Abstract
This study investigated the effect of cooking methods on the degradation of glucoraphanin (GR) in broccoli and the bioaccessibility of this compound through simulated gastrointestinal digestion. Broccoli was cooked using three different techniques: boiling, steaming and microwave cooking. Then, GR was extracted and quantified by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). In addition, the cooked samples were added to a system that simulates the digestion characteristics of the mouth, stomach and duodenum. Samples were drawn before and after the digestion, and GR bioaccessibility was calculated. GR losses were higher when broccoli was boiled (47.03%), followed by steaming (31.96%) and microwaving (11.87%). However, steamed broccoli presented the highest bioaccessibility (94.59%), followed by boiled (89.69%) and microwaved (60.88%). Taking into consideration the levels of remaining GR after cooking and its bioaccessibility after simulated digestion, steaming can be considered the best method and may result in the highest levels of this bioactive glucosinolate after broccoli intake.
El estudio investiga el efecto de diferentes métodos de cocción sobre la degradación de la Glucorafanina (GR) en brócoli y la bioaccesibilidad de este compuesto mediante un modelo de digestión gastrointestinal simulada. Los brócoli fueron cocidos usando tres diferentes técnicas: ebullición, cocción al vapor y mediante microondas. La GR se extrajo y se cuantificó mediante cromatografía líquida acoplada a espectrometría de masas en tándem (CL-EM/EM). Además las muestras cocidas se trataron con un sistema de digestión simulada incluyendo las fases de: boca, estomago e intestino delgado. El contenido en GR se analizó antes y después de las digestiones. Las pérdidas de la GR fueron mayores en los brócoli hervidos (47,03%), seguidos por los tratados al vapor (31,96%) y con microondas (11,87%). Los brócolis tratados al vapor presentaron los valores más altos de bioaccesibilidad (94,59%), seguidos por las muestras hervidas (89,69%) y las tratadas con microondas (60,88%). Teniendo en consideración los niveles de GR después de la cocción y su valor de bioaccesibilidad tras la digestión simulada, se puede concluir que el tratamiento al vapor fue el mejor método de cocción y las muestras así tratadas fueron las que contenían los niveles más altos del compuesto bioactivo después de la ingesta de los broccoli.
1. Introduction
In recent years, scientific research has focused on the health benefits associated with the consumption of Brassica vegetables. Many epidemiological studies have shown that the consumption of three to five servings of Brassica vegetables per week may reduce the risk of bladder, breast, colon, kidney and prostate cancers (Cohen, Kristal, & Stanfordm, Citation2000; Vig, Rampal, Singh Thind, & Arora, Citation2009). Brassica vegetables, like many other plants, contain a large number of antioxidants and vitamins, but, distinctively, they also contain high levels of glucosinolates (GLSs). This is a class of ~300 sulphurous compounds (Luciano & Holley, Citation2009), which have a common structure, consisting of a glucose combined with an oxime sulfonate by a β-D-thioglucosidic bound, and a variable side chain (R). Brassica vegetables also contain an enzyme known as thioglucoside glucohydrolase, EC 3.2.1.147 (myrosinase). In homeostasis, GLSs are compartmentalized in vacuoles, while myrosinase is located in the cytoplasm of the plant cell (Grob & Matile, Citation1979). Upon tissue damage, GLSs are released and cleaved by myrosinase in the presence of moisture. This degradation results in the formation of glucose plus an intermediate thiohydroximate-O-sulfonate component, which is extremely unstable and rearranges, releasing sulphate plus one or more of the following: isothiocyanates (ITCs), thiocyanates, nitriles, epithionitriles, oxazolidine-thiones and elementary sulphur (Vig et al., Citation2009).
Intact GLS can be also hydrolysed in the colon by microbial thioglucosidase activity (Cheng, Hashimoto, & Uda, Citation2004). At neutral pH, the reaction proceeds spontaneously to the formation of ITCs (Tiedink et al., Citation1991). Although parent GLS show little bioactivity, ITCs possess the ability to inhibit cancer development, by multiple anticarcinogenic mechanisms, such as inhibition of carcinogen-activating enzymes, induction of carcinogen-detoxifying enzymes, triggering apoptosis and arrest of cell-cycle progression (Nastruzzi et al., Citation1996).
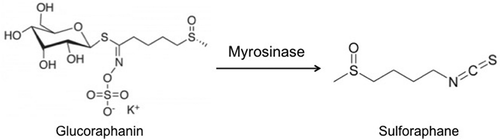
Researchers have shown the anticarcinogenic properties of sulforaphane (SFN), which is derived from glucoraphanin (GR) (), the main GLS found in broccoli (Fahey, Zalcmann & Talalay, Citation2001). Given the anticancer properties of SFN, it is of paramount importance to understand the bioavailability characteristics of this compound after ingestion. Therefore, several factors must be considered:
Figure 1. Glucoraphanin conversion to sulforaphane by the enzyme myrosinase.
Figura 1. Conversión de la glucurafanina en sulforafano por la enzima mirosinasa.
GR loss during cooking;
bioaccessibility of glucoraphanin;
and denaturation of myrosinase as a result of heat treatment.
The aim of this study was to examine the total loss of GR during broccoli cooking through boiling, steaming and microwaving and its bioaccessibility employing an in vitro gastrointestinal digestion model. GR bioaccessibility represents the fraction of compound that is released from the food matrix in the gastrointestinal tract without any structural modification and that becomes available for intestinal absorption. This is an important feature since it quantifies the fraction of a bioactive compound that can be converted in SFN by colonic bacteria, absorbed by the intestinal epithelium, and act on different organs of the human body (Gil-Izquierdo, Zafrilla, & Tomás-Barberá, Citation2002).
2. Material and methods
2.1. Chemicals
Sinigrin, potassium chloride (KCl), potassium thiocyanate (KSCN), monosodium phosphate (NaH2PO4), sodium sulphate (NaSO4), sodium chloride (NaCl), sodium bicarbonate (NaHCO3), urea, α-amilase, hydrochloric acid (HCl), pepsin, pancreatin, bile salts and sinigrin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile, methanol and formic acid were purchased from Carlo Erba (Milano, Italy). Deionized water (<18 MΩ cm resistivity) was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). Chromatographic solvents and water were degassed for 20 min using a Branson 5200 (Branson Ultrasonic Corp., Danbury, CT, USA) ultrasonic bath.
2.2. Sampling and processing of broccoli
Six fresh broccoli crowns (Brassica oleracea var. italica), with an average weight of 546 g, were purchased from a local market. Each broccoli was divided into single inflorescences, which were subsequently mixed, washed, dried, divided into portions of 100 g and cooked using three different methods. First, broccoli was boiled for 10 min. Boiling is the most common method for cooking broccoli. Another set of sample was cooked in a microwave oven AVM 420/WP/WH (Whirlpool, Benton Harbor, MI, USA) for 4 min at 700 W. Finally, the third treatment used steam cooking for 15 min. All tests were performed in triplicates.
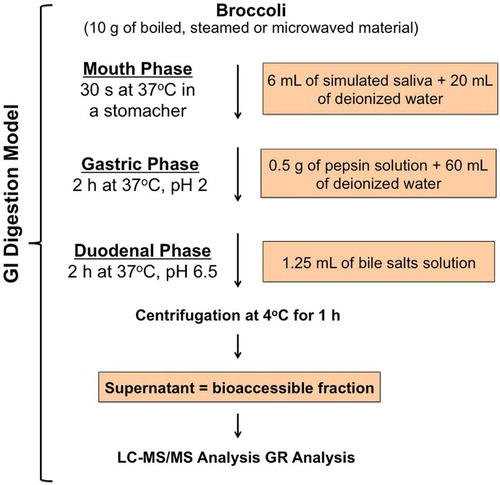
2.3. In vitro gastrointestinal digestion
The method consisted of three sequential steps that simulate the mouth mastication, gastric and small intestine digestion () as described by Gil-Izquierdo et al. (Citation2002) with slight modifications. The artificial saliva was composed by: 10 mL of KCl (89.6 g/L), 10 mL of KSCN (20 g/L), 10 mL of NaH2PO4 (88.8 g/L), 10 mL of NaSO4 (57 g/L), 1.7 mL of NaCl (175.3 g/L) and 20 mL of NaHCO3 (84.7 g/L). Then, 8 mL of a 25 g/L urea solution was added to the mixture and the volume was brought to 500 mL with water. Finally, 250 mg of α-amilase (≥10 units/mg) was added. The artificial saliva produced had a final pH of 6.8 ± 0.2. Broccoli portions (10 g) were sampled from each treatment and introduced in a plastic bag containing 20 mL of deionized water and 6 mL of artificial saliva. The mixture was homogenized during 30 s using a Stomacher 80 Biomaster (International PBI, Milano, Italy). Immediately after, 0.5 mL of pepsin solution (0.1 g of pepsin – ≥250 units/mg – in 25 mL of HCl 0.1N) and 60 mL of deionized water were added, and the pH was adjusted with HCl 6N (pH = 2). Then, the mixture was incubated at 37°C on an orbital shaker (250 rpm) (Infors AG CH-4103, Bottmingen, Switzerland) for 2 h. After the gastric digestion, small intestine digestion was simulated. The pH was increased to 6.5 with NaHCO3 0.5N. Then, the mixture was added with 1.25 mL of bile salts and pancreatin solution (0.1 g of pancreatin 4× USP specifications and 0.625 g of bile salts in 25 mL of NaHCO3 0.1N). This digestion solution was incubated at 37°C on an orbital shaker (250 rpm) for 2 h. After these steps, the mixture was centrifuged at 2000 × g at 4°C during 1 h. The final solution was filtered under vacuum through a Whatman Grade n°1 filter paper. The filtered liquid contained the GR available for intestinal absorption. This procedure was performed in triplicate for each treatment.
2.4. Analysis of glucoraphanin by LC-MS/MS
GR concentration was determined before and after the simulation of gastrointestinal digestion according to Park et al., (Citation2014) with a few modifications. Briefly, the samples collected before digestion were divided into portions of 10 g and freeze-dried. Then, 0.025 mg of each freeze-dried sample was extracted in 0.75 mL of 70% MeOH/30% H2O at 70°C for 30 min in an ultrasonic bath. The samples were centrifuged (13.200 × g, 10 min, 4°C) and 0.45 mL of supernatant was collected. The solvent of the extracted mixture was evaporated using a rotary evaporator under vacuum at 35°C. The dry material obtained was redissolved in 0.25 mL of deionized water and centrifuged (13.200 × g, 10 min, 4°C) before injection in the chromatography apparatus. The extraction after the simulated digestion process was performed by collecting 20 mL from the bioavailable liquid portion, which was freeze-dried and subsequently redissolved in 2 mL of deionized water. This solution was centrifuged (13.200 × g, 10 min, 4°C) for further analysis. The quantification of GR was carried out by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), following the method proposed by Zimmermann, Gerends, and Krumbein (Citation2007). Chromatographic separation was performed using an LC apparatus equipped with two micropumps Series 200 (Perkin Elmer, Canada Shellton, Waltham, MA, USA) and a Gemini C18 column (150 × 4.60 mm) (Phenomenex, Torrance, CA, USA). The eluents were (A) water + 0.1% formic acid and (B) CH3CN, at a constant flow of 0.2 mL/min. The gradient programme was as follows: 0 min 0% B, 10 min 15% B, 15 min 40% B, 20 min 50% B, from 25 min to 30 min 0% B. Injection volume was 20 µL. MS/MS analyses were performed on an API 3500 triple quadrupole mass spectrometer (Applied Biosystems, Burlington, ON, Canada) equipped with a TurboIonSpray source. The mass spectrometric conditions were as follows: IonSpray voltage −4500 V; declustering potential 35 V; desolvation gas temperature 450°C; collision energy −18 V. Analyses were performed in the negative ion mode in multiple reaction monitoring using the following precursor/product ion combinations: m/z 436→97 for GR. The quantification of GR was done by comparison with a calibration curve of sinigrin within the range of 0.01–1 mM.
3. Results and discussion
3.1. Cooking losses and glucoraphanin bioaccessibility
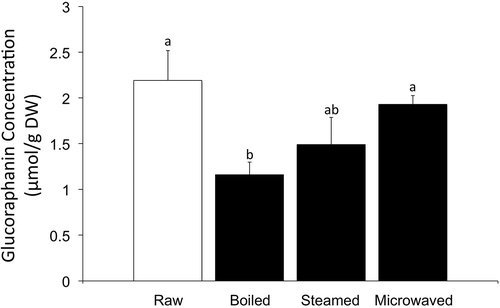
As presented in , the concentration of GR in raw broccoli was 2.19 µmol/g on a dry weight (DW) basis. When the samples were treated with different cooking techniques, GR concentration decreases in all cases. Particularly, the GR level in boiled samples was the lowest (1.16 µmol/g DW), whereas samples processed by steam (1.49 µmol/g DW) and microwave cooking (1.93 µmol/g DW) contained higher concentrations of the GLSs. During boiling, broccoli samples were in direct contact with water, which may have dissolved part of the GR total content. Jones, Frisina, Winkler, Imsic, and Tomkins (Citation2010) found that 83.56%, 93.9% and 70.28% of GR remained in broccoli samples that were microwaved, steamed and boiled for 5 min, respectively. Results presented in the present article show lower GR concentrations after steaming (68.04%) and boiling (52.97%), while microwaving has shown similar remaining concentration of the GLS (88.13%). These disparities can be explained by the differences in methodology from both studies. Broccoli samples were boiled and steamed for longer periods of time (10 and 15 min, respectively) in the present study, which may explain the higher losses in GR content. However, the microwave cooking technique used in both studies was similar, where Jones et al. used 5 min at 700 W and the present method used 4 min at 700 W.
Figure 3. Glucoraphanin concentration in raw, boiled, steamed and microwaved broccoli. Samples were freeze-dried and extracted with 70% MeOH/30% H2O at 70°C for 30 min in an ultrasonic bath. Quantities were analysed by LC-MS/MS. Different letters represent a significant difference (P < 0.05) among treatments.
Figura 3. Concentración de la glucorafanina en los broccoli crudos, hervidos, tratados al vapor y con microondas. Las muestras liofilizadas se extrajeron con una mezcla de metanol-agua 70–30 a 70°C durante 30 minutos en un baño de ultrasonidos. El análisis de realizó mediante CL-EM/EM. Las diferentes letras representan la significatividad del resultado (P < 0,05) entre los tratamientos.
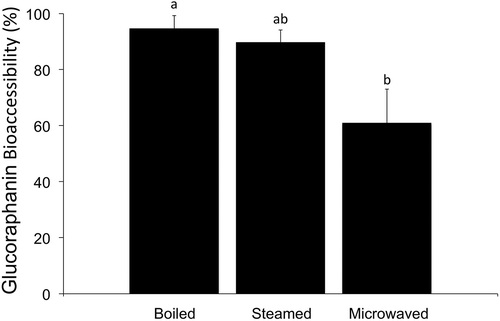
After cooking, 10 g samples from each cooking method were submitted to in vitro digestion. From all cooking techniques, steaming presented the highest GR bioaccessibility (94.59%), followed by boiling (89.69%) and microwave cooking (60.88%) (). From our knowledge, this is the first time that the influence of cooking methods on the bioaccessibility of the bioactive compound GR was determined using a simulated gastrointestinal digestion model. GR bioaccessibility from all the treated samples in this study was very high and can be compared with the bioaccessibility of readily available bioactive compounds such as polyphenols (Ramírez-Moreno, Hervert-Hernández, Sánchez-Mata, Díez-Marqués, & Goñi, Citation2011).
Figure 4. Percentage of bioaccessible glucoraphanin extracted from broccoli submitted to different cooking techniques. Different letters represent a significant difference (P < 0.05) among treatments.
Figura 4. Porcentaje de bioaccesibilidad de la glucorafanina extraída de los broccoli tratados con diferentes técnicas de cocción. Las diferentes letras representan la significatividad del resultado (P < 0.05) entre los tratamientos.
Vallejo, Gil-Izquierdo, Pérez-Vicente, and García-Viguera (Citation2004) found that 69% of the total GLS content of broccoli inflorescences was lost after in vitro digestion. This value is much higher than the results found in the present study, where GR bioaccessibility was ≥60.88% and, therefore, GR loss was ≤39.12%. However, the former authors have reported GR losses from raw broccoli, which may explain this discrepancy. Myrosinase remains active in raw broccoli during digestion and may have converted GR in other compounds during the 2-h incubation period. Cooking temperatures used in this study are able to inactivate myrosinase to a great extent (Oerlemans, Barrett, Suades, Verkerk, & Dekker, Citation2006; Wang, Farnham, & Jeffery, Citation2012), which may result in increased GR bioaccessibility.
Overall, the method that preserved the highest fraction of bioaccessible GR was steam cooking, which delivered 64.35% of the original GR (from raw broccoli) content at the intestinal simulation. Although microwaving was the best method to maintain the GR concentration after cooking, it presented the lowest rate of bioaccessibility, which resulted in 53.61% of the original GR content from raw broccoli. Similarly, 47.51% of the original GR concentration was bioaccessible in the intestinal fluid after final digestion. However, most of the GLS was lost during the cooking process, whereas nearly all GR left was bioaccessible.
Intestinal bacteria have been found to have myrosinase-like activity and are able to form ITCs from GLSs (Combourieu, Elfoul, Delort, & Rabot, Citation2001; Rabot, Guérin, Nugon-Boudon, & Szylit, Citation1995). Clinical studies have revealed that the yield of bioavailable SFN is threefold higher in slightly cooked than in fully cooked broccoli due to the lower conversion capacity of GLS-to-ITC of microbial myrosinase in comparison to the plant-derived enzyme (Rungapamestry, Duncan, Fuller, & Ratcliffe, Citation2007a, Citation2007b). However, many cultures are used to consume fully cooked broccoli rather than raw. Therefore, steaming was found as the best cooking technique to deliver the highest amount of GR into the intestine under the conditions applied in this study. This GLS may be subsequently transformed into SFN by the human intestinal microflora. As mentioned before, SFN has been widely described as a potent anticarcinogen and may prevent the occurrence of various cancers.
It is still difficult to predict the amount of GR that will be converted into SFN when cooked broccoli is consumed and if the SFN content produced is able to result in health benefits. Bheemreddy and Jeffery (Citation2007) have demonstrated that colonic bacteria of rats consuming pure GR (no myrosinase) were able to convert at least 13.5% of GR in SFN and 2% in SFN nitrile (a less potent bioactive compound). Moreover, the authors also found 5% of intact GR, meaning that the GLS can be absorbed by the GI tract and excreted through the urine. Unfortunately, this study was only able to identify 20% of metabolites/intact GR from the original GR oral dose. Further studies should be performed to understand the GR-to-SFN conversion rate by colonic bacteria in humans.
4. Conclusion
GR levels in broccoli were reduced by all cooking methods performed in this study. Boiled and steamed broccoli presented the highest levels of bioaccessible GR. Taking into consideration the levels of remaining GR after cooking and its bioaccessibility after simulated digestion, it can be concluded that steaming was the overall best method and may result in the highest levels of the bioactive GLS after broccoli intake. To our knowledge, this is the first time that the influence of cooking methods on the bioaccessibility of GR was determined using a simulated gastrointestinal digestion model.
Additional information
Funding
References
- Bheemreddy, R. M., & Jeffery, E. H. (2007). The metabolic fate of purified glucoraphanin in F344 rats. Journal of Agricultural and Food Chemistry, 55, 2861–2866. doi:10.1021/jf0633544
- Cheng, D.-L., Hashimoto, K., & Uda, Y. (2004). In vitro digestion of sinigrin and glucotropaeolin by single strains of Bifidobacterium and identification of the digestive products. Food and Chemical Toxicology, 42, 351–357. doi:10.1016/j.fct.2003.09.008
- Cohen, J. H., Kristal, A. R., & Stanfordm, J. L. (2000). Fruit and vegetable intakes and prostate cancer risk. Journal of the National Cancer Institute, 92, 61–68. doi:10.1093/jnci/92.1.61
- Combourieu, B., Elfoul, L., Delort, A. M., & Rabot, S. (2001). Identification of new derivatives of sinigrin and glucotropaeolin produced by the human digestive microflora using 1H NMR spectroscopy analysis of in vitro incubations. Drug Metabolism & Disposition, 29, 1440–1445.
- Fahey, J. W., Zalcmann, A. T., & Talalay, P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry, 56, 5–51. doi:10.1016/S0031-9422(00)00316-2
- Gil-Izquierdo, A., Zafrilla, P., & Tomás-Barberá, F. A. (2002). An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. European Food Research and Technology, 214, 155–159. doi:10.1007/s00217-001-0428-3
- Grob, K., & Matile, P. H. (1979). Vacuolar location of glucosinolates in horseradish root cells. Plant Science Letters, 14, 327–335. doi:10.1016/S0304-4211(79)90281-5
- Jones, R. B., Frisina, C. L., Winkler, S., Imsic, M., & Tomkins, R. B. (2010). Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chemistry, 123, 237–242. doi:10.1016/j.foodchem.2010.04.016
- Luciano, F. B., & Holley, R. A. (2009). Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H7. International Journal of Food Microbiology, 131, 240–245. doi:10.1016/j.ijfoodmicro.2009.03.005
- Nastruzzi, C., Cortesi, R., Esposito, E., Menegatti, E., Leoni, O., Iori, R., & Palmieri, S. (1996). In vitro cytotoxic activity of some glucosinolate-derived products generated by myrosinase hydrolysis. Journal of Agricultural and Food Chemistry, 44, 1014–1021. doi:10.1021/jf9503523
- Oerlemans, K., Barrett, D. M., Suades, C. B., Verkerk, R., & Dekker, M. (2006). Thermal degradation of glucosinolates in red cabbage. Food Chemistry, 95, 19–29. doi:10.1016/j.foodchem.2004.12.013
- Park, S., Arasu, M. V., Lee, M.-K., Chun, J.-H., Seo, J. M., Lee, S.-W., … Kim, S.-J. (2014). Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chemistry, 145, 77–85. doi:10.1016/j.foodchem.2013.08.010
- Rabot, S., Guérin, C., Nugon-Boudon, L., & Szylit, O. (1995, July). Glucosinolate degradation by bacterial strains isolated from a human intestinal microflora. In Proceedings of the 9th International Rapeseed Congress (GCIRC, Ed.), pp. 212–214. The Dorset Press, Dorchester, UK.
- Ramírez-Moreno, E., Hervert-Hernández, D., Sánchez-Mata, M. C., Díez-Marqués, C., & Goñi, I. (2011). Intestinal bioaccessibility of polyphenols and antioxidant capacity of pulp and seeds of cactus pear. International Journal of Food Sciences and Nutrition, 62, 839–843. doi:10.3109/09637486.2011.580731
- Rungapamestry, V., Duncan, A. J., Fuller, Z., & Ratcliffe, B. (2007a). Effect of meal composition and cooking duration on the fate of sulforaphane following consumption of broccoli by healthy human subjects. British Journal of Nutrition, 97, 644–652. doi:10.1017/S0007114507381403
- Rungapamestry, V., Duncan, A. J., Fuller, Z., & Ratcliffe, B. (2007b). Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proceedings of the Nutrition Society, 66, 69–81. doi:10.1017/S0029665107005319
- Tiedink, H. G. M., Malingre, C. E., Van Broekhoven, L. W., Jongen, W. M. F., Lewis, J., & Fenwick, G. R. (1991). Role of glucosinolates in the formation of N-nitroso compounds. Journal of Agricultural and Food Chemistry, 39, 922–926. doi:10.1021/jf00005a024
- Vallejo, F., Gil-Izquierdo, A., Pérez-Vicente, A., & García-Viguera, C. (2004). In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. Journal of Agricultural and Food Chemistry, 52, 135–138. doi:10.1021/jf0305128
- Vig, A. P., Rampal, G., Singh Thind, T., & Arora, S. (2009). Bio-protective effects of glucosinolates – A review. Food Science and Technology, 42, 1561–1572.
- Wang, G. C., Farnham, M., & Jeffery, E. H. (2012). Impact of thermal processing on sulforaphane yield from broccoli (Brassica oleracea L. ssp. italica). Journal of Agricultural and Food Chemistry, 60, 6743–6748. doi:10.1021/jf2050284
- Zimmermann, N. S., Gerends, L., & Krumbein, A. (2007). Identification of desulphoglucosinolates in Brassicaceae by LC/MS/MS: Comparison of ESI and atmospheric pressure chemical ionisation-MS Mol. Nutritional and Food Research, 51, 1537–1546.