 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We planned to investigate the protective effect of soy isoflavone (SIF) genistein (GEN), and daidzein (DAI), and their mixed solution against carbaryl-induced toxicity in PC12 cells. PC12 cells were exposed to carbaryl, pretreated, co-treatment, or posttreatment with three different SIF solutions for indicated times. Exposure to SIF resulted a better survival and cell morphology which were subjected to carbaryl toxicity. Treatment with SIF was also found to reduce malondialdehyde content, superoxide dismutase inhibitory rate, reactive oxygen species generation, acetylcholine levels in medium, and maintain the integrity of mitochondrial membrane potential. It was also found to increase GSH level. In conclusion, SIF can effectively mitigate adverse effects induced by carbaryl. GEN and DAI have a combined effect that is greater than the effect obtained with each isoflavone alone, although they have different effects against carbaryl-induced damage. SIF enhances metabolism of choline and plays a role as both antioxidant and neuroprotectant.
RESUMEN
Nuestro plan fue investigar el efecto protector de las isoflavonas de la soja (SIF), la genisteína (GEN) y la daidzeína (DAI), además de su solución mixta contra la toxicidad inducida por carbaril en células PC12. Las células PC12 estuvieron expuestas a carbaril, tratadas previamente, tratamiento conjunto o tratadas posteriormente con tres soluciones diferentes de SIF durante los tiempos indicados. La exposición a SIF resultó en una mayor supervivencia y morfología celular, las cuales estuvieron sujetas a la toxicidad del carbaril. También se encontró que el tratamiento con SIF redujo el contenido de MDA, el índice de inhibición de SOD, la generación de ROS, los niveles de acetilcolina a intermedio, además de mantener la integridad del potencial de membrana mitocondrial. También se encontró que hizo aumentar el nivel de GSH. En conclusión, SIF puede mitigar de forma eficiente los efectos adversos inducidos por el carbaril. GEN y DAI tienen un efecto combinado que es mayor que el efecto obtenido con cada isoflavona por separado, aunque tienen efectos distintos contra el daño inducido por carbaril. SIF mejora el metabolismo de la colina, además de jugar un papel importante como antioxidante y neuroprotector.
ABBREVIATIONS: SIF, soy isoflavone; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; GSH, glutathione; SOD, superoxide dismutase; MDA, malondialdehyde; DCFH-DA, 2′,7′-dichlorofluorescin diacetate; ROS, reactive oxygen species; ACh, acetylcholine; AChE, acetylcholine esterase; SEM, scanning electron micrographs; DMSO, dimethyl sulfoxide; PMSF, phenylmethylsulfonyl fluoride; RIPA, radio immunoprecipitation assay; DMEM, Dulbecco’s modified eagle medium; FBS, fetal calf serum; PBS, phosphate-buffered saline; BCA, bicinchoninic acid; MMP, mitochondrial membrane potential; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide; RFI, relative fluorescence intensity; nAChR, nicotinic acetylcholine receptor.
Introduction
Pesticides are widely used in agriculture all over the world, whereas they cause widespread contamination of air, water, soil, and agricultural products (Xu, Liu, & Lu, Citation2014), eventually leading to long-term accumulation in ecosystems including humans (Yang, Wild, & Russell, Citation1995). The residues with highly toxic substances have been found to cause serious problems to human health even at very low concentrations (Heath, Citation1997; Wang, Wang, Timchalk, & Lin, Citation2008). Carbamate pesticides, widely applied as insecticides, herbicides, and fungicides, may cause a variety of symptoms in mammals and humans, and which were originally designed to target nervous system of insects can obviously also cause neurotoxicity to humans, resulting in the accumulation of the neurotransmitter acetylcholine (ACh) in the body, which can lead to fatal consequences (Moser, Padilla, Simmons, Haber, & Hertzberg, Citation2012).
Carbaryl (1-naphthyl N-methylcarbamate, CAS No. 63-25-2), one of carbamate pesticides, has been applied for the control of pests in forestry and agriculture. This pesticide can inhibit the activity of acetylcholinesterase (AChE) in an irreversible manner and thereby cause insect death (Fahmy, Fukuto, Myers, & March, Citation1970). It is also used to control many kinds of pests in crops such as beans, bananas, potatoes, and domestic vegetables. Carbaryl is mildly toxic for humans and domestic animals (class II) (Mahajan et al., Citation2007) and is capable of altering the antioxidant defense system in diverse organisms (Ferrari, Venturino, & D’Angelo, Citation2007; Matos, Fontaı´nhas-Fernandes, Peixoto, Carrola, & Rocha, Citation2007). In addition to its neurotoxicity, it was found to cause heart malformation (Lin, Hui, & Cheng, Citation2007) and increase the mortality rate during development of zebra fish (Danio rerio) (Todd & Van Leeuwen, Citation2002). Incidences of human exposures to carbaryl were not uncommon (Branch & Jacqz, Citation1986).
In order to ameliorate or avoid possible harm to humans and animals, it is of great importance to study how to prevent against pesticides-induced nervous damage (Choi et al., Citation2013). In a pursuit to find strategy for negating carbaryl toxicity in humans, we stumbled upon the choice of soy isoflavone (SIF). It is believed that SIF, such as daidzein (DAI) and genistein (GEN), has an important role in potential health benefits (Xu et al., Citation2015). GEN is neuroprotective in an in vivo model of global cerebral ischemia in gerbils (Donzelli et al., Citation2010). In addition, the other main SIF, DAI, showed the same effects, protecting embryonic rat primary cortical neurons from ischemic-like injury in vitro and reducing ischemia/reperfusion-induced myocardial damage in vivo (Schreihofer & Redmond, Citation2009). GEN and DAI have a synergistic effect that is greater than the effect obtained with each isoflavone alone (Rando, Ramachandran, Rebecchi, Ciana, & Maggi, Citation2009).
Because the nature of the effects of carbamate pesticides on neuronal nicotinic acetylcholine receptor (nAChRs) is mainly inhibitory (Smulders, Bueters, Van Kleef, & Vijverberg, Citation2003), we have focused on the mechanism of inhibition by carbaryl. The PC12 cell line, from a rat pheochromocytoma, is a useful model system for neurobiological and neurochemical studies establish by Greene and Tischler (Citation1976). In this study, we investigated potential protective effects of GEN, DAI, and their mixed solution against carbaryl-induced damage in PC12 cells. We found that SIF could play neuroprotective role, its mechanism should be achieved through enhancing PC12 cells antioxidation and metabolism of choline.
Materials and methods
Materials and reagents
GEN, DAI, carbaryl, trypsin, 2′,7′-dichlorofluorescin diacetate (DCFH-DA), and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical (St. Louis, MO, USA). MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was purchased from Promega (Madison, USA). Dulbecco’s modified eagle medium (DMEM), fetal calf serum (FBS), penicillin–streptomycin, and phosphate-buffered saline (PBS) were obtained from Gibco (Grand Island, NY, USA). Cell-culture plates and cell-culture dishes were purchased from Corning Incorporated (USA). PC12 cells were provided by Shanghai Cellular Institute of China Scientific Academy.
Phenylmethylsulfonyl fluoride (PMSF), radio immunoprecipitation assay lysis buffer, bicinchoninic acid (BCA) protein assay kit, and mitochondrial membrane potential (MMP) assay kit with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1)) were purchased from Beyotime Institute of Biotechnology (Shanghai, China).
Superoxide dismutase (SOD) assay kit, malondialdehyde (MDA) assay kit, glutathione (GSH) assay kit, and ACh assay kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Cell culture
PC12 cells were cultured on collagen-coated dishes in DMEM medium supplemented with 10% FBS, 100.00 U/mL penicillin, and 100.00 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2 incubator (Feng et al., Citation2012).
Cell viability assay
Carbaryl, GEN, and DAI were dissolved in DMSO (the concentration of DMSO in the final culture medium was <0.1%). GEN–DAI mixed solution contented 1:1 concentration ratio on GEN and DAI. PBS solution had the same concentration of DMSO as SIF solutions (DMSO < 0.1%).
PC12 cells (1 × 105 cells/mL) were seeded into a 96-well plate and grown in the incubator for 24 h, then exposed to GEN (20.00 μg/mL), DAI (20.00 μg/mL), or the mixed solution (20.00 μg/mL). The control group was treated with PBS.
In protective experiments, PC12 cells were divided into five groups as shown in . Control group (G1): After seeded 24 h, PC12 cells were treated with PBS. Carbaryl group (G2): After seeded 24 h, PC12 cells were treated with carbaryl (100.00 μg/mL). Pretreatment group (G3): After seeded 12 h, PC12 cells were treated with GEN, DAI, or the mixed solution and incubated for 12 h, then treated with carbaryl (100.00 μg/mL). Co-treatment group (G4): After seeded 24 h, PC12 cells were treated with carbaryl (100.00 μg/mL) and treated with GEN, DAI, or the mixed solution simultaneously. Posttreatment group (G5): After seeded 24 h, PC12 cells were treated with carbaryl (100.00 μg/mL) and incubated for 12 h, then treated with GEN, DAI, or the mixed solution. All treatments were incubated for 48 h (Liu, Liu, Xu, Liu, & Li, Citation2015).
Table 1. Grouping emperiment.
Tabla 1. Experimiento grupal.
PC12 cells viability after various treatments were assessed by the MTS (1.90 mg/mL MTS and 300 μM phenazine ethosulfate) assay. Following the treatment, 20 μL of MTS was added into each well for 40–50 min in incubation at 37°C. Absorbances were read at a wavelength of 490 nm by a microplate reader (BioTek Instruments, US), and cell viability was expressed as the percentage of viable cells in the treated groups compared to the control group.
Scanning electron micrograph examination
Surface morphologies of PC12 cells in G1, G2, and G3 were observed by S-3400N Scanning Electron Micrographs (SEM) (Hitachi, Japan) and images were taken.
GSH, MDA content, and SOD inhibition rate
PC12 cells in five groups were treated by drugs in the same way as indicated above. Following the treatments, cells were homogenized in ice-cold lysis buffer with 1% PMSF, and protein concentrations of the resulting supernatant were determined by the BCA protein assay kit. GSH, MDA contents, and SOD inhibition rate were measured according to the direction of the assay kit following the manufacturer’s protocol.
ROS measurement
DCFH-DA was dissolved in DMEM without FBS and kept in a dark place. The final concentration was 10.00 μM (Rosenkranz et al., Citation1992).
PC12 cells in five groups were treated by drugs in the same way as indicated above. When determining, media from PC12 cells were removed, then PC12 cells were preincubated with 10.00 μM DCFH-DA (80 μL/well) for 15 min. Cells were then washed by DMEM, the relative fluorescence intensity (RFI) of the fluorophore was detected. Reactive oxygen species (ROS) generation was measured (excitation, 485 nm; emission, 528 nm) using a microplate fluorometer. Therefore, the RFI calculated according to the following equation:
ACh assay
ACh content was detected by using ACh assay kit. Briefly, the media from PC12 cells were collected, then related reagents were added, the absorbance of each sample was read at 550 nm by a microplate reader.
Monitoring of the MMP
Protective effect of SIF against carbaryl-induced damage in PC12 cells resulted in a change of MMP, which could be measured using a fluorescence probe JC-1. JC-1 working solution was dissolved in ultrapure-grade water and kept in a dark place. PC12 cells in five groups after being incubated for 48 h were incubated at 37°C for another 20 min with 200-μL JC-1, then washed twice with PBS (without DMSO) and placed in fresh medium.
The relative intensities of red and green fluorescence were simultaneously examined. J-aggregates in the mitochondria (only in living cells) were detected as a red color by a fluorescence microscope using excitation/emission = 525/590 nm. J-monomers in the cytoplasm were detected as a green color to excitation/emission = 490/530 nm (Sivanesan, Palanisamy, Vanessa, James, & Appu, Citation2013). Mitochondrial depolarization is indicated by an increase in the green/red fluorescence intensity ratio.
Statistical analysis
The data analyzed by SPSS 16.0 were mean ± SD based on Duncan’s Multiple Range (SSR) test at P < 0.05 level (*) and P < 0.01 level (**), respectively.
Results
Protection effect of SIF against carbaryl-induced in PC12 cells viability and morphology
SIF may have nutritional or cytotoxic effect in PC12 cells, thus being interference to our readouts. To exclude this nonspecific interference, we determined the effects of GEN, DAI, and the mixed solution on PC12 cells by the MTS assay. PC12 cells viability was (102.92 ± 6.68)%, (110.33 ± 8.67)%, and (89.09 ± 0.62)% of the control (100.00 ± 1.97)% after 48 h of cell incubation with 20.00 μg/mL DAI, GEN, and mixed solution, respectively. The effect of GEN, DAI, or their combinations showed no significant effect on PC12 cells proliferation or cytotoxicity (P > 0.05).
Carbaryl at a concentration of 100.00 μg/mL for 24 h led to (49.99 ± 13.07)% viability. In G3, G4, and G5, DAI and the mixed solution showed significant protective effects in PC12 cells (P < 0.05). However, supplement with GEN seems to have no effect on carbaryl-induced damage in any group (P > 0.05) ().
Table 2. Soy isoflavone ameliorates oxidative stress after carbaryl.
Tabla 2. Las isoflavonas de la soja mejoran el estrés oxidativo después del carbaril.
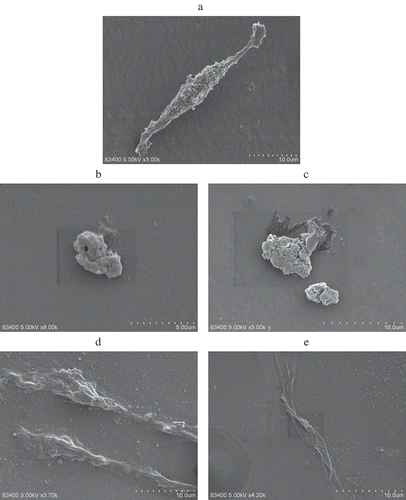
SEM of PC12 cell in the normal showed normal size and shape (. Processed by carbaryl, PC12 cell signaled the characteristics of apoptosis such as shrinking and detaching (. Pretreated with GEN showed some improvement in size of PC12 cell, though overall morphology was still shrinking (. Compared with GEN pretreatment group, the PC12 cell injury was ameliorated with DAI supplement, recovery of neural form is obvious (. It is clear that the mixed solution treatment group showed significant improvement in both size and shape in carbaryl-damaged PC12 cell that resulted in an obvious restoration (.
Figure 1. Scanning electron micrographs of the PC12 cells. (a) The control group; (b) 100.00 μg/mL carbaryl treated; (c) exposed to 100.00 μg/mL carbaryl for prior to 20.00 μg/mL GEN treatment for 12 h; (d) exposed to 100.00 μg/mL carbaryl for prior to 20.00 μg/mL DAI treatment for 12 h; (e) exposed to 100.00 μg/mL carbaryl for prior to 20.00 μg/mL combinations treatment for 12 h.
Figura 1. Micrografía por barrido electrónico de las células PC12. (a) El grupo control; (b) 100,00 μg/mL de carbaril tratado; (c) expuesto a 100,00 μg/mL de carbaril previo a 20,00 μg/mL de tratamiento GEN durante 12 h; (d) expuesto a 100,00 μg/mL de carbaril previo a 20,00 μg/mL de tratamiento DAI durante 12 h; (e) expuesto a 100,00 μg/mL de carbaril previo a 20,00 μg/mL de tratamiento de combinaciones durante 12 h.
Soybean isoflavone enhanced the activity of endogenous antioxidant system
As shown in , carbaryl-induced changes in PC12 cells are endogenous to antioxidant system.
MDA increased from 5.50 ± 0.68 to 105.19 ± 14.49 nmol/mgprot with carbaryl-treated group in G2. Both DAI and mixed solution in G3, G4, and G5 markedly decreased MDA content compared to carbaryl group (P < 0.01). However, GEN supplement was less effective besides in G5. In the present study, SOD inhibitory rate increased from (19.00 ± 1.66)% to (66.27 ± 1.39)% when exposed to 100.00 μg/mL carbaryl. And then, SOD inhibitory rate greatly decreased when exposed to DAI or the mixed solution (P < 0.05). GSH content in PC12 cells decreased sharply after exposure to carbaryl. The control group was 206.48 ± 7.28 μmol/gprot; however, carbaryl group was 44.08 ± 0.69 μmol/gprot. GSH increased obviously at DAI and mixed solution treatment in G3 and G4 (P < 0.01). Compared to G5, GSH content indicated the longer time exposure to DAI or the mixed solution, the better effects of treatments. However, GEN was also less effective (P > 0.05).
In G3, G4, and G5, all soybean isoflavone treatments showed a lower RFI than carbaryl-treated group (P < 0.05). These indicated the protective effects of soybean isoflavone against carbaryl-induced damage in PC12 cells were suppression of ROS generation.
Effect of SIF on ACh concentration of carbaryl-treated PC12 cells
ACh concentration in culture medium was obviously increased from 28.49 ± 1.41 to 561.76 ± 30.92 μg/mL with carbaryl treatment. All the soybean isoflavone treatments showed a decrease than carbaryl group (P < 0.01); however, DAI treatments in G3, G4, and G5 were the most remarkable.
Effect of SIF on mitochondrial function incarbaryl-treated PC12 cells
After treatment with carbaryl, the ratio of J-monomer/J-aggregates increased to 0.95 ± 0.27. Treatment with DAI, or the mixed solution, was found to preserve MMP evident from the red fluorescence seen in carbaryl-treated group, the ratio of J-monomer/J-aggregates was significantly decreased (P < 0.01). Results of JC-1 staining were given in .
Discussion
Environmental pollution affecting human has become the order of the day. Pesticides used in agriculture easily find their way into human through the food chain as well as directly from the environment. These chemicals induce toxicity by producing prooxidants in cells (Tellez-Bañuelos, Santerre, Casas-Solis, Bravo-Cuellar, & Zaitseva, Citation2009).
We first supplemented PC12 cells with SIF or carbaryl to test the effect of supplementation on cell viability. Our results showed the concentrations of SIF on PC12 cells were able to protect the cells from pesticide damage and had no significant effect on PC12 cells proliferation or cytotoxicity.
Exposed to 100.00 μg/mL carbaryl, PC12 cells viability rates decreased, and cell morphology changed. Carbaryl could also be generating an oxidative stress situation and inducing antioxidant responses in PC12 cells. Pesticide-induced oxidative stress is the ultimate manifestation of a multistep pathway, resulting in an imbalance between prooxidant and antioxidant defense mechanisms (Banerjee, Seth, & Ahmed, Citation2001), and affects normal cellular redox status (Habib, Kumar, Manikar, Zutshi, & Fatma, Citation2011). Carbaryl treatment resulted in a significant increase in the levels of MDA, SOD inhibition rate, and RFI, this suggested induction of oxidative stress to PC12 cells. The ROS can be detoxified by an endogenous antioxidant system, comprising a group of enzymes and low molecular weight antioxidants (Winston & Giulio, Citation2015). SOD neutralizes the highly reactive superoxide radical produced by the cell especially under stress condition. Jokanović (Citation2001) reported that a decrease of 20–30% in GSH levels may affect the antioxidant response and lead to oxidative damage and cellular death. GSH decrease induced by carbaryl has been associated with direct ROS scavenging activity.
We further used PC12 cells exposed to carbaryl in presence or absence of SIF GEN, DAI, or their mixed solution for indicated times. PC12 cells endogenous antioxidant system ability enhanced when exposed to carbaryl in presence of SIF. In addition, the recovery of cell viabilities by DAI or mixed solution in carbaryl-induced damaged PC12 cells was the consequence of oxidative stress inhibition rather than cell proliferation stimulation. In the present study, both DAI and GEN significantly affected carbaryl-induced ROS generation; however, GEN seems to have no effect on SOD inhibition or GSH content in carbaryl-induced damage in PC12 cells, differently from DAI. This could be ascribed to the molecular structure of DAI that may have affected bioavailability of this compound to cells or alternatively there might be a higher biological activity of DAI with respect to GEN (Foti et al., Citation2005). The protection was not dose dependent and this could be caused by problems related to the mechanism of uptake. DAI, different from GEN, lacks the hydroxyl group in C-5 position, so giving a lower steric hindrance. This could be the cause of a higher concentration of DAI next to the membrane surface, while this was not observed for the structurally closely related isoflavone, GEN (Lehtonen, Adlercreutz, & Kinnunen, Citation1996). Muthaiah, Venkitasamy, Michael, Chandrasekar, and Venkatachalam (Citation2013) studied oxidative stress by exposure to carbaryl and reported treatment with naringenin can reduce the oxidative stress by decreasing the ROS.
Our work proved that carbaryl inhibits the activity of the AChE and induces an increase in ACh concentration in PC12 cells medium. ACh leads to free-radical production dependent on tyrosine kinase. GEN behaves as a tyrosine kinase inhibitor at higher concentration while at lower concentration it exerts estrogenic activity (Guo et al., Citation2015). GEN is also a positive modulator of α7 nAChRs (Cho et al., Citation2005; Eric et al., Citation2005) that increases α7 nAChR current response. GEN effects are primarily mediated by a direct allosteric effect on the α7 nAChR (Izumi et al., Citation2010). GEN inhibited the ability of ACh to increase ROS production in cells (Oldenburg et al., Citation2002).
Mitochondria are a major source of ROS generated and play a key role in the development of apoptotic and necrotic cell death. JC-1 staining showed a clear red to green shift in fluorescence which is the hallmark of loss of MMP, loss of which would release proapoptotic factors. Results of the present study provided overwhelming support to our hypothesis as treatment with soybean isoflavone was found to counter the effects of carbaryl. It effectively maintained the MMP.
An observation of the present study clearly demonstrates that the administration of DAI enhanced specific activities of antioxidative enzymes and levels GSH content as well as reduced peroxidative damage, leading to the enhancement of the overall antioxidative status, which in turn result in the protective effect against carbaryl-induced damage in PC12 cells. Moreover, a supplement of GEN though could not increase endogenous antioxidant system activity, it may inhibit the ability of ACh to increase ROS production. It may be concluded that the enhanced antioxidative potential possibly attributes to the chemopreventive nature of DAI, while decreased ROS production possibly attributes to the tyrosine kinase inhibited nature of GEN.
Conclusion
Depending on the data obtained in the present study, exposure of carbaryl to PC12 cells at a concentration of 100.00 μg/mL for 24 h caused significant changes in oxidative stress markers and certain morphological changes. These changes were observed in the form of the improvement of values in the groups, which were administered SIF. Results from biochemical parameters demonstrated that exposure to SIF resulted in a better survival of PC12 cells which were subsequently subjected to carbaryl toxicity. DAI has strong radical scavenging activity due to its molecular structure. Supplement with GEN could inhibit the ability of ACh to increase ROS production. It is obviously a protective effect of SIF against carbaryl-induced damage in PC12 cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Banerjee, B.D., Seth, V., & Ahmed, R.S. (2001). Pesticide-induced oxidative stress: Perspective and trends. Reviews on Environmental Health, 16, 1–40. doi:10.1515/REVEH.2001.16.1.1
- Branch, R.A., & Jacqz, E. (1986). Subacute neurotoxicity following long-term exposure to carbaryl. The American Journal of Medicine, 80, 741–745. doi:10.1016/0002-9343(86)90837-5
- Cho, C.H., Song, W., Leitzell, K., Teo, E., Meleth, A.D., Quick, M.W., & Lester, R.A. (2005). Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. The Journal of Neuroscience, 25, 3712–3723. doi:10.1523/jneurosci.5389-03.2005
- Choi, R.C.Y., Zhu, J.T.T., Yung, A.W.Y., Lee, P.S.C., Xu, S.L., Guo, A.J.Y., … Tsim, K.W.K. (2013). Synergistic action of flavonoids, baicalein, and daidzein in estrogenic and neuroprotective effects: A development of potential health products and therapeutic drugs against Alzheimer’s disease. Evidence-Based Complementary and Alternative Medicine, 2013, 1–10. doi:10.1155/2013/635694
- Donzelli, A., Braida, D., Finardi, A., Capurro, V., Valsecchi, A.E., Colleoni, M., & Sala, M. (2010). Neuroprotective effects of genistein in Mongolian gerbils: Estrogen receptor-β involvement. Journal of Pharmacological Sciences, 114, 158–167. doi:10.1254/jphs.10164FP
- Eric, C., Andreas, W., Kyung-Hye, H., Roch, O., Jean-Charles, H., Geraldine, A., … Christian, F. (2005). Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and src-family kinases. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 25, 9836–9849. doi:10.1523/jneurosci.3497-05.2005
- Fahmy, M.A., Fukuto, T.R., Myers, R.O., & March, R.B. (1970). The selective toxicity of new n-phosphorothioyl-carbamate esters. Journal of Agricultural and Food Chemistry, 18, 793–796. doi:10.1021/jf60171a014
- Feng, X., Zhou, Q., Liu, C., Tao, M.L., Cao, J.G., & Zhong, Z.H. (2012). Protective effect of 7-difluoromethoxy-5,4ʹ-di-hydroxyl isoflavone against the damage induced by glutamate in PC12 cells. International Journal of Molecular Medicine, 30, 1159–1165. doi:10.3892/ijmm.2012.1109
- Ferrari, A., Venturino, A., & D’Angelo, A.M.P.D. (2007). Effects of carbaryl and azinphos methyl on juvenile rainbow trout (Oncorhynchus mykiss) detoxifying enzymes. Pesticide Biochemistry and Physiology, 88, 134–142. doi:10.1016/j.pestbp.2006.10.005
- Foti, P., Erba, D., Riso, P., Spadafranca, A., Criscuoli, F., & Testolin, G. (2005). Comparison between daidzein and genistein antioxidant activity in primary and cancer lymphocytes. Archives of Biochemistry and Biophysics, 433, 421–427. doi:10.1016/j.abb.2004.10.008
- Greene, L.A., & Tischler, A.S. (1976). Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Sciences of the United States of America, 73, 2424–2428. doi:10.1073/pnas.73.7.2424
- Guo, T.L., Germolec, D.R., Zheng, J.F., Kooistra, L., Auttachoat, W., Smith, M.J., … Elmore, S.A. (2015). Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicologic Pathology, 43, 435–448. doi:10.1177/0192623314526318
- Habib, K., Kumar, S., Manikar, N., Zutshi, S., & Fatma, T. (2011). Biochemical effect of carbaryl on oxidative stress, antioxidant enzymes and osmolytes of cyanobacterium calothrix brevissima. Bulletin of Environmental Contamination and Toxicology, 87, 615–620. doi:10.1007/s00128-011-0410-0
- Heath, C.W. (1997). Pesticides and cancer risk. Cancer, 80, 1887–1888. doi:10.1002/(sici)1097-0142(19971115)80:10<1887::aid-cncr2>3.0.co;2-l
- Izumi, M., Sweeney, A.M., Demartini, D., Weaver, J.C., Powers, M.L., Tao, A., … Morse, D.E. (2010). Changes in reflectin protein phosphorylation are associated with dynamic iridescence in squid. Journal of the Royal Society Interface, 7, 549–560. doi:10.1098/rsif.2009.0299
- Jokanović, M. (2001). Biotransformation of organophosphorus compounds. Toxicology, 166, 139–160. doi:10.1016/S0300-483X(01)00463-2
- Lehtonen, J.Y.A., Adlercreutz, H., & Kinnunen, P.K.J. (1996). Binding of daidzein to liposomes. Biochimica Et Biophysica Acta, 1285, 91–100. doi:10.1016/s0005-2736(96)00154-x
- Lin, C.C., Hui, M.N.Y., & Cheng, S.H. (2007). Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicology and Applied Pharmacology, 222, 159–168. doi:10.1016/j.taap.2007.04.013
- Liu, J.B., Liu, W.C., Xu, M.-L., Liu, J.Y., & Li, L.-Y. (2015). Neuroprotective effects of soybean protein isolate hydrolysates against neuronal oxidative damage in PC12 neuronal cells. Modern Food Science and Technology, 31, 8–12, 57. doi:10.13982/j.mfst.1673-9078.2015.4.002
- Mahajan, R., Blair, A., Coble, J., Lynch, C.F., Hoppin, J.A., Sandler, D.P., & Alavanja, M.C.R. (2007). Carbaryl exposure and incident cancer in the Agricultural Health Study. International Journal of Cancer, 121, 1799–1805. doi:10.1002/ijc.22836
- Matos, P., Fontaı´nhas-Fernandes, A., Peixoto, F., Carrola, J., & Rocha, E. (2007). Biochemical and histological hepatic changes of Nile tilapia Oreochromis niloticus exposed to carbaryl. Pesticide Biochemistry and Physiology, 89, 73–80. doi:10.1016/j.pestbp.2007.03.002
- Moser, V.C., Padilla, S., Simmons, J.E., Haber, L.T., & Hertzberg, R.C. (2012). Impact of chemical proportions on the acute neurotoxicity of a mixture of seven carbamates in preweanling and adult rats. Toxicological Sciences, 129, 126–134. doi:10.1093/toxsci/kfs190
- Muthaiah, V.P., Venkitasamy, L., Michael, F.M., Chandrasekar, K., & Venkatachalam, S. (2013). Neuroprotective role of naringenin on carbaryl induced neurotoxicity in mouse neuroblastoma cells. Journal of Pharmacology and Pharmacotherapeutics, 4, 192–197. doi:10.4103/0976-500x.114599
- Oldenburg, O., Qin, Q., Sharma, A.R., Cohen, M.V., Downey, J.M., & Benoit, J.N. (2002). Acetylcholine leads to free radical production dependent on KATP channels, Gi proteins, phosphatidylinositol 3-kinase and tyrosine kinase. Cardiovascular Research, 55, 544–552. doi:10.1016/s0008-6363(02)00332-2
- Rando, G., Ramachandran, B., Rebecchi, M., Ciana, P., & Maggi, A. (2009). Differential effect of pure isoflavones and soymilk on estrogen receptor activity in mice. Toxicology and Applied Pharmacology, 237, 288–297. doi:10.1016/j.taap.2009.03.022
- Rosenkranz, A.R., Schmaldienst, S., Stuhlmeier, K.M., Chen, W., Knapp, W., & Zlabinger, G.J. (1992). A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. Journal of Immunological Methods, 156, 39–45. doi:10.1016/0022-1759(92)90008-h
- Schreihofer, D.A., & Redmond, L. (2009). Soy phytoestrogens are neuroprotective against stroke-like injury in vitro. Neuroscience, 158, 602–609. doi:10.1016/j.neuroscience.2008.10.003
- Sivanesan, D., Palanisamy, M., Vanessa, H., James, K.D., & Appu, R. (2013). Induction of apoptosis in hela cells via caspase activation by resveratrol and genistein. Journal of Medicinal Food, 16, 139–146. doi:10.1089/jmf.2012.0141
- Smulders, C.J., Bueters, T.J., Van Kleef, R.G., & Vijverberg, H.P. (2003). Selective effects of carbamate pesticides on rat neuronal nicotinic acetylcholine receptors and rat brain acetylcholinesterase. Toxicology and Applied Pharmacology, 193, 139–146. doi:10.1016/j.taap.2003.07.011
- Tellez-Bañuelos, M.C., Santerre, A., Casas-Solis, J., Bravo-Cuellar, A., & Zaitseva, G. (2009). Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish & Shellfish Immunology, 27, 105–111. doi:10.1016/j.fsi.2008.11.002
- Todd, N.E., & Van Leeuwen, M. (2002). Effects of sevin (carbaryl insecticide) on early life stages of zebrafish (Danio rerio). Ecotoxicology and Environmental Safety, 53, 267–272. doi:10.1006/eesa.2002.2231
- Wang, H., Wang, J., Timchalk, C., & Lin, Y. (2008). Magnetic electrochemical immunoassays with quantum dot labels for detection of phosphorylated acetylcholinesterase in plasma. Analytical Chemistry, 80, 8477–8484. doi:10.1021/ac801211s
- Winston, G.W., & Giulio, R.T.D. (2015). Prooxidant and antioxidant mechanisms in aquatic organisms. Journal of Immunotoxicology, 19, 1–11. doi:10.1016/0166-445x(91)90033-6
- Xu, M.-L., Liu, J.-B., & Lu, J. (2014). Determination and control of pesticide residues in beverages: A review of extraction techniques, chromatography, and rapid detection methods. Applied Spectroscopy Reviews, 49, 97–120. doi:10.1080/05704928.2013.803978
- Xu, M.-L., Liu, J., Zhu, C., Gao, Y., Zhao, S., Liu, W., & Zhang, Y. (2015). Interactions between soy isoflavones and other bioactive compounds: A review of their potentially beneficial health effects. Phytochemistry Reviews, 14, 459–467. doi:10.1007/s11101-015-9398-0
- Yang, F., Wild, J.R., & Russell, A.J. (1995). Nonaqueous biocatalytic degradation of a nerve gas mimic. Biotechnology Progress, 11, 471–474. doi:10.1021/bp00034a018
