ABSTRACT
The present study was designed to analyze the formation mechanics of heat-induced myofibrillar gels from rabbit psoas major at 0.6 mol/L KCl (pH 6.5) and 0.2 mol/L KCl (pH 6.0; n = 3). Morphological structure changes were observed using phase-contrast microscopy, transmission electron microscopy, scanning electron microscopy (SEM) and gel properties were determined through water-holding capacity (WHC), gel strength and dynamic rheological test. The results revealed that myofibrillar filaments aggregated and formed larger oligomers by side-by-side interactions when heated from 25 to 65°C. In higher ionic strength at higher pH, the filaments formed a more compact homogeneous network structure and the G′ value was much lower with higher WHC and gel strength, suggesting that the myofibril gel properties could be described by ∆G′. Based on the present study, it is suggested that the desired structural and textural attributes of myofibril gel can be manipulated by temperature, ionic strength and pH.
RESUMEN
El presente estudio se diseñó para analizar la formación de los mecanismos de geles miofibrilares inducidos con calor de psoas mayor de conejo (PM) a 0,6 mol/L de KCl (pH 6,5) y 0,2 mol/L de KCl (pH 6,0) (n=3). Se observaron cambios en la estructura morfológica utilizando microscopio de contraste de fase, microscopio electrónico de transmisión (TEM) y microscopía electrónica de barrido (SEM), además se determinaron las propiedades del gel examinando la capacidad de retención de agua, la resistencia del gel y el ensayo reológico dinámico. Los resultados revelaron que los filamentos miofibrilares agregaron y formaron oligómeros más grandes mediante interacciones conjuntas cuando se calentaron de 25°C a 65°C. Con la mayor fuerza iónica y el mayor pH, los filamentos formaron una estructura de red homogénea más compacta y el valor G’ fue más bajo con los mayores valores de WHC y resistencia del gel, sugiriendo que las propiedades miofibrilares del gel podrían describirse con ∆G’. En base al presente estudio, se sugiere que los atributos estructurales y texturales deseados del gel miofibrilar pueden ser manipulados con la temperatura, la fuerza iónica y el pH.
1. Introduction
Gel-forming ability is one of the most important functional properties of meat proteins in which myofibrillar proteins play the most important role. The heat-induced gelation of myofibrillar proteins is one of the key factors that determine the characteristics of meat products such as texture, sensory quality, emulsifying stability, water-holding capacity (WHC), and meat tenderness (Xiong, Citation2000).
The mechanics of heat-induced gelation of myofibrillar proteins has been studied for more than half a century. Ferry and Myers (Citation1961) were the earliest to study it and made a hypothesis about it. Hermansson, Harbitz, and Langton (Citation1986) investigated the gelation of myosin treated at different pH and ionic strengths and found different gel structures. Sharp and Offer (Citation1992) investigated the gathering manner of rabbit skeletal myosin molecules after heating at 30–60°C for 30 min using transmission electron microscopy (TEM), and they believed that the head–head interactions, head–tail interactions and tail–tail interactions were the basic mechanics of gelation. Sano, Noguchi, Matsumoto and Tsuchiya (Citation1990) thought that light meromyosin played the main role in myosin gelation. Boyer, Joandel, Ouali, and Culioli (Citation1996) observed different gel network structures of myofibrils and myosin from rabbit psoas major (PM) and Semimembranosus proprius muscle at different ionic strengths. The heat-induced gelation was associated with the formation of sufficient intermolecular protein bonds such as hydrogen bonds, ionic linkages, hydrophobic interactions and covalent bonds (Lanier, Yongsawatdigul, & Carvajal-Rondanelli, Citation2013). The formation of heat-induced gels is a complex thermodynamic process that is related to the muscle sources and types and different cooking conditions such as temperature, pH, protein concentrations and ionic strength (Lanier et al., Citation2013) and most of the researches focused on the study of myosin heat-induced gelation.
The typical myofibrillar proteins which could participate in the gelation were myosin, actin, actomyosin, the fragments of myofibrils and myofibrils (Lanier et al., Citation2013; Lesiów & Xiong, Citation2001; Xiong, Citation2000). The mechanics of gelation of myosin molecules was clear in previous studies (Sharp & Offer, Citation1992), while the mechanics of gelation of myofibrils was not reported. This research focuses on the morphological structure changes of myofibrils in the process from sol to gel with different temperature (from 25 to 65°C) and ionic strengths (0.6 mol/L KCl at pH 6.5 vs. 0.2 mol/L KCl at pH 6.0). It also aims to provide more insights into the gelation mechanics of myofibrils from rabbit PM, allowing the manipulation of processing conditions to obtain products with the desired structural and textural attributes.
2. Materials and methods
2.1. Materials
Three male New Zealand White rabbits (3 months old, 2–2.5 kg) were purchased from the Jiangsu Academy of Agricultural Science. The rabbits should fully rest overnight to reduce stress before slaughter. After percussive stunning, their neck blood vessels were cut, and they were fully bled. Then, they were immediately skinned, headed, gutted and washed with tap water and distilled water. The separated rabbit PM without visible fat and connective tissue was cooled at 4°C for 0.5 h before use.
2.2. Preparation of myofibrils from rabbit PM
The PM was minced and mixed together to prepare myofibril suspension. Myofibrils were extracted from rabbit PM mix according to the procedures of Parsons and Knight (Citation1990) and Xiong, Lou, Wang, Moody, and Harmon (Citation2000) with some modifications. All solutions used for the myofibril preparation were kept at 4°C to minimize protein denaturation. Rigor buffer, which contained 0.1 mol/L KCl, 2 mmol/L MgCl2, 1 mmol/L EGTA, 0.5 mmol/L dithiothreitol, and 10 mmol/L K2HPO4 at pH 7.0, was previously prepared. Minced muscle (20 g) was suspended in 200 mL of rigor buffer by homogenization in a Waring Blender (BLENDER LB20E*, WARING BLENDER, USA) at 6000 rpm for 30 s. The mixture was centrifuged at 2000 × g for 10 min at 4°C in Avanti J-E high-performance centrifuge (Avanti J-E, Beckman Coulter, USA). The sediment was suspended again using the same rigor buffer conditions. It was then homogenized in a homogenizer (T25 digital, ULTRA TURRAX IKA, Germany) operated at a moderate speed setting of 12,000 rpm for 1 min. The last two steps were repeated more than three times. It was then centrifuged again and the sediment was carefully separated for use.
The extracted myofibrils were separately incubated in the rigor buffer mentioned above, 25 mmol/L potassium phosphate buffer (pH 6.5) with 0.6 mol/L KCl and 25 mmol/L potassium phosphate buffer (pH 6.0) with 0.2 mol/L KCl.
The protein concentration of extracted myofibrils was determined by the Biuret method (Gornall, Bardawill, & David, Citation1949) according to Xiong et al. (Citation2000). The samples were prepared in triplicate per treatment. BSA was used as the standard protein. The myofibril suspension was kept at 0–4°C and used within 3 days.
2.3. Observation using phase-contrast microscopy
2.3.1. Preparation of myofibrils
All of the myofibril suspensions prepared above (other than the one in the rigor buffer) were diluted to 0.3 mg/mL and incubated from 20 to 70°C at the rate of 1°C/min in a water bath. The myofibril suspensions were taken out when the temperature reached 25, 35, 45, 55 or 65°C and were cooled on ice with another suspension at 0°C as a control. The myofibrils in each of these conditions would be used in the following steps when irrigating myofibrils.
2.3.2. Irrigation of myofibrils
The myofibrils were irrigated according to Parsons and Knight’s (Citation1990) and Xiong et al.’s (Citation2000) methods without the step of making a pool of about 12 mm wide, 18 mm long and 500 μm deep with the tape.
Then, 8 μL of the diluted myofibril suspension to be examined was applied to one end of the channel on the slide and taken up into the full length of the channel by capillary force. Another 8 μL of rigor buffer was applied to both ends of the channel to prevent evaporation. Then, the slide was inverted for 2 min to allow the suspension to settle and attach to the underside of the coverslip. The ultrastructure of the myofibrils was observed using a phase-contrast microscopy (BX51, OLYMPUS, Japan) equipped with a 100× oil immersion planachromatic objective.
2.4. TEM
All of the myofibril suspensions prepared above (other than the one in the rigor buffer) were diluted to 10 μg/mL and incubated from 20 to 70°C at a rate of 1°C/min in a water bath. Then, the myofibril suspensions were taken out when the temperature reached 25, 35, 45, 55 or 65°C and were cooled on ice with another sample at 0°C as a control.
The myofibril suspensions were dropped onto wax plates and immediately covered with copper wire meshes plated with carbon films. The drops were in contact with the carbon films. After applying a filter paper wick for 30 s to the edge of the copper wire mesh to absorb extra liquid, the samples were negatively stained with 3% phosphotungstic acid aqueous solution for 1 min when slightly dry, and then, the extra solution was absorbed with a piece of filter paper. The copper wire meshes (the specimens) were illuminated under a filament lamp for 10 min to dry before the observations were carried out using a TEM (H-7650, Hitachi High-technologies Corporation, Japan) at an accelerating voltage of 80 kV (Sharp & Offer, Citation1992).
2.5. Preparation of heat-induced rabbit PM myofibrillar gel
The protein concentrations of the myofibril suspensions (other than the one in the rigor buffer) were adjusted to 40 mg/mL. Then, 2 mL of every suspension was loaded into five plastic centrifugal tubes of 5 mL. These samples were incubated from 20 to 70°C at a rate of 1°C/min in a water bath. When the temperature reached 25, 35, 45, 55 or 65°C, the corresponding tubes were transferred and incubated overnight (12 h) at 4°C for WHC determination and scanning electron microscopy (SEM) observations.
2.6. SEM
The gels were cut into 5-mm thick pieces and fixed with 2.5% glutaraldehyde. The fixed specimens were dehydrated in graded ethanol solutions (50%, 70%, 90%, 95% and 100%) before replacing the solution with tertiary butyl alcohol. Then, they were freeze-dried and coated with 10-nm thick uniform layers of gold to observe the structure in a Hitachi S-3000 N SEM (S-3000 N, Hitachi High-technologies Corporation, Tokyo, Japan) at an accelerating voltage of 15 kV (Gordon & Barbut, Citation1989).
2.7. Rheological measurements
The protein concentration was adjusted to 10 mg/mL. A Physica MCR301 Rotational Rheometer (Physica MCR301 Rotational Rheometer, Anton Paar, Austria) was employed to measure the rheological properties of the rabbit PM myofibrils. Measurements were conducted within the linear viscoelastic range at a strain of 0.02 and a frequency of 0.1 Hz. Approximately 1.5 mL myofibril suspensions were loaded onto the lower plate and the myofibril suspensions were heated at a rate of 1°C/min from 20 to 85°C between parallel plates (pp50) (gap = 0.5 mm), maintained at 85°C for 3 min and then cooled at a rate of 5°C/min from 85 to 20°C. The storage modulus (G′), loss modulus (G″) and phase angle (δ) were recorded according to the changes in temperature every 60 s. ∆G′ was estimated by the following equation:
The G′ values at 80 and 20°C were expressed as plateau G′ and initial G′, respectively.
2.8. WHC
The WHC of the gels was determined according to Kocher and Foegeding (Citation1993). The heat-induced rabbit PM myofibrillar gels were centrifuged at 10,000 × g for 10 min at 4°C and approximately 5 g gel samples were used. The exact weights (g) of the centrifuge tubes and the one with the gels before or after centrifugation were all recorded. Then, the WHC (%) of the gel was determined according to the following formula:
where W is the weight (g) of the centrifuge tubes, W1 is the weight (g) of the centrifuge tubes with the heat-induced gel before centrifugation and W2 is the exact weight (g) of centrifuge tubes with the heat-induced gel after centrifugation and after the supernatant is decanted. All treatments were performed in triplicate.
2.9. Gel strength
A Texture Analyzer (TA-XT2i, Stable Micro Systems Ltd., UK) was employed. Gels in the glass vial were extruded with a stainless steel probe and approximately 5 g gel samples were used. Testing parameters were set as follows: a pretest speed of 2.0 mm/s, a test speed of 1.0 mm/s, a posttest speed of 1.0 mm/s, a percent compression of 50%, a trigger force of 1.0 g and a testing time of 5 s. The penetration force, defined as the force required to rupture the gels (the first major peak), was expressed as gel strength (gf). The samples were prepared in triplicate.
2.10. Statistical analysis
All samples were prepared in triplicate per condition. The data were analyzed with the Statistical Analysis System (SAS Institute Inc., Cary, NC, USA). Means of each treatment were statistically analyzed by SAS. And differences were compared using Duncan’s multiple-range test at significance level of 0.05.
3. Results and discussion
3.1. Observation of myofibrils from rabbit PM using phase-contrast microscopy
The myofibrils prepared at different temperatures and ionic strengths were observed using phase-contrast microscopy, and the microstructural changes are shown above.
In 0.6 mol/L KCl, when the temperature was low (), the filaments were almost intact with vague Z-lines and swollen A-bands which showed noticeable protein extraction (Xiong et al., Citation2000) as protein extraction followed initial fiber swelling (Xiong, Citation2000). As the temperature increased (–)), fracturing and decomposition and cross-linking began with the Z-lines fading and the diffuse A-bands narrowing, which suggestted that protein extraction occurred in the myofibrillar filaments. This was consistent with the studies by Xiong et al. (Citation2000). The obscure mass in the background might show some type of cross-links of the swollen myofibrillar filament fragments. In ), there were two to three or even more filaments existing side by side with evident cross-linking, which pattern was also reported in the study of Parsons and Knight (Citation1990). Furthermore, the swollen filaments fractured and decomposed from both ends as sarcomere units, and then, the fragments were separated from the main myofibrillar filaments into the background.
Figure 1. Phase-contrast micrographs of myofibrillar protein molecules in 0.6 mol/L (a–f) and 0.2 mol/L KCl (g–l) during heating (bar = 30 μm). (a, g) 0–4°C, (b, h) 25°C, (c, i) 35°C, (d, j) 45°C, (e, k) 55°C, and (f, l) 65°C.
Figura 1. Micrografías de contraste de fase de las moléculas de proteína miofibrilar en 0,6 mol/L (a–f) y 0,2 mol/L de KCl (g–l) durante el calentamiento (barra = 30 μm). (a, g) 0–4°C, (b, h) 25°C, (c, i) 35°C, (d, j) 45°C, (e, k) 55°C, y (f, l) 65°C.
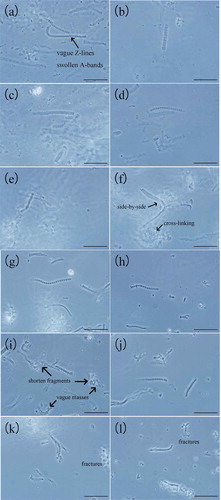
In 0.2 mol/L KCl (–)), the myofibrils seemed thicker and much more regular and distinct than the ones in 0.6 mol/L KCl. The filaments became shorter as the temperature rose up with more fractures. At 35°C ()), the A-bands were swollen and the filaments shortened with some of the fragments gathering together into vague bulks and masses due to cross-bridging among them (Offer & Trinick, Citation1983). Additionally, it was reasonable to infer that the extraction of the A-bands occurred at their ends and that fracture and decomposition occurred at both ends of the filaments or somewhere near the ends of the filaments, which was consistent with the results of Offer and Trinick (Citation1983). At higher temperatures (,)), there were many myofibrillar fragments around in the background either gathering together or isolated and the cross-linking and the fractures were not as obvious as the ones in 0.6 mol/KCl, which indicated that the extraction of protein in myofibrils was not as thorough as the ones in 0.6 mol/KCl.
3.2. Microstructures of the myofibrils from rabbit PM using TEM
At 0–4°C ()), it was observed myofibrillar proteins (usually contain myosin and actomyosin) dispersed into slender filament of various sizes and formed a relatively homogeneous dissociated threadlike network structure with globular protein aggregates adhering to the regularly staggered filaments, which agreed with Boyer et al.’s study (Boyer et al., Citation1996). The diameters of the globular protein aggregates which contained heads of myosin monomer and actin ranged from approximately 14 to 28 nm. The thickness of most filaments crossing the entire view was approximately 8–10 nm. Changes in the morphological structure occurred as the temperature increased. The slender filaments broke into shorter and smoother ones with more globular proteins gathering into globular protein groups. The tendency of aggregation of the filaments via side-by-side interactions was more obvious as the temperature increased. Most filaments assembled regularly into clusters in the same direction and bound with each other in side-by-side strands ()). It could be inferred from the relatively loose filaments on the edges of the strands and the scattered filaments at both ends that, as the temperature increased, more filaments gathered and assembled into strands via side-by-side interactions and the whole structure became smoother and more compact than before. Cross-links, oligomers and copolymers (Huxley, Citation1963) formed almost completely, and few single filaments could be observed ()). The final extensive gel network structure formed with complicated protein–protein cross-links in it ()).
Figure 2. Microstructures of myofibrillar protein molecules in 0.6 mol/L (a–f) and 0.2 mol/L KCl (g–l) during heating (bar = 200 nm). (a, g) 0–4°C, (b, h) 25°C, (c, i) 35°C, (d, j) 45°C, (e, k) 55°C, and (f, l) 65°C.
Figura 2. Microestructuras de las moléculas de proteína miofibrilar en 0,6 mol/L (a–f) y 0,2 mol/L de KCl (g–l) durante el calentamiento (barra = 200 nm). (a, g) 0–4°C, (b, h) 25°C, (c, i) 35°C, (d, j) 45°C, (e, k) 55°C y (f, l) 65°C.
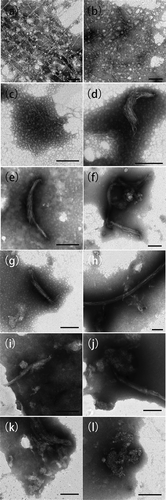
At 0.2 mol/L KCl ()), the fine and smooth myofibrillar filaments had already arranged side by side into a neat juxtaposing cylindrical pattern at 0–4°C with more copolymers or globular aggregates forming around the filaments. The filament bundles became rough and thick, and the gel formed gradually before it reached the myosin denaturation temperature (below 40°C). As shorter fibrils moved more easily than longer ones during heating, the short filaments gathered into strands and distributed in a regular pattern at the beginning of the heating. When heated to 55°C ()), bridging and cross-linking among filament strands became evident and the entire network structure began to form until the final network structure formed ().
3.3. Microstructures of myofibrillar gel from rabbit PM using SEM
The SEM was performed to investigate the ultrastructure features of gel formed by rabbit PM myofibrils at different temperatures and ionic strengths.
When the myofibrillar proteins were heated from 20 to 65°C in 0.6 mol/L KCl, the filaments bended and fractured into shorter strands and tended to show coarse aggregation. Some of the scattered slender filaments existed in side-by-side arrangements with something villous or globular bulging over the surfaces of the myofibrillar proteins ()). As temperature increased, most filaments arranged in side-by-side assemblies and showed a tendency of forming regular holes in the more tightly structure (, )). As showed in ), the filaments evidently fractured into shorter and thicker rods and regular holes almost formed with significantly mass distinct cross-links, forming a relatively compact structure, which was similar to the study of Iwasaki, Washio, Yamamoto and Nakamura (Citation2005b) where the strands treated above 55°C were thicker than those treated at 40°C, as observed by AFM, and the strands above 55°C were formed via the side-by-side interactions of several filaments. When heated to 65°C, the short rods of the knobby-surface myofibrillar fragments (Iwasaki, Washio, & Yamamoto, Citation2005a) assembled themselves in order into a dense gel matrix with smaller holes in it, most likely because of the compact and extensive cross-links ()). Some studies showed that myosins or myofibrils in 0.6 mol/L NaCl or KCl could form good three dimensional network gels (Ishioroshi, Samejima, Arie, & Yasui, Citation1980; Morita, Choe, Yamamoto, Samejima, & Yasui, Citation1987; Sun & Arntfield, Citation2011; Xiong, Citation1992). Shimada, Takai, Ejima, Arakawa and Shiraki (Citation2015) hypothesized that the dissociation of soluble filaments and thermal unfolding at high temperature, which resulted in the formation of soluble oligomers and binding interactions, provided new potential mechanics insight into the heat-induced myosin gelation in high-salt solution, while Hermansson et al. (Citation1986) and Tornberg (Citation2005) found that coarsely aggregated myosin gel structures were formed in 0.6 mol/L.
Figure 3. SEM images showing the effect of different denaturation temperatures on myofibrillar proteins in 0.6 mol/L (a–e) and 0.2 mol/L KCl (f–j) (bar = 20 μm). (a, f) 25°C, (b, g) 35°C, (c, h) 45°C, (d, i) 55°C and (e, j) 65°C.
Figura 3. Imágenes SEM que muestran el efecto de las diferentes temperaturas de desnaturalización en las proteínas miofibrilares en 0,6 mol/L (a–e) y 0,2 mol/L de KCl (f–j) (barra = 20 μm). (a, f) 25°C, (b, g) 35°C, (c, h) 45°C, (d, i) 55°C y (e, j) 65°C.
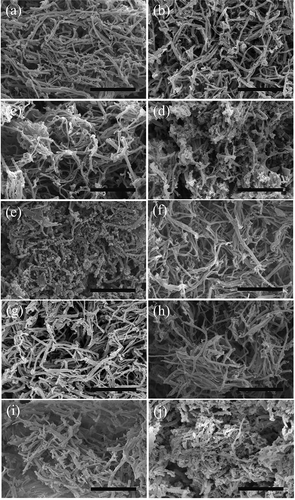
In 0.2 mol/L KCl, the aggregation existed even before heating and the myofibrillar matrix (–)) showed a tighter fine-stranded structure in a more irregular and disordered manner than the ones at 0.6 mol/L KCl, which agreed with Boyer et al.’s study (Boyer et al., Citation1996). At higher temperature, the matrix emerged in a weak and loose way with increasingly obvious cross-links and aggregates forming irregular holes, and few filaments fractured into sections ()). When heated to 65°C, flared cross-bridge projections from the surfaces of the thicker filaments were obvious, while the matrix became quite irregularly tight. Only some of the rough filaments fractured into sections or rods ()). Trespalacios and Pla (Citation2007) showed that the gel network formed in 1% NaCl (equivalent to 0.3 mol/L KCl) was loose with large, irregular holes. Samejima, Oka, Yamamoto, Asghar, and Yasui (Citation1986) found that, in low ionic strength systems, cardiac myosin could form a strong three-dimensional gel structure with fine filaments and uniform porosity when heated to 65°C, which were different from those of the gel from skeletal myosin. Yamamoto, Samejima, and Yasui (Citation1988) observed that, at low salt concentrations, the myosin filaments were polymerized, similar to the natural thick filaments of muscle before heating, and it would form an even stronger gel upon heating.
3.4. Gel properties of myofibril solutions from rabbit PM
3.4.1. Rheological properties of myofibril solutions during heating
In 0.6 mol/L KCl ()), the sharp decrease in δ from 50°C and the simultaneous increase in G′ from 55°C indicated the transition from sol to gel, leading to network formation. That was consistent with , ), where the myofibril filaments clearly fractured into short rods and regular holes formed with markedly mass distinct cross-links among the myofibrils, tending to form a relatively compact network structure. The rheological thermogram ()) indicated that the transition temperature during gelation was approximately 58°C (Wu, Xiong, Chen, Tang, & Zhou, Citation2009). As the temperature increased to 65°C, a denser elastic gel matrix had formed through compact and extensive cross-links and diversified protein–protein interactions ()) with the δ lower than 5° (Liu et al., Citation2010).
Figure 4. Dynamic rheological test curves of myofibrillar proteins. (a) In 0.6 mol/L KCl and (b) in 0.2 mol/L KCl.
Figura 4. Curvas de los ensayos reológicos dinámicos de las proteínas miofibrilares. (a) en 0,6 mol/L de KCl, (b) en 0,2 mol/L de KCl.
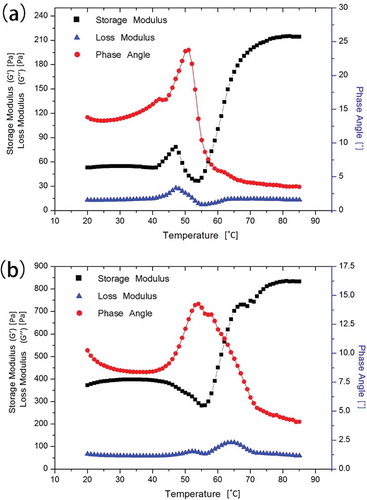
In 0.2 mol/L KCl, G′ decreased to its minimum at 55°C and continued increasing with a slight decrease before 80°C, which was most likely caused by the formation of covalent bonds (Egelandsdal, Martinsen, & Autio, Citation1995; Niwa, Chen, Wang, Kanoh, & Nakayama, Citation1988). δ Exhibited almost the exact opposite pattern of G′, except for the initial obvious decrease which indicated preliminary protein–protein interactions and network development (Liu et al., Citation2010). Further heating above 55°C resulted in a sudden decrease in δ indicating that the transition from sol to gel occurred, which occurred at a higher temperature than the one in 0.6 mol/L KCl; this result is consistent with the SEM results in .
The G′ value during cooling process () showed the same increase trend as the heating process, which showed that the G′ value in 0.2 mol/L (pH 6.0) increased more greatly than that in 0.6 mol/L (pH 6.5). And during cooling, the gradual increase suggested that slow continued hydrogen bonds occurred, which was important in stabilization of bound water and increasing gel strength by their great numbers (Lanier et al., Citation2013; Sun & Arntfield, Citation2011).
Figure 5. The storage modulus (G′) of PM myofibrillar proteins during cooling from 85 to 20°C at a rate of 5°C/min.
Figura 5. módulo de almacenamiento (G’) de proteínas miofibrilares PM durante enfriamiento de 85°C a 20°C a una velocidad de 5°C/min.
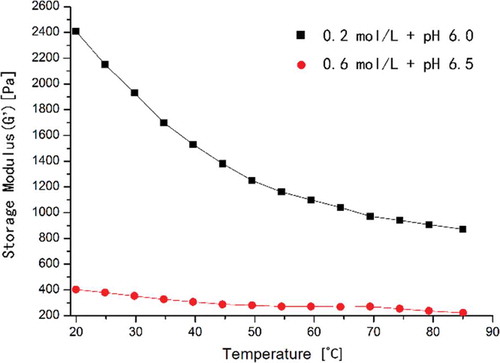
From , it was observed that the G′ value in 0.6 mol/L KCl (pH 6.5) was much lower with better gel properties, such as the WHC and gel strength (), than the ones in 0.2 mol/L KCl (pH 6.0) during the entire heating process. The differences of WHC and gel strength between two conditions were significant. It indicated that the properties of the myofibril protein gel could be described by ∆G′ () rather than the G′ value. The lower maximum δ and the higher initial G′ and the lower final gel strength in 0.2 mol/L KCl than the ones in 0.6 mol/L KCl (, ) showed that the incomplete dissolution and denaturation of myofibrillar proteins in 0.2 mol/L KCl resulted in insufficient ordered aggregation to form a comprehensive and good gel network. At high ionic strengths, sufficient amounts of proteins were highly solubilized and diffused throughout the protein matrix, forming a better gel network with complete structural uniformity when compared with the MP gel formed in 0.3 mol/L NaCl (Chin, Go, & Xiong, Citation2009).
Table 1. WHC and gel strength of the heat-induced myofibrillar protein gel at 65°C and ∆G′ at 80°C.
Tabla 1. WHC y resistencia de gel de la proteína miofibrilar provocado por el calor del gel a 65°C y ∆G’ a 80°C.
Previous studies showed that higher ionic strength produced more elastic gels, and 0.6 mol/L KCl was the most commonly used ionic strength (Hermansson et al., Citation1986; Stone & Stanley, Citation1994). The type of muscle, ionic strength, protein concentration, pH, addition of adenosine triphosphate or pyrophosphate and filament length all effected the gelation process (Egelandsdal, Fretheim, & Samejima, Citation1986; Westphalen, Briggs, & Lonergan, Citation2006, Citation2005). G′ showed a marked concentration dependence, while δ primarily varied with the concentration at low temperatures, and the transition temperature was not responsive to pH (Egelandsdal et al., Citation1986; Westphalen et al., Citation2006).
3.4.2. WHC of the heat-induced myofibrillar gel at 65°C
shows that, in 0.6 mol/L KCl (pH 6.5), the WHC was higher than that in 0.2 mol/L KCl (pH 6.0) at 65°C. More free water was retained in compact networks formed by sufficient soluble proteins in 0.6 mol/L KCl than in 0.2 mol/L KCl because the solubility of the myofibrillar proteins increased with increasing ionic strength. Electrostatic repulsions were enhanced, and the myofibrillar structures were loosened at higher ionic strengths, allowing more water to be trapped (Trespalacios & Pla, Citation2007). These behaviors could explain why the WHC in 0.6 mol/L KCl was higher than the one in 0.2 mol/L KCl in . As the temperature increased, the proteins denatured, and the protein solubility decreased, which was in accordance with the results of Shimada et al. (Citation2015).
The water holding and binding capacities of processed meat are based on the capability of myosin to form well-structured thermal gels that can effectively bound and immobilize water through capillary forces (Ishioroshi, Samejima, & Yasui, Citation1983; Wu et al., Citation2009). The pH, ionic strength, temperature, heating rate, protein concentrations and other factors influenced the WHC (Camou, Sebranek, & Olson, Citation1989; Chou & Morr, Citation1979; Huff-Lonergan & Lonergan, Citation2005; Kristinsson & Hultin, Citation2003; Westphalen et al., Citation2005; Yongsawatdigul & Park, Citation1999). Westphalen et al. (Citation2005) found a direct linear relationship between WHC and pH, which was consistent with Offer and Trinick’s findings (Offer & Trinick, Citation1983), and attributed the relationship to the possibility of increased protein–water hydrogen bonding at high pH values. Liu et al. (Citation2010) also found that the WHC of myosin gel decreased as the pH decreased close to the pI and proteins tended to coagulate due to increased protein–protein interactions. Xiong and Brekke (Citation1989) showed that increasing the negative charges on proteins above the pI resulted in strengthened protein–water interactions and weakened protein–protein interactions, which contributed to improving the WHCs of myofibril gels. Salt-free gels exhibited greater water absorbing abilities than gels in 150 mmol/L NaCl, and shifting the salt concentrations of the gel-bathing solutions also affected the water uptake of the gels considerably (Kristinsson & Hultin, Citation2003). Negative charges on proteins contributed to the water uptake and retention in terms of pH and ionic strength (Kristinsson & Hultin, Citation2003). Damodaran (Citation2007) studied water in proteins and found that –CO– and –NH became positive and negative polarization centers, respectively, forming multilayer water systems in polypeptide chains of denatured proteins. As the temperature decreased, free water would be entrapped by the structure partly due to hydrogen bonds between proteins (Chou & Morr, Citation1979; Damodaran, Citation2007).
3.4.3. Gel strength of heat-induced myofibrillar gel at 65°C
In 0.6 mol/L KCl (pH 6.5), the gel strength was much higher than that in 0.2 mol/L KCl (pH 6.0) at 65°C ().
Gel strength, which was evaluated as rigid, was influenced by many factors according to previous studies (Camou et al., Citation1989; Ishioroshi et al., Citation1983; Lesiów & Xiong, Citation2001; Liu et al., Citation2010), such as the heating rate, pH, ionic strength, the lengths of the filaments before heating and the addition of other components like ATP, most of which mainly functioned by affecting protein–protein attractive interactions (hydrogen bonds, ionic bonds, sulfhydryl bonds and hydrophobic interactions), repulsive interactions (mainly electrostatic but also sometimes steric) and protein–water interactions (Liu, Zhao, Xiong, Xie, & Qin, Citation2008). The gel strength decreased as the heating rate increased (Camou et al., Citation1989). The effect of the pH on the rigidity tended to be influenced by the ionic strength (Ishioroshi et al., Citation1983; Lefevre, Fauconneau, Ouali, & Culioli, Citation2002). As pH was raised, gel strength gradually increased until reaching a maximum. The high pH resulted in a higher gel strength and more open structures (Barbut, Citation1997). The myofibril solubility increased with pH because proteins were highly charged and protein–water interaction increased at pH far away from the pI (Lefevre et al., Citation2002). A drastic decrease in rigidity was caused by the addition of ATP (Ishioroshi et al., Citation1983). Liu and Xiong (Citation1997) proved that the gel strength of chicken myofibrillar proteins increased with increased salt concentration via the redistribution of charges on the surfaces of proteins, which affected the protein–protein electrostatic interactions and the protein stabilities, thus promoting the unfolding of the proteins to form gels. Samejima, Lee, Ishioroshi, and Asghar (Citation1992) found that, in gels from rabbit myofibrils of skeletal muscle proteins, the gel strength increased as the temperature increased in the range of 30–50°C and remained stable upon subsequent heating. The gel strength depended primarily on the type and quantity of myofibrillar protein cross-links formed during heating, which was associated with environmental ionic strength due to its effect on the solubility of the myofibrillar proteins (Foegeding, Allen, & Dayton, Citation1986). That is, the main reason for various gel strengths is the solubility of the proteins, which explained the higher gel strength in 0.6 mol/L KCl in . In some studies, gel strength was described as a function of salt concentration (Hermansson et al., Citation1986), and some even expressed gel strength using the storage modulus (Liu et al., Citation2010).
When studying the gelation of bovine myosin, Hermansson et al. (Citation1986) found that the rigidities of fine-stranded gel structures formed at low ionic strength (0.25 mol/L KCl) were higher than those of coarsely aggregated gel structures formed at high ionic strength (0.6 mol/L KCl). As the pH increased, the gel strength and gelation rate decreased (Liu et al., Citation2010). Ishioroshi et al. (Citation1983) found that the rigidity of the myosin gel in 0.2 mol/L KCl at pH 6.0 was greater than that in 0.6 mol/L KCl at pH 6.5 after incubation for 20 min at 65°C, which is somewhat contrary to our research, and that could be the result of the interaction between myosin molecules and other myofibrillar proteins (Lefevre et al., Citation2002).
4. Conclusions
From the obtained results, it can be concluded that myofibrillar filaments in the combinations of 0.6 mol/L KCl (pH 6.5) and 0.2 mol/L KCl (pH 6.0) aggregated and formed longer and wider oligomers by side-by-side interactions as the temperature was increased. In the combination of 0.6 mol/L KCl and pH 6.5, the filaments formed a more compact and homogeneous network structure than the one in the combination of 0.2 mol/L KCl and pH 6.0 by SEM results. The gel strength and WHC of the myofibrillar filament gel in 0.6 mol/L KCl was much better than that in the 0.2 mol/L KCl. However, the storage modulus (G′) was much lower in 0.6 mol/L KCl compared to the 0.2 mol/L KCl during the entire heating process, suggesting that the properties of the myofibrillar protein gel could be described as a function of ∆G′ (defined as the percentage of the increased G′).
Acknowledgments
This research work was supported by Herbivore Livestock fattening and Technique of High Quality Meat Produce Research of South China (201303144).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Barbut, S. (1997). Microstructure of white and dark turkey meat batters as affected by pH. British Poultry Science, 38(2), 175–182.
- Boyer, C., Joandel, S., Ouali, A., & Culioli, J. (1996). Ionic strength effects on heat-induced gelation of myofibrils and myosin from fast- and slow-twitch rabbit muscles. Journal of Food Science, 61, 1143–1148. doi:10.1111/jfds.1996.61.issue-6
- Camou, J., Sebranek, J., & Olson, D. (1989). Effect of heating rate and protein concentration on gel strength and water loss of muscle protein gels. Journal of Food Science, 54, 850–854. doi:10.1111/jfds.1989.54.issue-4
- Chin, K.B., Go, M.Y., & Xiong, Y.L. (2009). Konjac flour improved textural and water retention properties of transglutaminase-mediated, heat-induced porcine myofibrillar protein gel: Effect of salt level and transglutaminase incubation. Meat Science, 81, 565–572. doi:10.1016/j.meatsci.2008.10.012
- Chou, D.H., & Morr, C.V. (1979). Protein-water interactions and functional properties. Journal of the American Oil Chemists’ Society, 56, A53–A62. doi:10.1007/BF02671785
- Damodaran, S. (2007). Amino acids, peptides, and proteins. In O.R. Fennema (Ed.), Food chemistry (pp. 217–329). London: CRC Press.
- Egelandsdal, B., Fretheim, K., & Samejima, K. (1986). Dynamic rheological measurements on heat‐induced myosin gels: Effect of ionic strength, protein concentration and addition of adenosine triphosphate or pyrophosphate. Journal of the Science of Food and Agriculture, 37, 915–926. doi:10.1002/(ISSN)1097-0010
- Egelandsdal, B., Martinsen, B., & Autio, K. (1995). Rheological parameters as predictors of protein functionality: A model study using myofibrils of different fibre-type composition. Meat Science, 39, 97–111. doi:10.1016/0309-1740(95)80011-5
- Ferry, J.D., & Myers, H.S. (1961). Viscoelastic properties of polymers. Journal of the Electrochemical Society, 108, 142C–143C. doi:10.1149/1.2428174
- Foegeding, E., Allen, C., & Dayton, W. (1986). Effect of heating rate on thermally formed myosin, fibrinogen and albumin gels. Journal of Food Science, 51, 104–108. doi:10.1111/jfds.1986.51.issue-1
- Gordon, A., & Barbut, S. (1989). The effect of chloride salts on the texture, microstructure and stability of meat batters. Food Structure, 8(2), 14.
- Gornall, A.G., Bardawill, C.J., & David, M.M. (1949). Determination of serum proteins by means of the biuret reaction. Journal of Biological Chemistry, 177, 751–766.
- Hermansson, A.-M., Harbitz, O., & Langton, M. (1986). Formation of two types of gels from bovine myosin. Journal of the Science of Food and Agriculture, 37, 69–84. doi:10.1002/(ISSN)1097-0010
- Huff-Lonergan, E., & Lonergan, S.M. (2005). Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Science, 71, 194–204. doi:10.1016/j.meatsci.2005.04.022
- Huxley, H.E. (1963). Electron microscope studies on the structure of natural and synthetic protein filaments from striated muscle. Journal of Molecular Biology, 7, 281–IN30. doi:10.1016/S0022-2836(63)80008-X
- Ishioroshi, M., Samejima, K., Arie, Y., & Yasui, T. (1980). Effect of blocking the myosin-actin interaction in heat-induced gelation of myosin in the presence of actin. Agricultural and Biological Chemistry, 44, 2185–2194.
- Ishioroshi, M., Samejima, K., & Yasui, T. (1983). Heat-induced gelation of myosin filaments at a low salt concentration. Agricultural and Biological Chemistry, 47, 2809–2816.
- Iwasaki, T., Washio, M., & Yamamoto, K. (2005a). Atomic force microscopy of thermally treated myosin filaments. Journal of Agricultural and Food Chemistry, 53, 4589–4592. doi:10.1021/jf0500381
- Iwasaki, T., Washio, M., Yamamoto, K., & Nakamura, K. (2005b). Rheological and morphological comparison of thermal and hydrostatic pressure-induced filamentous myosin gels. Journal of Food Science, 70, e432–e436. doi:10.1111/jfds.2005.70.issue-7
- Kocher, P., & Foegeding, E. (1993). Microcentrifuge-based method for measuring water-holding of protein gels. Journal of Food Science, 58, 1040–1046. doi:10.1111/jfds.1993.58.issue-5
- Kristinsson, H., & Hultin, H. (2003). Role of pH and ionic strength on water relationships in washed minced chicken-breast muscle gels. Journal of Food Science, 68, 917–922. doi:10.1111/jfds.2003.68.issue-3
- Lanier, T.C., Yongsawatdigul, J., & Carvajal-Rondanelli, P. (2013). Surimi gelation chemistry 4. In J. W. Park (Ed.), Surimi and surimi seafood. London: CRC Press.
- Lefevre, F., Fauconneau, B., Ouali, A., & Culioli, J. (2002). Thermal gelation of brown trout myofibrils from white and red muscles: Effect of pH and ionic strength. Journal of the Science of Food and Agriculture, 82(4), 452–463. doi:10.1002/(ISSN)1097-0010
- Lesiów, T., & Xiong, Y.L. (2001). Mechanism of rheological changes in poultry myofibrillar proteins during gelation. Avian and Poultry Biology Reviews, 12, 137–149. doi:10.3184/147020601783698486
- Liu, G., & Xiong, Y.L. (1997). Gelation of chicken muscle myofibrillar proteins treated with protease inhibitors and phosphates. Journal of Agricultural and Food Chemistry, 45, 3437–3442. doi:10.1021/jf9700485
- Liu, R., Zhao, S.-M., Liu, Y.-M., Yang, H., Xiong, S.-B., Xie, B.-J., & Qin, L.-H. (2010). Effect of pH on the gel properties and secondary structure of fish myosin. Food Chemistry, 121, 196–202. doi:10.1016/j.foodchem.2009.12.030
- Liu, R., Zhao, S.-M., Xiong, S.-B., Xie, B.-J., & Qin, L.-H. (2008). Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Science, 80, 632–639. doi:10.1016/j.meatsci.2008.02.014
- Morita, J.I., Choe, I.S., Yamamoto, K., Samejima, K., & Yasui, T. (1987). Heat-induced gelation of myosin from leg and breast muscles of chicken. Agricultural and Biological Chemistry, 51(11), 2895–2900.
- Niwa, E., Chen, E., Wang, T., Kanoh, S., & Nakayama, T. (1988). Extraordinarity in the temperature-dependence of physical parameters of kamaboko [elastic fish paste]. Nippon Suisan Gakkaishi, 54, 1789–1793. doi:10.2331/suisan.54.1789
- Offer, G., & Trinick, J. (1983). On the mechanism of water holding in meat: The swelling and shrinking of myofibrils. Meat Science, 8, 245–281. doi:10.1016/0309-1740(83)90013-X
- Parsons, N., & Knight, P. (1990). Origin of variable extraction of myosin from myofibrils treated with salt and pyrophosphate. Journal of the Science of Food and Agriculture, 51, 71–90. doi:10.1002/(ISSN)1097-0010
- Samejima, K., Lee, N.H., Ishioroshi, M., & Asghar, A. (1992). Protein extractability and thermal gel formability of myofibrils isolated from skeletal and cardiac muscles at different post-mortem periods. Journal of the Science of Food and Agriculture, 58, 385–393. doi:10.1002/(ISSN)1097-0010
- Samejima, K., Oka, Y., Yamamoto, K., Asghar, A., & Yasui, T. (1986). Effects of temperature, actin-myosin ratio, pH, and salt and protein concentrations on heat-induced gelling of cardiac myosin and reconstituted actomyosin. Agricultural and Biological Chemistry, 50, 2101–2110.
- Sano, T., Noguchi, S.F., Matsumoto, J.J., & Tsuchiya, T. (1990). Thermal gelation characteristics of myosin subfragments. Journal of Food Science, 55, 55–58. doi:10.1111/jfds.1990.55.issue-1
- Sharp, A., & Offer, G. (1992). The mechanism of formation of gels from myosin molecules. Journal of the Science of Food and Agriculture, 58, 63–73. doi:10.1002/(ISSN)1097-0010
- Shimada, M., Takai, E., Ejima, D., Arakawa, T., & Shiraki, K. (2015). Heat-induced formation of myosin oligomer-soluble filament complex in high-salt solution. International Journal of Biological Macromolecules, 73, 17–22. doi:10.1016/j.ijbiomac.2014.11.005
- Stone, A., & Stanley, D. (1994). Muscle protein gelation at low ionic strength. Food Research International, 27, 155–163. doi:10.1016/0963-9969(94)90157-0
- Sun, X.D., & Arntfield, S.D. (2011). Gelation properties of chicken myofibrillar protein induced by transglutaminase crosslinking. Journal of Food Engineering, 107, 226–233. doi:10.1016/j.jfoodeng.2011.06.019
- Tornberg, E. (2005). Effects of heat on meat proteins–Implications on structure and quality of meat products. Meat Science, 70, 493–508. doi:10.1016/j.meatsci.2004.11.021
- Trespalacios, P., & Pla, R. (2007). Simultaneous application of transglutaminase and high pressure to improve functional properties of chicken meat gels. Food Chemistry, 100, 264–272. doi:10.1016/j.foodchem.2005.09.058
- Westphalen, A., Briggs, J., & Lonergan, S. (2005). Influence of pH on rheological properties of porcine myofibrillar protein during heat induced gelation. Meat Science, 70, 293–299. doi:10.1016/j.meatsci.2005.01.015
- Westphalen, A.D., Briggs, J.L., & Lonergan, S.M. (2006). Influence of muscle type on rheological properties of porcine myofibrillar protein during heat-induced gelation. Meat Science, 72, 697–703. doi:10.1016/j.meatsci.2005.09.021
- Wu, M., Xiong, Y., Chen, J., Tang, X., & Zhou, G. (2009). Rheological and microstructural properties of porcine myofibrillar protein-lipid emulsion composite gels. Journal of Food Science, 74, E207–E217. doi:10.1111/jfds.2009.74.issue-4
- Xiong, Y., & Brekke, C. (1989). Changes in protein solubility and gelation properties of chicken myofibrils during storage. Journal of Food Science, 54, 1141–1146. doi:10.1111/jfds.1989.54.issue-5
- Xiong, Y., Lou, X., Wang, C., Moody, W., & Harmon, R. (2000). Protein extraction from chicken myofibrils irrigated with various polyphosphate and NaCl solutions. Journal of Food Science, 65, 96–100. doi:10.1111/jfds.2000.65.issue-1
- Xiong, Y.L. (1992). A comparison of the rheological characteristics of different fractions of chicken myofibrillar proteins. Journal of Food Biochemistry, 16, 217–227. doi:10.1111/jfbc.1992.16.issue-4
- Xiong, Y.L. (2000). Meat processing. In S.S. Nakai & H.W. Modler (Eds.), Food proteins: Processing applications (pp. 89–145). New York, NY: Wiley-VCH.
- Yamamoto, K., Samejima, K., & Yasui, T. (1988). Heat-induced gelation of myosin filaments. Agricultural and Biological Chemistry, 52, 1803–1811.
- Yongsawatdigul, J., & Park, J. (1999). Thermal aggregation and dynamic rheological properties of Pacific whiting and cod myosins as affected by heating rate. Journal of Food Science, 64, 679–683. doi:10.1111/jfds.1999.64.issue-4
