtenido por HPLC-PDA.
In the present study, we found that, in boiling water defrosting methods, carotenoids were the best conserved biomolecules, which is consistent with other reports (Yuan et al., Citation2009; Zhang & Hamauzu, Citation2004), although more prolonged defrosting time did result in a decrease in carotenoid concentration (). Moreover, Gliszczyńska-Świglo et al. (Citation2006) reported that boiling 300 g of broccoli for 5 min in 1 L of water caused an increase in total carotenoids as compared with fresh broccoli. Meanwhile, Miglio et al. (Citation2008) reported similar results for broccoli boiled under moderate flame until desired tenderness was reached. In the present study, defrosting broccoli in boiling water for moderate time (40 s) also resulted in an increase in the carotenoid content, which is likely due to an improved extractability resulting from disruption of carotenoid-protein complexes, inactivation of carotene-oxidizing enzymes, and/or loss of soluble solids into the water (Lessin & Schwartz, Citation1997). Chandler and Schwartz (Citation1988) also reported an increase in carotenoid extractability after thermal treatment, presumably due to a change in tissue morphology and, thus, a greater contact between the organic solvent and the carotenoids, resulting in an enhanced release of these biomolecules.
Table 4. Concentrations of individual carotenoids present in frozen and thawed broccoli.
Tabla 4. Concentraciones de carotenoides individuales presentes en brócoli congelado y descongelado.
On the other hand, microwave-defrosting methods (MM1 and MM2) negatively affected the total carotenoid content of frozen broccoli. Zhang and Hamauzu (Citation2004) reported a decrease of total carotenoids in florets cooked in microwave for 120 and 300 s in comparison with fresh broccoli florets. Moreover, Chen and Chen (Citation1993) also reported a significant loss of total carotenoids in sweet potato leaves in a time-dependent manner when samples were microwaved with high output power (700 W). Nevertheless, Yuan et al. (Citation2009) reported no significant loss of total carotenoid content in microwave-cooked samples (1000 W, 5 min). In the present study, microwave-defrosting resulted in a greater carotenoid loss than prolonged defrosting with boiling water, which is also consistent with previously described reports.
Chlorophylls were both negatively affected by microwaving, with losses between ~19% and 37%. These results are in agreement with Yuan et al. (Citation2009), who reported a significant loss of total chlorophyll (16% less) after microwave treatment for 5 min at full power. Moreover, chlorophylls showed a loss of 26% after a short boiling time, nevertheless, had no significant changes when boiled for a more prolonged time. As earlier discussed for vitamin C, this behavior could be related to the balance between increase in thermal-induced extractability and degradation of the compound.
In agreement to reports by Gliszczyńska-Świglo et al. (Citation2006) and Miglio et al. (Citation2008), lutein and β-carotene showed an increase after moderate time boiling (26% and 28% more, respectively). Violaxanthin showed significant losses only after microwaving, but its concentration raised with SM1 and showed no significant changes with SM2. On the other hand, β-cryptoxanthin showed an increase of ~24% after moderate time boiling, but decreased with all other treatments. Interestingly, neoxanthin was the only carotenoid that did not show a significant increase by effect of any thermal treatment; on the contrary, losses were observed with every treatment, especially when microwaved (between ~32% and ~42%). A similar loss behavior was observed in carotenes and xanthophylls.
Lutein concentration presented herein was in the range of reported data, though, β-carotene values were lower than those reported in the literature (Gliszczyńska-Świglo et al., Citation2006; Miglio et al., Citation2008; Zhang & Hamauzu, Citation2004).
Sensory evaluation of thawed broccoli
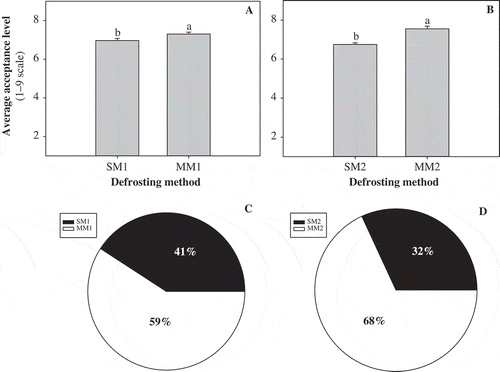
The objective of the sensory evaluation was to determine which of the defrosting methods of broccoli resulted on the highest acceptability of the product by consumers. The effect of the defrosting method on the average overall liking of broccoli by participants is shown in . Paired preference tests showed that, in more than 50% of the cases, participants preferred samples thawed by microwaving methods (). Firmness was one of the most mentioned attributes by the consumers to explain their choice. In agreement with these results, Bongoni et al. (Citation2014) also found that more firm broccoli was preferred over less firm, more cooked samples.
Figure 4. Average acceptance level of cold-served (A) and hot-served (B) broccoli and paired preference of cold-served (C) and hot-served (D) broccoli by consumers. Different letters in the same chart indicate statistical difference by the LSD test (p < 0.05). Abbreviations: Stove Method 1 (SM1, broccoli boiled for 40 s); Stove Method 2 (SM2, broccoli boiled until second boiling was observed); Microwave Method 1 (MM1, broccoli microwaved for 150 s); Microwave Method 2 (MM2, broccoli microwaved for 300 s).
Figura 4. Nivel de aceptación promedio de brócoli servido frío (A) y caliente (B) y preferencia pareada de brócoli servido frío (C) y caliente (D) por parte de los consumidores. Diferentes letras en la misma gráfica indican diferencia estadísticamente significativa por la prueba LSD (p < 0,05). Abreviaciones: Método por estufa 1 (SM1, brócoli hervido por 40 s); Método por estufa 2 (SM2, brócoli hervido hasta que se observó un segundo hervor); Método por microondas 1 (MM1, brócoli descongelado en microondas por 150 s); Método por microondas 2 (MM2, brócoli descongelado en microondas por 300 s).
Conclusions
Results presented herein demonstrate that the nutritional content of frozen broccoli is dramatically affected by defrosting and that modifications in its content of bioactive compounds are dependent upon the applied defrosting method and the chemical nature of each compound. Additionally, consumers’ acceptability also varies depending on the defrosting method. Carotenoids decreased after microwaving but showed a higher extractability when frozen broccoli was defrosted for a short period of time with boiling water. Though, hydrosoluble compounds such as phenolic compounds, vitamin C, and glucosinolates were mainly affected by water-based methods, short-time microwaving showed a moderate or no effect on them. Moreover, microwave-based methods were preferred by consumers. Thereafter, to best preserve nutritional and organoleptic properties of frozen broccoli, consumers may defrost broccoli by a short-time microwaving, which caused less overall losses of bioactive compounds than the other tested methods.
Acknowledgments
This study is based upon research supported by research funds from the Tecnológico de Monterrey – Research Chair Initiative (CAT 161). We also would like to thank Frigorizados La Huerta, S.A. de C.V. for providing broccoli samples. Author D.V.-G. also acknowledges the scholarship (296572) from the Consejo Nacional de Ciencia y Tecnología (CONACYT, México).
References
- Armah, C. N., Traka, M. H., Dainty, J. R., Defernez, M., Janssens, A., Leung, W., … Mithen, R. F. (2013). A diet rich in high-glucoraphanin broccoli interacts with genotype to reduce discordance in plasma metabolite profiles by modulating mitochondrial function. American Journal of Clinical Nutrition, 98, 712–722. doi:10.3945/ajcn.113.065235
- Bahadoran, Z., Tohidi, M., Nazeri, P., Mehran, M., Azizi, F., & Mirmiran, P. (2012). Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: Randomized double-blind clinical trial. Journal of Food Science and Nutrition, 63(7), 767–771.
- Barbieri, G., Pernice, R., Maggio, A., De Pascale, S., & Fogliano, V. (2008). Glucosinolates profile of Brassica rapa L. subsp. Sylvestris L. Janch. Var. esculenta Hort. Food Chemistry, 107, 1687–1691. doi:10.1016/j.foodchem.2007.09.054
- Becerra-Moreno, A., Alanís-Garza, P. A., Mora-Nieves, J. L., Mora-Mora, J. P., & Jacobo-Velázquez, D. A. (2014). Kale: An excellent source of vitamin C, pro-vitamin A, lutein and glucosinolates. CyTA-Journal of Food, 12(3), 298–303. doi:10.1080/19476337.2013.850743
- Bernhardt, S., & Schlich, E. (2006). Impact of different cooking methods on food quality: Retention of lipophilic vitamins in fresh and frozen vegetables. Journal of Food Engineering, 77, 327–333. doi:10.1016/j.jfoodeng.2005.06.040
- Bongoni, R., Verkerk, R., Steenbekkers, B., Dekker, M., & Stieger, M. (2014). Evaluation of different cooking conditions on broccoli (Brassica oleracea var. italica) to improve the nutritional value and consumer acceptance. Plant Foods for Human Nutrition. doi:10.1007/s11130-014-0420-2
- Borges-Marques, R. M., Von Atzingen, M. C., Pinto, M., & Silva, M. E. (2004). Sensory evaluation and ascorbic acid of frozen and fresh vegetables. Ciencia y Tecnología Alimentaria, 4(4), 240–245.
- Chandler, L. A., & Schwartz, S. J. (1988). Isomerization and losses of trans-β-carotene in sweet potatoes as affected by processing treatments. Journal of Agricultural and Food Chemistry, 36, 129–133. doi:10.1021/jf00079a033
- Chandrika, U. G., Jansz, E. R., & Warnasuriya, N. D. (2005). Identification and HPLC quantification of carotenoids of the fruit pulp of Chrysophyllumroxburghii. Journal of the National Science Foundation of Sri Lanka, 33(2), 93–98.
- Chen, B. H., & Chen, Y. Y. (1993). Stability of chlorophylls and carotenoids in sweet potato leaves during microwave cooking. Journal of Agricultural and Food Chemistry, 41, 1315–1320. doi:10.1021/jf00032a029
- Chen, H.-J., Inbaraj, B. S., & Chen, B.-H. (2012). Determination of phenolic acids and flavonoids in Taraxacum formosanum kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. International Journal of Molecular Sciences, 13, 260–285. doi:10.3390/ijms13010260
- Cieslik, E., Leszczyńska, T., Filipiak-Florkiewicz, A., Sikora, E., & Pisulewski, P. M. (2007). Effects of some technological processes on glucosinolate contents in cruciferous vegetables. Food Chemistry, 105, 976–981. doi:10.1016/j.foodchem.2007.04.047
- Ciska, E., & Kozlowska, H. (2001). The effect of cooking on the glucosinolates content in white cabbage. European Food Research and Technology, 212, 582–587. doi:10.1007/s002170100293
- Fahey, J. W., Zhang, Y., & Talalay, P. (1997). Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proceedings of the National Academy of Sciences of the United States of America, 94, 10367–10372. doi:10.1073/pnas.94.19.10367
- Francisco, M., Velasco, P., Moreno, D. A., Garcia-Viguera, C., & Cartea, M. E. (2010). Cooking methods of Brassica rapa affect the preservation of glucosinolates, phenolics and vitamin C. Food Research International, 43, 1455–1463. doi:10.1016/j.foodres.2010.04.024
- Fratianni, A., Cinquanta, L., & Panfili, G. (2010). Degradation of carotenoids in orange juice during microwave heating. Food Science and Technology, 43, 867–871.
- Gallaher, C. M., Gallaher, D. D., & Peterson, S. (2012). Development and validation of a spectrophotometric method for quantification of total glucosinolates in cruciferous vegetables. Journal of Agricultural and Food Chemistry, 60, 1358–1362. doi:10.1021/jf2041142
- Gliszczyńska-Świglo, A., Ciska, E., Pawlak-Lemańska, K., Chmielewski, J., Borkowski, T., & Tyrakowska, B. (2006). Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Additives and Contaminants, 23, 1088–1098. doi:10.1080/02652030600887594
- Hansen, M., Moller, P., Sorensen, H., & Cantwell de Trejo, M. (1995). Glucosinolates in broccoli stored under controlled atmosphere. Journal of the American Socierty for Horticultural Science, 120, 1069–1074.
- Howard, L. A., Wong, A. D., Perry, A. K., & Klein, B. P. (1999). β-carotene and ascorbic acid retention in fresh and processed vegetables. Journal of Food Science, 64, 929–936. doi:10.1111/j.1365-2621.1999.tb15943.x
- James, D., Devaraj, S., Bellur, P., Lakkanna, S., Vicini, J., & Boddupalli, S. (2012). Novel concepts of broccoli sulforaphanes and disease: Induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutrition Reviews, 70(11), 654–665. doi:10.1111/j.1753-4887.2012.00532.x
- Jeffery, E. H., & Araya, M. (2009). Physiological effects of broccoli consumption. Phytochemistry Reviews, 8, 283–298. doi:10.1007/s11101-008-9106-4
- Johnson, I. (2002). Glucosinolates in the human diet. Bioavailability and implications for health. Phytochemistry Reviews, 1, 183–188. doi:10.1023/A:1022507300374
- Khachik, F., Beecher, G. R., Goli, M. B., & Lusby, W. R. (1991). Separation, identification, and quantification of carotenoids in fruits, vegetables and human plasma by high performance liquid chromatography. Pure and Applied Chemistry, 63(1), 71–80. doi:10.1351/pac199163010071
- Khachik, F., Beecher, G. R., & Whittaker, N. F. (1986). Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography. Journal of Agricultural and Food Chemistry, 34(4), 603–616. doi:10.1021/jf00070a006
- Lee, H. S., Castle, W. S., & Coates, G. A. (2001). High-performance liquid chromatography for the characterization of carotenoids in the new sweet orange (Earlygold) grown in Florida, USA. Journal of Chromatography A, 913(1–2), 371–377. doi:10.1016/S0021-9673(00)01029-3
- Lee, S. K., & Kader, A. A. (2000). Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biology and Technology, 20, 207–220. doi:10.1016/S0925-5214(00)00133-2
- Lessin, W. J., & Schwartz, S. J. (1997). Quantification of cis-trans isomers of provitamin A carotenoids in fresh and processed fruits and vegetables. Journal of Agricultural and Food Chemistry, 45, 3728–3732. doi:10.1021/jf960803z
- Mahn, A., & Reyes, A. (2012). An overview of health-promoting compounds of broccoli (Brassica oleracea var. italica) and the effect of processing. Food Science and Technology International, 18(6), 503–514. doi:10.1177/1082013211433073
- Martínez-Hernández, G. B., Artés-Hernández, F., Colares-Souza, F., Gómez, P. A., García-Gómez, P., & Artés, F. (2013). Innovative cooking techniques for improving the overall quality of a kailan-hybrid broccoli. Food and Bioprocess Technology, 6, 2135–2149. doi:10.1007/s11947-012-0871-0
- Mendes-Pinto, M. M., Ferreira, A. C. S., Oliveira, M. B. P. P., & De Pinho, P. G. (2004). Evaluation of some carotenoids in grapes by reversed- and normal-phase liquid chromatography: A qualitative analysis. Journal of Agricultural and Food Chemistry, 52(10), 3182–3188. doi:10.1021/jf0499469
- Miglio, C., Chiavaro, E., Visconti, A., Fogliano, V., & Pellegrini, N. (2008). Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. Journal of Agricultural and Food Chemistry, 56, 139–147. doi:10.1021/jf072304b
- Moreno, D. A., Carvajal, M., López-Berenguer, C., & García-Viguera, C. (2006). Chemical and biological characterisation of nutraceutical compounds of broccoli. Journal of Pharmaceutical and Biomedical Analysis, 41, 1508–1522. doi:10.1016/j.jpba.2006.04.003
- Müller, H. (1997). Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection. Zeitschrift für Lebensmittel-Untersuchung und –Forschung, 204, 88–94. doi:10.1007/s002170050042
- Murashima, M., Watanabe, S., Zhuo, X.-G., Uehara, M., & Kurashige, A. (2004). Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors, 22(1–4), 271–275. doi:10.1002/biof.5520220154
- Nicoli, M. C., Anese, M., & Parpinel, M. (1999). Influence of processing on the antioxidant properties of fruit and vegetables. Trends in Food Science and Technology, 10(3), 94–100. doi:10.1016/S0924-2244(99)00023-0
- Oerlemans, K., Barrett, D. M., Bosch-Suades, C., Verkerk, R., & Dekker, M. (2006). Thermal degradation of glucosinolates in red cabbage. Food Chemistry, 95, 19–29. doi:10.1016/j.foodchem.2004.12.013
- Podsędek, A. (2007). Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Science and Technology, 40(1), 1–11. doi:10.1016/j.lwt.2005.07.023
- Porter, Y. (2012). Antioxidant properties of green broccoli and purple-sprouting broccoli under different cooking conditions. Bioscience Horizons, 5, hzs004–hzs004. doi:10.1093/biohorizons/hzs004
- Riso, P., Martini, D., Visioli, F., Martinetti, A., & Porrini, M. (2009). Effect of broccoli intake on markers related to oxidative stress and cancer risk in healthy smokers and nonsmokers. Nutrition and Cancer, 61(2), 232–237. doi:10.1080/01635580802425688
- Robbins, R. J. (2003). Phenolic acids in foods: An overview of analytical methodology. Journal of Agricultural and Food Chemistry, 51, 2866–2887. doi:10.1021/jf026182t
- Rungapamestry, V., Duncan, A. J., Fuller, Z., & Ratcliffe, B. (2007). Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proceedings of the Nutrition Society, 66, 69–81. doi:10.1017/S0029665107005319
- Saha, S., Hollands, W., Teucher, B., Needs, P. W., Narbad, A., Ortori, C. A., … Kroon, P. A. (2012). Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Molecular Nutrition and Food Research, 56, 1906–1916. doi:10.1002/mnfr.201200225
- Sánchez-Rangel, J. C., Benavides, J., Heredia, J. B., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2013). The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Analytical Methods, 5, 5990–5999. doi:10.1039/c3ay41125g
- Taylor, K. L., Brackenridge, A. E., Vivier, M. A., & Oberholster, A. (2006). High-performance liquid chromatography profiling of the major carotenoids in Arabidopsis thaliana leaf tissue. Journal of Chromatography A, 1121(1), 83–91. doi:10.1016/j.chroma.2006.04.033
- Vallejo, F., Tomás-Barberán, F. A., & García-Viguera, C. (2002). Potential bioactive compounds in health promotion from broccoli cultivars grown in Spain. Journal of the Science of Food and Agriculture, 82(11), 1293–1297. doi:10.1002/jsfa.1183
- Vallejo, F., Tomás-Barberán, F. A., & García-Viguera, C. (2003a). Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. European Food Research and Technology, 215, 310–316.
- Vallejo, F., Tomás-Barberán, F. A., & García-Viguera, C. (2003b). Phenolic compound contents in edible parts of broccoli inflorescences after domestic cooking. Journal of the Science of Food and Agriculture, 83, 1511–1516. doi:10.1002/jsfa.1585
- Vallejo, F., Tomás-Barberán, F., & García-Viguera, C. (2003c). Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. Journal of Agricultural and Food Chemistry, 51, 3029–3034.
- Verkerk, R., & Dekker, M. (2004). Glucosinolates and myrosinase activity in red cabbage (Brassica oleracea L. Var. Capitata f. rubra DC.) after various microwave treatments. Journal of Agricultural and Food Chemistry, 52, 7318–7323. doi:10.1021/jf0493268
- Wachtel-Galor, S., Wong, K. W., & Benzie, I. F. F. (2008). The effect of cooking on Brassica vegetables. Food Chemistry, 110, 706–710. doi:10.1016/j.foodchem.2008.02.056
- Wu, L., Noyan-Ashraf, M. H., Facci, M., Wang, R., Paterson, P. G., Ferrie, A., & Juurlink, B. H. J. (2004). Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proceedings of the National Academy of Sciences of the United States of America, 101(18), 7094–7099. doi:10.1073/pnas.0402004101
- Yuan, G.-F., Sun, B., Yuan, J., & Wang, Q.-M. (2009). Effects of different cooking methods on health-promoting compounds of broccoli. Journal of Zhejiang University Science B, 10(8), 580–588. doi:10.1631/jzus.B0920051
- Zhang, D., & Hamauzu, Y. (2004). Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chemistry, 88, 503–509. doi:10.1016/j.foodchem.2004.01.065
