ABSTRACT
In order to improve the nutritional value and quality of milk products, Helianthus tuberosus L. (HT) and Lupinus luteus (LL) fermented with lactic acid bacteria (LAB) (Pediococcus pentosaceus KTU05-9, P. acidilactici KTU05-7 and Lactobacillus sakei KTU05-6) were used, and the quality parameters (physicochemical, microbiological and sensory) of new fermented milk products were evaluated for the first time ever. Chemical analysis of fermented milk products showed a positive effect of the edible plants. The safety profile and nutritional value were higher in milk-LL products compared with fermented milk-HT products. Fermented milk-HT products were more acceptable because of higher taste pleasure, lower smoothness and external taste compared to fermented milk-LL products. The results clearly indicated that HT rich in inulin and LL rich in proteins can be used with selected LAB to improve the nutritional value and increase the assortment of fermented milk products.
RESUMEN
Se utilizaron Helianthus tuberosus L. (HT) y Lupinus luteus (LL) fermentadas con bacteria ácido-láctica (Pediococcus pentosaceus KTU05-9, Pediococcus acidilactici KTU05-7 y Lactobacillus sakei KTU05-6) para mejorar el valor nutricional y la calidad de los productos lácteos, además de los parámetros de calidad (fisicoquímicos, microbiológicos y sensoriales) de productos lácteos fermentados nuevos que fueron evaluados por primera vez. El análisis químico de los productos lácteos fermentados mostró un efecto positivo en las plantas comestibles. El perfil de seguridad y el valor nutricional fueron mayores en los productos lácteos con LL en comparación con los productos lácteos fermentados con HT. Los productos lácteos fermentados con HT tuvieron una mayor aceptación debido a su gusto placentero, menor homogeneidad y mejor sabor en comparación con los productos lácteos fermentados con LL. Los resultados indicaron claramente que HT rica en inulina y LL rica en proteínas pueden utilizarse con las LAB seleccionadas para mejorar el valor nutricional y aumentar la variedad de productos lácteos fermentados.
1. Introduction
Functional milk products enriched with different ingredients become more popular. Producers are looking for a possibility to create products not only acceptable by sensory properties, but also rich in biological active substances. Two edible plants, Helianthus tuberosus L. (HT) and Lupinus luteus (LL), were fermented with lactic acid bacteria (LAB) (Pediococcus pentosaceus KTU05-9, P. acidilactici KTU05-7, Lactobacillus sakei KTU05-6). These edible plants were never used for milk fermentation before and there are no data about processing of milk products using these plants, fermented with LAB. The study was performed for the first time ever and preliminary research analysis was performed.
L. luteus L. (LL) is a genus of Fabaceae (Leguminosae) family and is widely used in human nutrition and animal feeding since long time (Pedersen & Gylling, Citation2000). Lupine seeds are rich in proteins and represent a good nutritional balance of essential amino acids. Lupine contains high amounts of carotenoids, tocopherols and lecithin that are characterised as antioxidants (Kohajdova, Karovicova, & Schmidt, Citation2011; Lampart-Szczapa, Korczak, Nogala-Kalucka, & Zawirska-Wojtasiak, Citation2003).
The perennial herbaceous plant H. tuberosus L. (HT), also known as Jerusalem artichoke, is a widely available non-grain raw material (Swanton, Calvers, Clements, & Moore, Citation1992). It is rich in carbohydrate inulin (70–300 g kg‒1 of wet weight and around 500 g kg‒1 of dry weight), a polymer of the monosaccharide fructose (Rubel, Pérez, Genovese, & Manrique, Citation2014). Inulin consists of fructose units, terminated by a glucose unit, and can be readily hydrolysed to fructose and glucose. HT provides an assortment of health advantages. For instance, HT lowers blood pressure and decreases blood cholesterol level (Oliveira, Perego, Oliveira, & Converti, Citation2012). It is high in iron, potassium, protein, magnesium and fibre as well (Kim, Faqih, & Wang, Citation2001). Inulin is used in food industry as dietary fibre with probiotic properties (Roberfroid, Citation2000), fat replacer (Kim et al., Citation2001) and probiotic food carrier (Mantzouridou, Spanou, & Kiosseoglou, Citation2012).
LAB are used widely in the production of different foods. These bacteria are often used as starter cultures in the production of fermented meat and dairy products. Lactic acid is a key metabolite produced during fermentation and in food products it usually serves either as a preservative or as a flavouring agent (Vuyst & Vancanneyt, Citation2007; Widyastuti, Rohmatussolihat, & Febrisiantosa, Citation2014). On the other hand, microbiologically produced lactic acid is usually a mixture of l(+) and d(‒) isomers. As the latter cannot be metabolised by humans and can result in acidosis, it is important to assess the content of d(‒) lactic acid in fermented foods (Bartkiene, Serniene, Juodeikiene, Drungilas, & Valatkeviciene, Citation2014).
Sensory properties, such as specific odour, texture and taste, of fermented milk products are greatly influenced by LAB (Sobrino-López & Martín-Belloso, Citation2008). LAB are not only grown easily and on inexpensive media but also produce secondary metabolites such as bacteriocin-like inhibitory substances, which are able to inhibit the growth of spoilage bacteria and foodborne pathogens (Simova, Beshkova, & Dimitro, Citation2009). LAB are considered as safe food preservatives, which are easily degraded by gastrointestinal proteases (Facklam & Elliott, Citation1995). LAB are normally found in nutrient-rich environments and require fermentable carbohydrates, amino acids, fatty acids, salts and vitamins for their growth (Saeed & Salam, Citation2013).
The aim of this study was to investigate the quality of milk products enriched with H. tuberosus L. and L. luteus L. fermented with LAB (P. pentosaceus KTU05-9, P. acidilactici KTU05-7, L. sakei KTU05-6) and to analyse the influence of such edible plants on the quality parameters of fermented milk products.
2. Materials
2.1. Edible plant materials
Tubers of HT (protein 114 g kg‒1, moisture 192 g kg‒1, fat 10 g kg‒1, ash 58 g kg‒1, inulin 42 g kg‒1, etc.) were taken from the Lithuanian Institute of Horticulture (Babtai, Lithuania) in 2013. Seeds of LL (protein 424 g kg‒1, moisture 86 g kg‒1, fat 64 g kg‒1, starch 49 g kg‒1, oligosaccharides 109 g kg‒1, etc.) with low alkaloid content (<1 g kg‒1) were taken from the Lithuanian Institute of Agriculture (Vokė, Lithuania) in 2013. Before subjecting to fermentation, tubers of HT were cut into 1–2 mm slices, dried in the vacuum oven (Model SZG, China) at +45°C and ground to powder. Seeds of LL were ground in a laboratory mill (LM 3100; Perten Instruments AB, Sweden) to pass a 0.8–1.2 mm screen. The powder of dried HT and LL were used for solid state fermentation (SSF) with selected LAB.
2.2. Fermentation of plant material
For fermentation of HT and LL, different LAB were obtained from the Department of Food Science and Technology of Kaunas University of Technology (P. pentosaceus KTU05-9, P. acidilactici KTU05-7, L. sakei KTU05-6), which were previously isolated from fermented rye (Digaitiene, Hansen, Juodeikiene, & Josephsen, Citation2005). LAB were stored at −80°C and cultured at +30°C for 48 h in MRS broth (CM0359, Oxoid Ltd, Hampshire, UK), adding 40 mmol L−1 of fructose and 20 mmol L−1 of maltose prior to use. After enrichment, strains were diluted with saline up to 108 (CFU mL‒1). Three hundred grams of plant material were mixed with 450 mL of water and 5 mL of pure cultures of microorganisms and fermented for 24 h at temperatures 30°C (L. sakei KTU05-6), 32°C (P. acidilactici KTU05-7) and 35°C (P. pentosaceus KTU05-9).
2.3. Fermentation of milk with fermented plant material
For milk fermentation, 10% of fermented plants were added into the samples of milk (UHT, 32 g kg‒1 fat, 32 g kg‒1 protein, 47 g kg‒1 lactose, 60 kcal). Milk-HT and milk-LL samples were incubated for 24 h at temperatures 35°C (P. pentosaceus KTU05-9), 32°C (P. acidilactici KTU05-7) and 30°C (L. sakei KTU05-6).
2.4. Determination of total titratable acidity, pH and lactic acid isomers
Total titratable acidity (TTA) of fermented milk samples was determined according to ISO Method No. 11869, 2013.
The pH value of fermented milk samples was measured with a pH meter (PP-15, Sartorius, Germany).
l(+) and d(‒) lactic acid concentrations in fermented milk samples were determined by an enzyme test kit (R-Biopharm AG – Roche, Germany) (de Lima, Coelho, Blanco, & Contiero, Citation2009).
2.5. Determination of fat, ash and moisture content
Fat content of fermented milk samples was determined according to a gravimetric method (ICC Standard No. 1211, 2010). Briefly, the samples (5 g) were boiled in diluted hydrochloric acid, filtered, dried and extracted with petroleum ether. The residues of minerals were weighed and total ash content (g 100 g−1) was calculated.
For evaluation of moisture content, all samples (5 g) were dried at 105°C to constant weight (g 100 g−1). The moisture content was determined according to the ICC Standard Method 110/1 (ICC-Standard No.110/1, 1976).
2.6. Determination of total carbohydrates and lactose concentration
Total carbohydrates and lactose concentration were measured using high-pressure liquid chromatography (HPLC) method (Zeppa, Conterno, & Gerbi, Citation2001). Chromatographic separation was performed at a temperature of 65°C with a mobile phase of 0.01 mol L−1 H2SO4 at a flow rate of 0.6 mL min−1 on an Aminex HPX-87 H column (300 × 7.8 mm) equipped with a cation H+ microguard cartridge (Bio-Rad Laboratories, Hercules, CA, USA). HPLC equipment consisted of a quaternary pump, an online degasser (Series 200) and a refractive index detector (Series Flexar; Perkin Elmer, Norwalk, CT, USA). Data were collected and processed on a computer with the software Chromera® (Perkin Elmer). For HPLC analysis, 5 g of fermented milk sample was dispersed in 0.01 mol L−1 H2SO4 and adjusted to a final volume of 50 mL. The suspension was homogenized and centrifuged at 15,000 g for 20 min at 4°C. The supernatant was filtered through 0.45 μm membranes (Millex, Millipore, Sao Paulo, Brazil) and injected into the chromatograph, using a loop of 60 μL. Quantification was based on the external method using lactose (Sigma-Aldrich, St. Louis, MO, USA) as standard to obtain the calibration curve.
2.7. Calculation of energy value
Energy value of fermented milk samples was calculated based on the calories provided by the amount of proteins (4 kcal g‒1), carbohydrates (4 kcal g‒1) and fat (9 kcal g‒1).
2.8. Microbiological analysis of LAB
For calculation of LAB, 10 g of fermented milk samples were homogenized with 90 mL of saline (sodium chloride physiological solution 9 g kg−1) and serial dilutions (1 mL) 10–4–10–8 were inoculated on lactobacilli MRS agar (deMan, Ragosa, Sharpe, Oxoid Microbiology Products, Oxoid LTD., Basingstoke, Hampshire, UK). The plates were incubated under anaerobic conditions at temperatures 30°C (for L. sakei KTU05-6), 32°C (for P. acidilactici KTU05-7) and 35°C (for P. pentosaceus KTU05-9) for 72 h and the LAB (CFU mL‒1) were calculated.
2.9. Analysis of texture profile (firmness)
Texture profile analysis of fermented milk products (10 g) was conducted using Stevens LFRA Texture Analyzer as a constant speed cone penetrometer (Voland Corp., New York, NY, USA) using 10 mm penetration depth and 1.0 mm s‒1 speed and the force exerted on the probe. Fermented milk samples were stored at room temperature (23°C) prior to testing and the results were expressed in texture analysis units.
2.10. Sensory analysis of milk products
Sensory analysis was carried out by 10 panellists–experts of age 30–55 at the Sensory laboratory of Lithuanian University of Health Sciences. They were selected and taught to work according to ISO 8586–1 and had practical skills to evaluate milk products. Sensory profile of fermented milk products (20 g) was analysed using 10-point rating scale ranging from 1 (the lowest intensity) to 10 (the highest intensity). Attributes such as overall smell and taste, external taste, taste pleasure, smoothness and appearance (referred to colour intensity and absence of defects) were evaluated. Preliminary sensory acceptability of the products was scored using 9 mm score hedonic line scale, ranging from 1 (dislike extremely) to 9 (like extremely).
2.11. Statistical analysis
All the tests were repeated three times. In total, 24 samples of fermented milk were tested. Statistical data analysis was conducted using a Microsoft Excel’07 (Microsoft Corporation, Redmond, WA, USA) and the SPSS programme (Ver.17.0, 2006; SPSS Inc., Chicago, IL, USA) was used for the descriptive analysis (N, mean ± standard deviation), General Linear Modelling and ANOVA. Calculated mean values were compared using Bonferroni’s multiple range tests. For all statistical analyses, p ≤ 0.05 was considered as statistically significant.
3. Results and discussion
3.1. The impact of fermentation on pH, TTA and formation of lactic acid isomers
The pH values of analysed milk samples after 12 and 24 h of fermentation differed marginally (). After 12 h fermentation, the maximum pH drop (by 0.60) was seen in milk-LL samples fermented with P. acidilactici, whereas the least (by 0.10) pH changes were found in milk-HT samples fermented with P. acidilactici. After 24 h fermentation, milk-LL samples fermented with L. sakei stayed the most acidic, whereas milk-HT samples fermented with P. pentosaceus were the least acidic. However, L. sakei caused the lowest pH in fermented milk-HT samples as well. A high pH drop was observed in milk-HT and milk-LL samples after fermentation with milk-LL exhibiting lower pH values in the presence of LAB. The drop of pH showed that the LAB inoculated into the UHT milk was able to carry out acid fermentation which resulted in the production of lactic acid.
Table 1. Influence of fermentation on pH and TTA of milk product samples.
Tabla 1. La influencia de la fermentación en el pH y TTA de las muestras de productos lácteos.
TTA was assessed throughout the fermentation period. The results showed that TTA values of fermented milk samples ranged from 21.0 (milk-HT with L. sakei) to 33.0 (milk-LL with L. sakei) at the beginning of the experiment. The TTA values of all samples increased with fermentation. The highest TTA values were seen in both: milk-HT and milk-LL samples after 24 h fermentation with L. sakei. Fermentation phases are strongly dependent on the properties of the plant, fermentation conditions and types of bacteria in the inoculum (Szambelan, Nowak, & Chrapkowskaja, Citation2004). Research by Bartkiene, Skabeikytė, Juodeikienė, Vidmantienė, and Bašinskienė (Citation2014) reported that fermentation in the SSF of the L. luteus and H. tuberosus L. is effective when moisture of the substratum is less than 500 g kg‒1, pH is lower and the LAB count is higher. In our research, lactic acid produced by LAB starters induced lower pH and higher TTA of fermented LL. The higher TTA could be ascribed to lower pH due to a higher fermentation activity of the LAB used (Bello, Clarke, & Ryan, Citation2007). It could be assumed that oligosaccharides in milk-LL samples induced more intensive fermentation leading to higher amounts of lactic acid, which are reflected in values of TTA and pH ().
Analysing the effect of lactic acid isomers d(‒)/l(+) formation in fermented milk samples, we saw that the highest amount of lactic acid isomer d(‒) was reached in milk-LL samples fermented with P. pentosaceus (). The amount of lactic acid isomer d(‒) ranged from 0.103 ± 0.015% (in milk-HT with P. acidilactici) to 0.308 ± 0.015% (in milk-LL with P. pentosaceus). The amount of lactic acid isomer l(+) ranged from 0.126 ± 0.002% (in milk-HT with L. sakei) to 0.469 ± 0.002% and 0.469 ± 0.005% (in milk-LL with P. pentosaceus and L. sakei, respectively). We have also found that milk-LL samples produced more l(+) than d(‒) isomers compared to milk-HT samples. It could be assumed that production of lactic acid depends on the strain and substrate used (Mirdamadi et al., Citation2002). In our study, milk-LL with L. sakei and P. pentosaceus could be assigned as the most active l(+) lactic acid producers. However, in industrial fermentations, the use of various species of Lactobacillus is preferred, owing to higher rates of metabolism (John, Anisha, Madhavan, & Ashok, Citation2009). The l(+) lactic acid is the predominant metabolite formed during milk fermentation and has the same configuration as the lactic acid produced by the human body. Substantial quantities of d(‒) lactic acid were also found in our research. The d(‒) isomer reduces cell metabolism and causes acidosis in humans (Gurukripa Kowlgi & Chhabra, Citation2015). Because of the lower rate of metabolism of d(‒) compared to l(+) lactic acid and effects mentioned, the World Health Organization has recommended restriction in consumption of products containing high d(‒) lactic acid. The recommended d(‒) lactic acid intake is 650 g kg‒1 human body weight (Park, Lee, Kim, Jung, & Yang, Citation2012). Thus, our fermented milk products could be assumed as safe because of the higher amount of l(+) isomer and not exceeded daily intake of d(‒) isomer.
Table 2. Amount of d(‒) and l(+) lactates in fermented milk product samples.
Tabla 2. Cantidad de lactatos D (-) y L (+) en las muestras de productos lácteos fermentados.
3.2. Composition of fermented milk products
One of the tasks of the study was to estimate and compare the composition of fermented milk-HT and milk-LL samples. Carbohydrates and proteins are the principal components of food products and play an important role in fermented dairy products, which are widely used as healthy food and considered as important part of the diet (La Torre, Citation2003).
Chemical analysis of fermented milk product samples showed that the milk-LL samples were more nutritious and had lower moisture content compared to milk-HT samples (). Many other studies confirm the high nutritive value of lupine seeds, with the highest nutrient profile and fat content (Campos-Andrada, Santana, Felgueiras, Mimoso, & Empis, Citation1999; Dolezal, Citation2002; Gilbert, Särkilahti, Apajalahti, Acamovic, & Bedford, Citation2000; Kohajdova, Korovičova, & Schmidt, Citation2011). In our study, the highest fat content was seen in milk-LL samples fermented with P. acidilactici. It was higher by 0.14% and 0.15% (p ≤ 0.05) compared to fermented milk-HT samples.
Table 3. Parameters of fermented milk products.
Tabla 3. Parámetros de productos lácteos fermentados.
The nutritional value of legumes is related to the high protein content of the seeds. In our study, higher protein content was observed in milk-LL samples compared to milk HT samples. Protein content in milk-LL samples fermented with P. pentosaceus was higher by 1.62 (p ≤ 0.05), 1.63 (p ≤ 0.01) and 1.64% (p ≤ 0.01) compared with milk-HT samples.
Our results are in agreement with the findings of Ibeawuchi and Daylop (Citation1995), who stated that crude protein content increased after fermentation of pasteurized milk with starter culture. Martínez-Villaluenga, Frías, and Vidal-Valverde (Citation2006) also indicated white lupine (Lupinus albus) as one of the potential plants with high protein content. Hence, rich in proteins, L. luteus can be useful as a protein source for fermented milk products.
Total ash and carbohydrate content of fermented milk-LL samples was significantly (p ≤ 0.01) higher compared with milk-HT samples. Amount of carbohydrates in milk-LL samples ranged from 6.24% to 6.26% and was higher (about 0.73%) than in milk-HT samples where amount of carbohydrates ranged from 5.46% to 5.51%. The moisture content of fermented milk-HT samples was significantly higher than that of fermented milk-LL samples (p < 0.05). The moisture content of fermented milk-HT (87.76 ± 0.04) samples was significantly higher than that of milk-LL samples (85.23 ± 1.34) (p ≤ 0.05).
Compositional analysis of fermented milk products indicated that there were significant differences (p ≤ 0.05) between fat, moisture, total carbohydrates and pH after 12 h and between above-mentioned indexes and texture after 48 h fermentation ( and ). Significant differences were seen between TTA after 12 h and 24 h, between total ash and total protein content (p ≤ 0.001) and between pH after 24 h and lactose () concentration (p ≤ 0.01). As there were no significant differences between fermentation conditions, it could be assumed that the composition of fermented milk samples was mostly influenced by edible plant material. Therefore, L. luteus seeds could be the main plant source of proteins in the human diet as it can improve the nutritional quality of the products (Gomez, Oliete, Rosell, Pando, & Fernandez, Citation2008). Also, lupine does not contain gluten and it could be used as a functional ingredient in gluten-free foods (Scarafoni, Ronchi, & Duranti, Citation2009).
Figure 1. Changes of texture of fermented milk product samples.
Figura 1. Cambios en la textura de las muestras de productos lácteos fermentados.
P.p.: P. pentosaceus; P.a.: P. acidilactici; L.s.: L. sakei; HT: Helianthus tuberosus L.; LL: Lupinus luteus L.
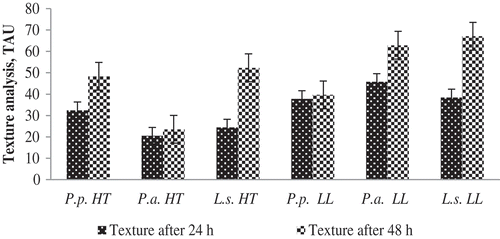
Fermentation with different LAB cultures caused significantly different (p ≤ 0.001) lactose values of fermented milk samples (). It was found that lactose values of the tested samples depended on the substrate used. In fermented milk-HT samples, lactose concentration was higher compared to milk-LL samples. Whereas the decrease of lactose value could be due to lactic acid formation. The highest lactose values were found in milk-HT and milk-LL samples fermented with L. sakei (4.65 ± 0.30 and 4.40 ± 0.36, respectively). Erbas, Certel, and Uslu (Citation2005) reported that lupine seeds contain high amounts of sugar (5.82%). In our study, the most intensive breakdown of carbohydrates was seen in milk-LL samples fermented with P. pentosaceus.
The energy value of fermented milk samples ranged from 60.24 ± 0.01 kcal (milk-HT, with P. acidilactici) to 70.70 ± 5.43 kcal (milk-LL with P. pentosaceus). In our opinion, the higher energy value in milk-LL samples resulted from a high content of proteins and total carbohydrates in milk-LL samples.
3.3. Microbiological analysis of LAB
LAB can play an important role in milk fermentation process. LAB growth and metabolic activities are needed to assure a high quality of the final product. These microorganisms produce lactic acid via lactose fermentation, which leads to a rapid decrease in pH. Cheese and fermented milk products depend largely on this fermentation process, which is also crucial for ensuring control of pathogenic and spoilage microorganisms (Mariángeles, Moineau, & Quiberon, Citation2012).
The microbiological analysis of fermented milk samples showed that LAB count varied from 9.6 × 105 to 2.7 × 109 (milk-HT) and from 6.0 × 104 to 8.0 × 108 CFU mL‒1 (milk-LL), respectively (). The highest LAB count was found using P. acidilactici for fermentation in the presence of both edible plants, whereas the count of P. pentosaceus was the least. Thus, it is possible to state that P. acidilactici growth in milk media is the most intensive regardless of the edible plant used. In other substrates, for example, in bread sourdough, the growth of P. acidilactici was more intensive than that of L. sakei (Bartkiene et al., Citation2013).
Different cultures (a single strain or a mixture of several strains) of microorganisms can be added into milk in order to provide specific characteristics of finished fermented milk product in a controlled and predictable manner. Despite the primary function of lactic acid starters to ferment lactose into lactic acid, they may also contribute to flavour, aroma and alcohol production while inhibiting spoilage microorganisms (Panesar, Citation2011).
3.4. Texture analysis
Texture analysis of fermented milk products is an important attribute, characterizing the quality of the products. It is related to sensory perception of the product as well (Ozcan, Citation2013; Sodini, Remeuf, Haddad, & Corrieu, Citation2004). The strongest texture after 24 h storage at room temperature was detected in milk-HT with P. acidilactici (20.56 ± 5.03), whereas the softest texture was detected in milk-LL with P. acidilactici (45.78 ± 20.60) (Figure 1). After 48 h fermentation, the strongest texture was detected in milk-HT with P. acidilactici. This could mean that the texture of fermented milk products is influenced by the edible plant used and fermentation with P. acidilactici is the most suitable for production of fermented milk. Sodini, Lucas, Oliveira, Remeuf, and Corrieu (Citation2002) confirm that starter cultures have influence on rheological parameters of fermented milk products as well.
3.5. Sensory analysis
The changes during fermentation affect products’ physical properties such as appearance, texture, flavour, aroma, acidity and overall acceptability (Ghosh & Chattopadhyay, Citation2012). After the analysis of the sensory attributes, it was found that milk-HT samples fermented with P. pentosaceus were the most acceptable (7.0 ± 1.0 points) and this could be explained by the highest taste pleasure, the lowest smoothness and external taste of the final product (). Despite the low evaluation of appearance, these products were more palatable to consumers. This confirms the fact that fermented milk-HT has a great potential in food applications, especially in the development of functional foods, including functional milk products.
Table 4. Influence of HT and LL on sensory attributes of fermented milk products.
Tabla 4. La influencia de HT y LL en los atributos sensoriales de los productos lácteos fermentados.
Milk-HT samples fermented with L. sakei were the least acceptable and this could be influenced by the highest external taste. The increased external taste could be influenced by the plant’s aromatic characteristic that had lowered the overall acceptability of the final product.
Sensory analysis has shown that acceptability of fermented milk products was influenced not only by edible plant material used but also by LAB used for fermentation. The LAB used in this study also had an influence on odour and flavour intensity, taste pleasure, smoothness and appearance of the final product.
To our knowledge, there are no studies present in the literature concerning fermented milk products made with H. tuberosus L. and L. luteus. In most other investigations, LAB and plants (HT and LL) were used in different alimentary matrices (Bartkiene et al., Citation2014; Bello et al., Citation2007). Hence, it is difficult to compare our results with those reported for products from the same category, due to differences in the applied experimental conditions, raw materials and strains of LAB used for fermentation. Our data are valuable and show that HT and LL fermented via solid-state fermentation can be used as pre-ferment in the processing of milk products.
4. Conclusions
In this study, three LAB strains (P. pentosaceus, P. acidilactici and L. sakei) were applied to a novel fermentation medium of milk with a perspective of industrial production of fermented milk products.
Chemical analysis of fermented milk products showed that addition of L. luteus L. had a positive effect on energy value, fat, protein, ash and total carbohydrate content. The products were more nutritious and had lower moisture content, softer texture and higher acidity. Milk-LL fermented with L. sakei and P. pentosaceus produced more l(+) than d(‒) isomers than those of milk-HT products, whereas the higher amounts of L(+) and the lower amounts of d(‒) isomers formed during milk-HT fermentation with P. acidilactici (p ≤ 0.01).
Milk-HT-fermented products were more acceptable and palatable because of the higher taste pleasure, lower smoothness and external taste compared to milk-LL-fermented products. The acceptability of fermented milk products is dependent on the LAB used as well. Thus, milk fermented with P. acidilactici was more acceptable because of texture and appearance. In addition, the highest amount of LAB was found in milk-HT fermented with P. acidilactici compared to milk-LL products.
This investigation has demonstrated that some plants which are part of our diet can be used in combination with LAB for the production of novel fermented milk products. Advantages of the novel fermented milk products are their high nutritional value, low d(‒) lactic acid content and high sensory attributes. Continuous growth of the milk industry may depend largely upon the introduction of new milk fermented products with creative concepts, which are built on consumer appeal.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bartkiene, E., Schleining, G., Rekstyte, T., Krungleviciute, V., Juodeikiene, G., Vaiciulyte-Funk, L., & Maknickiene, Z. (2013). Influence of the addition of lupin sourdough with different lactobacilli on dough properties and bread quality. International Journal of Food Science & Technology, 48, 2613–2620. doi:10.1111/ijfs.12257
- Bartkiene, E., Serniene, L., Juodeikiene, G., Drungilas, E., & Valatkeviciene, Z. (2014). The safety, technology and sensory aspects of pasteurized and raw milk treated by solid-state fermented grain extrudates inoculated with certain lactobacilli. Veterinarija Ir Zootechnika, 65, 3–10.
- Bartkiene, E., Skabeikytė, E., Juodeikienė, G., Vidmantienė, D., & Bašinskienė, L. (2014). The use of solid state fermentation for food. Veterinarija Ir Zootechnika, 66, 1–11.
- Bello, D., Clarke, F., & Ryan, C. (2007). Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. Journal of Cereal Science, 45, 309–318. doi:10.1016/j.jcs.2006.09.004
- Campos-Andrada, M.P., Santana, F.M.C., Felgueiras, I., Mimoso, M.J., & Empis, J.M.A. (1999, June) Nutritional value of Lupinus angustifolius and L. cosentinii accessions with diverse genetic origin. In Proceedings of the 9th International Lupin Conference. Germany
- de Lima, C.J.B., Coelho, L.F., Blanco, K.C., & Contiero, J. (2009). Response surface optimization of D(-) lactic acid production from Lactobacillus SMI8 using corn steep liquor and yeast autolysate as nitrogen sources. African Journal of Food Science, 3, 257–261. doi:10.4314/ajb.v8i21.66061
- Digaitiene, A., Hansen, A., Juodeikiene, G., & Josephsen, J. (2005). Microbial population in Lithuanian spontaneous rye sourdoughs. Ekologia I Technika, 5, 193–198.
- Dolezal, P. (2002). Effect of supplements of Lactobacillus plantarum DSM 12771 on the quality of ensiled alfalfa and grass with a high content of dry matter. Mendel University of Agriculture and Forestry in Brno, 5, 37–44.
- Erbas, M., Certel, M., & Uslu, M.K. (2005). Some chemical properties of white lupin seeds (Lupinus albus L). Food Chemistry, 89, 341–34. doi:10.1016/j.foodchem.2004.02.040
- Facklam, R., & Elliott, J.A. (1995). Identification, classification and clinical relevance of catalase-negative, gram-positive cocci excluding the streptococci and enterococci. Clinical Microbiology. Reviews, 8, 479–495.
- Ghosh, D., & Chattopadhyay, P. (2012). Application of principal component analysis (PCA) as a sensory assessment tool for fermented food products. Journal of Food Science and Technology, 49, 328–334. doi:10.1007/s13197-011-0280-9
- Gilbert, C., Särkilahti, L., Apajalahti, J., Acamovic, T., & Bedford, M.R. (2000). Microbial populations in caecal contents from chicks fed lupin-based diets, with and without enzyme supplementation. In Proceedings of XXI World’s Poultry Congress (CD). Montreal, Canada.
- Gomez, M., Oliete, B., Rosell, C.M., Pando, V., & Fernandez, E. (2008). Studies on cake quality made of wheat – chickpea flour blends. Food Science and Technology, 41, 1701–1709. doi:10.1016/j.lwt.2007.11.024
- Gurukripa Kowlgi, N., & Chhabra, L. (2015). D-lactic acidosis: An underrecognized complication of short bowel syndrome. Gastroenterology Research and Practice, 2015, 1–8. doi:10.1155/2015/476215
- Ibeawuchi, J.A., & Daylop, D.M. (1995). Composition and quality of fresh cow milk offered for sale in plateau State Nigeria. Nigerian Journal of Animal Production, 22, 81–85.
- John, R.P., Anisha, G.S., Madhavan, N.K., & Ashok, P. (2009). Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnology Advances, 27, 145–152. doi:10.1016/j.biotechadv.2008.10.004
- Kim, Y., Faqih, M.N., & Wang, S.S. (2001). Factors affecting gel formation of inulin. Carbohydrate Polymers, 46, 135–145. doi:10.1016/S0144-8617(00)00296-4
- Kohajdova, Z., Karovičova, J., & Schmidt, Š. (2011). Lupin composition and possible use in bakery – A review. Czech Journal of Food Science, 29, 203–211.
- La Torre, L. (2003). Rheology and sensory profiling of set-type fermented milks made with different commercial probiotic and yogurt starter cultures. International Journal of Dairy Technology, 56, 163–170. doi:10.1046/j.1471-0307.2003.00098.x
- Lampart-Szczapa, E., Korczak, J., Nogala-Kalucka, M., & Zawirska-Wojtasiak, R. (2003). Antioxidant properties of lupin seed products. Food Chemistry, 83, 279–285. doi:10.1016/S0308-8146(03)00091-8
- Mantzouridou, F., Spanou, A., & Kiosseoglou, V. (2012). An inulin-based dressing emulsion as a potential probiotic food carrier. Food Research International, 46, 260–269. doi:10.1016/j.foodres.2011.12.016
- Mariángeles, B.M., Moineau, S., & Quiberon, A. (2012). Bacteriophages and dairy fermentations. Bacteriophage, 2, 149–158. doi:10.4161/bact.21868
- Martínez-Villaluenga, C., Frías, J., & Vidal-Valverde, C. (2006). Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of ɑ-galactosides. Food Chemistry, 9(8), 291–299.
- Mirdamadi, S., Sadeghi, H., Sharafi, N., Fallahpour, M., Mohseni, A.F., & Bakhtiari, M.R. (2002). Comparison of lactic acid isomers produced by fungal and bacterial strains. Iranian Biomedical Journal, 6, 69–75.
- Oliveira, R.P.S., Perego, P., Oliveira, M.N., & Converti, A. (2012). Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT - Food Science and Technology, 47, 358–363. doi:10.1016/j.lwt.2012.01.031
- Ozcan, T. (2013). Determination of yogurt quality by using rheological and textural parameters. 2nd International Conference on Nutrition and Food Sciences IPCBEE. doi:10.7763/IPCBEE.2013.V53.23
- Panesar, P.S. (2011). Fermented dairy products: starter cultures and potential nutritional benefits. Food and Nutrition Sciences, 2, 47–51. doi:10.4236/fns.2011.21006
- Park, S.J., Lee, S.Y., Kim, T.W., Jung, Y.K., & Yang, T.H. (2012). Biosynthesis of lactate-containing polyesters by metabolically engineered bacteria. Biotechnology, 7, 199–212. doi:10.1002/biot.201100070
- Pedersen, S.M., & Gylling, M. (2000). Lupin proteins for fermentation – economic and technology assessment. In Proceedings of 1st World Conference on Biomass for Energy and Industry. London: James and James (Science Publishers) Ltd.
- Roberfroid, M. (2000). Non digestible oligosaccharides. Critical Reviews in Food Science and Nutrition, 40, 461–480. doi:10.1080/10408690091189239
- Rubel, I.A., Pérez, E.E., Genovese, D.B., & Manrique, G.D. (2014). In vitro prebiotic activity of inulin-rich carbohydrates extracted from Jerusalem artichoke (Helianthus tuberosus L.) tubers at different storage times by Lactobacillus paracasei. Food Research International, 62, 59–65. doi:10.1016/j.foodres.2014.02.024
- Saeed, A.H, & Salam, A.I. (2013). Current limitations and challenges with lactic acid bacteria: A review. Food and Nutrition Sciences, 4, 73–87. doi:10.4236/fns.2013.411A010
- Scarafoni, A., Ronchi, A., & Duranti, M. (2009). A real-time PCR method for the detection and quantification of lupin flour in wheat flour-based matrices. Food Chemistry, 115, 1088–1093. doi:10.1016/j.foodchem.2008.12.087
- Simova, E.D., Beshkova, D.B., & Dimitro, Z.P. (2009). Characterization and antimicrobial spectrum of bacteriocins produced by lactic acid bacteria isolated from traditional Bulgarian dairy products. Journal of Applied Microbiology, 106, 692–701. doi:10.1111/j.1365-2672.2008.04052.x
- Sobrino-López, A., & Martín-Belloso, O. (2008). Use of nisin and other bacteriocins for preservation of dairy products. International Dairy Journal, 18, 329–343. doi:10.1016/j.idairyj.2007.11.009
- Sodini, I., Lucas, A., Oliveira, N., Remeuf, F., & Corrieu, G. (2002). Effect of milk base and starter culture on acidification, texture, and probiotic cell counts in fermented milk processing. Journal of Dairy Science, 85, 2479–2488. doi:10.3168/jds.S0022-0302(02)74330-0
- Sodini, I., Remeuf, F., Haddad, S., & Corrieu, G. (2004). The relative effect of milk base, starter, and process on yogurt texture: A review. Critical Reviews in Food Science and Nutrition, 44, 113–137. doi:10.1080/10408690490424793
- Swanton, C.J., Calvers, P.B., Clements, D.R., & Moore, M.J. (1992). The biology of Canadian weeds. 101. Helianthus tuberosus L. Canadian Journal of Plant Science, 72, 1367–1382. doi:10.4141/cjps92-169
- Szambelan, K., Nowak, J., & Chrapkowskaja, K.J. (2004). Comparison of the bacterial and yeast ethanol fermentation yield from Jerusalem artichoke (Helianthus tuberosus L.) tubes, pulp and juice. Acta Scientiarium Polonarium. Technology Alimentarium, 3, 45–53.
- Vuyst, L.D., & Vancanneyt, M. (2007). Biodiversity and identification of sourdough lactic acid bacteria. Food Microbiology, 24, 120–127. doi:10.1016/j.fm.2006.07.005
- Widyastuti, Y., Rohmatussolihat, & Febrisiantosa, A. (2014). The role of lactic acid bacteria in milk fermentation. Food & Nutrition Sciences, 5, 435–442. doi: 10.4236/fns.2014.54051
- Zeppa, G., Conterno, L., & Gerbi, V. (2001). Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. Journal of Agricultural and Food Chemistry, 49, 2722–2726. doi:10.1021/jf0009403
