ABSTRACT
Five potatoes varieties both raw and microwave (800 W, 8 min) and fried (180°C, 5 min) were evaluated. Colored-fleshed potatoes showed between 2- and 2.5-fold higher total polyphenols (TP, 2880.5–3241.6 mg GAE/kg DW) than the light-yellow. Chlorogenic acid was predominant. However, related to the others identified phenols, microwave and fried presented different phenolic profile. Vitamin C and total antioxidant capacity were also higher on colored ones with values in the range of 260.7–511.6 mg AA/kg DW and 344.1–527.8 mg GAE/kg DW respectively. Microwave and frying reduced TPs and vitamin C. Conversely, microwave increased total antioxidant capacity in 1–2-fold while frying reduced more than half compared to raw potatoes. Microwave and fried-colored potatoes represent an interesting alternative compared to light-yellow potatoes due to its contribution of phytochemicals to the consumer’s diet.
RESUMEN
Se evaluaron cinco variedades de papa tanto crudas como cocidas en microondas (800 W, 8 min) y fritas (180°C, 5 min). Las de pulpa coloreada presentaron entre 2 y 2,5 veces más polifenoles totales (2880,5 a 3241,6 mg EAG/kg PS), que la de pulpa amarilla. El ácido clorogénico se encontró en mayor proporción. Con relación a los restantes fenoles identificados, las hervidas y fritas presentaron un perfil diferente. La vitamina C y capacidad antioxidante total también fueron superiores en las de pulpa coloreada, con valores de entre 260,7 a 511,6 mg AA/kg PS y 344,1 y 527,8 mg EAG/kg PS respectivamente. Los procesos de cocción redujeron el contenido de polifenoles totales y vitamina C. Por el contrario, la capacidad antioxidante de la papa hervida al microondas fue entre 1 y 2 veces superior a la de las crudas, mientras que en las fritas fue de alrededor de la mitad. Las papas de pulpa coloreada representan una alternativa interesante para los consumidores, comparadas con las de pulpa amarilla, debido a su mayor aporte de fitoquímicos a la dieta.
1. Introduction
Potato (Solanum tuberosum L.) is considered one of the most important crop linked to human consumption only surpassed by rice, wheat and maize (Singh & Saldana, Citation2011). The importance of this crop resulted in a strong genetic selection process, determining the current knowledge of some 5000 varieties, among which the fleshed and/or skin-colored ones, whose nutritional and cosmetic characteristics have attracted the interest of industry and consumers. Potato is an important source of carbohydrates which represents about 75% of the total dry matter, being starch the most abundant (Jansen, Flamme, Schüler, & Vandrey, Citation2001). Besides the contribution of carbohydrates, potato is an important source of phytonutrients and also provides high-quality proteins, vitamins (C, B3 and B6) and minerals such as potassium, phosphorous and magnesium (André et al., Citation2007; Ezekiel, Singh, Sharma, & Kaur, Citation2013). Within the identified genetic materials, the fleshed and/or skin colored are particularly attractive because they are rich in compounds that protect human cells against damage caused by free radicals, prevent the oxidation of low-density lipoprotein cholesterol and contributes to the lower incidence of certain cancers, neurodegenerative diseases, osteoporosis and diabetes (Lachman & Hamouz, Citation2005). Potatoes were considered the third most important source of phenols after apples and oranges due to the phenolic compounds present both in skin and flesh with greater concentration in skin, especially in colored varieties (Chun et al., Citation2005; Ezekiel et al., Citation2013). Phenolic compounds are responsible for taste, color and nutritional value (Cheynier, Citation2005; Perla, Holm, & Jayanty, Citation2012). Phenolic acids, mainly chlorogenic and in smaller proportion caffeic acid, cinnamic acid, p-coumaric acid, ferulic acid, and sinapic acid (Friedman, Citation1997) and flavonoids principally catechin and epicatechin (Brown, Culley, Yang, Durst, & Wrolstad, Citation2005) have been identified. Potatoes also contain flavones aglycones, a major group of plant phenols, which are potent antioxidants. Scientific studies have shown that red-fleshed potatoes has acylated pelargonidin glycosides while purple-fleshed has acylated malvidin glycosides, petunidin, peonidin and delphinidin (Brown et al., Citation2005; Lachman et al., Citation2009). These compounds are responsible of the strong antioxidant power of colored fleshed potatoes.
Fried potatoes made both domestically or industrially, are highly consumed followed by boiling, microwave and baking potatoes. In this sense, the high consumption of chips and the new interests of consumers in innovative, nutrient rich and autochthonous products have arisen the interest of consumers by colored potatoes. However, not all the potato germplasms are appropriate to be cooked on the different forms and misuse resulting in low quality products affecting the consumer acceptability. Potato quality is defined by dry matter contents, starch, reducing sugars and TPs. In the case of potatoes intended for the snack manufacture, dry matter content is of great importance because it determines the absorption of oil during frying, affecting texture and flavor of the final product. Thus, potatoes to be spent on the development of fries should have dry matter content above 20% (Jenkins & Nelson, Citation1992). Moreover, the different cooking processes have a different impact on phytonutrient content availability (Navarre, Shakya, Holden, & Kumar, Citation2010).
The present study is aimed to characterize five potato varieties, through the analysis of some phytochemicals, besides determine how they are affected by the two major cooking processes (microwave and fried) and to know if these varieties could be recommended as new ones for cooking.
2. Material and methods
2.1. Plant material
Five colored potato varieties, obtained from the breeding program of the National Agricultural Research Institute (INIA, Uruguay) harvested in autumn were studied.
Varieties used had light-yellow (cod. 050521), purple (cod. 050214), purple-white (cod. 8050219), red (8050214) and red-white (cod. 8050213) fleshed.
After harvest, potatoes were cured in a ventilated and shading shed, with a temperature of 15°C and 85% RH, during 30 days before processing.
2.2. Sample preparation
Three replicates of 15 tubers, previously selected considering a uniform size and the absence of damages, each per variety were washed with tap water, peeled, randomly divided and cut into chips (~0.5-mm thick) and cubes (~1-cm thick). Chips were obtained using a mandolin (Tescoma, Madrid, Spain) while cubes were manually cut using a sharp knife (Ilko, Chile).
Slices were fried while cubes were microwaved under time and temperature condition described later. Samples were maintained in plastics containers with tap water until cooking to prevent browning. All the measurements of the analyzed parameters were conducted in triplicate.
2.3. Raw potato
About 15 g of cubes from the central portion of tuber, for homogeneous samples, were separated and frozen in an −80°C ultralow temperature freezer (Labotec, Montevideo, Uruguay). Then, they were lyophilized during 48 h (Labotec, 01.JLG, Montevideo, Uruguay) and maintained in plastic sealed bags in a box with moisture absorbers until potato characterization.
2.4. Microwave potato
Three samples of about 200 g of potato cubes were washed in cold water, centrifuged in a manual domestic centrifuge (Ilko, Santiago, Chile) and placed in plastic containers with 500 mL of water. Then, cubes were placed in a domestic microwave (Daewoo KOG-8A2B, Barcelona, Spain) at 800 W during 8 min, minimal time needed to complete the cooking according to preliminary tests. After that, about 80 g of sample were separated and lyophilized for further analysis.
2.5. Fried potato
In the same way, three samples of about 200 g of chips were washed in cold water, centrifuged in a manual domestic centrifuge (Ilko, Santiago, Chile), superficially dried with paper towels and fried during 5 min in a domestic fryer (Moulinex, Uno, Shanghai, China) with sunflower oil (Optimo, Cousa, Montevideo, Uruguay), preheated at 180°C. The time required for frying was previously determined. After frying, chips were evenly distributed on a tray with paper towels. Also in this case, about 80-g samples were separated and lyophilized for further analysis.
2.6. Dry matter determination
Five grams of potato cubes were placed in aluminum foil baskets and subsequently taken to the oven (T6, Heraues Instruments, London, U.K) at 105°C until reached a constant weight, accomplished after 48 h (Cacace, Huarte, & Monti, Citation1994). Weight was determined with an analytical balance (Hinotek, FA/JA, Ningbo, China). The results were expressed as percentage (%) of the initial weight.
2.7. Total polyphenols
Total pholyphenols content was determined following the methodology proposed by Singleton and Rossi (Citation1965) modified by Falagán et al. (Citation2016), using an extract obtained by homogenizing 0.3 g of lyophilized sample with 3 mL of methanol by an Ultraturrax T25 basic (IKA, Berlin, Germany) for 2 min. Extract was subsequently centrifuged (Sigma 1–13, Osterode, Germany) at 15,000×g at 4°C during 10 min. Then, a 19.2-µL aliquot was mixed with 29 µL of Folin Ciocalteau reagent 1 N in a 96-well flat bottom plate (Biofil, TCP 011096, Guangdong, China). After 3 min, 192 µL of a 75 g/L sodium carbonate (Na2CO3) solution was added and immediately mixed. Plates were covered and incubated in dark conditions in an orbital shaker at 25°C and 150 rpm for 2 h. Absorbance was measured in microplate reader (TECAN-Infinite M200, Reading, UK) at 760 nm.
TP content was quantified using commercial standards of gallic acid (Sigma-Aldrich, St. Louis MO, U.S.A) and results were expressed .as mg of gallic acid equivalents (GAE)/kg in dry weight (DW).
2.8. Individual polyphenol determination
Chlorogenic, p-coumaric, caffeic, ellagic and ferulic acid determination was made by high liquid performance chromatography (HPLC). For extraction, 1.5 g of lyophilized samples homogenized for 2 min with 10 mL a solution of 80% methanol; 19.5% ultrapure water and 0.5% acetic acid (CH3COOH) with an Ultraturrax T25 basic (IKA, Berlin, Germany), as it has been proposed by André et al. (Citation2009). Subsequently, samples were centrifuged 10 min at 9000×g at 4°C. The supernatant was filtered through a 0.45-µm polytetrafluoroethylene (PTFE) membrane syringe filter (Millex, Sigma-Aldrich, Carlsbad, U.S.A). An aliquot of 10 µL was injected in a HPLC (Series 1100 Agilent Technologies, Waldbronn, Germany) equipped with a photodiode array detector and with a Gemini NX column C18 of 250 mm × 4.6 mm, 5 µm (Phenomenex, Torrance CA, U.S.A). The elution solvents were: 0.1% formic acid (CH2O2) in water (A) and 0.1% CH2O2 in acetonitrile (B). Samples were eluted according to the following binary gradient: starting with 0% B in order to reach 9% B at 10 min, 13% B at 40 min, 35% B at 80 min, 100% B at 88 min, 0% B at 95 min. The elution was performed at 40°C with a flow rate of 1 mL/min (André et al., Citation2009; Navarre, Pillai, Shakya, & Holden, Citation2011). Compounds were identified and quantified by conventional retention times using standards with a purity ≥98% provided by Sigma-Aldrich (St. Louis MO, U.S.A). Results were expressed as mg/kg DW.
2.9. Vitamin C determination
Ascorbic and dehydroascorbic acids (AA and DHA, respectively) were measured according to the method proposed by Zapata and Dufour (Citation1992) with slight modifications. For the extraction, 2 g of lyophilized sample were homogenized (Ultraturrax T25 basic, IKA, Berlin, Germany) in 10 mL of 0.1 M citric acid (C6H8O7); 0.05% ethylenediaminetetraacetic acid (EDTA); 4 mM sodium fluoride (NaF) on methanol water (5:95 v/v) cold buffer during 3 min. Then, the extract was filtered through cheesecloth and the pH was adjusted to 2.35–2.40 using a saturated sodium hydroxide (NaOH) solution. Subsequently, the extract was purified by solid-phase extraction (Sep Pak cartridges C18, Waters, Dublin, Ireland) and filtered through a 0.45-µm PTFE membrane syringe filter (Millex, Sigma-Aldrich, Carlsbad, USA). Prior to injection, 750 µL of the extract were derivatized with 250 µL of 1,2-phenylenediamine 7.7 M in an amber vial. The mixture was maintained on ice for 37 min to complete the reaction. After that, 20-µL aliquot was injected in a HPLC (Series 1100 Agilent Technologies, Waldbronn, Germany) equipped with a photodiode array detector and with a Gemini NX C18 column of 250 mm × 4.6 mm, 5 µm (Phenomenex, Torrance CA, U.S.A). This analysis was performed at isocratic condition using 5 mM hexadecyl trimethyl ammonium bromide (C19H42BrN); 50 mM potassium phosphate monobasic (KH2PO4) and 5% methanol (pH 4.59) at 1.8 mL/min as mobile phase.
AA and DHA were quantified using commercial standards (Sigma-Aldrich, St. Louis MO, U.S.A). Results were expressed as mg ascorbic acid (AA)/kg DW.
2.10. Total antioxidant capacity
Total antioxidant capacity was determined through the evaluation of the free radical scavenging capacity according to the 2,2 diphenyl-1-picryhydrazyl (DPPH) free radical assay (Brand-Williams, Cuveliar, & Berset, Citation1995). Determinations were performed based on the methodology described by Bobo-García et al. (Citation2015) with some modification, by mixing 21 µL of the same extract used on TP analysis, with 194 µL of methanolic DPPH solution (118 mg/L) on 96-well microplate (Biofil, TCP 011096, Guangdong, China). Before absorbance reading (λ = 515), in a microplate reader (TECAN-Infinite M200, Reading, U.K). Samples were allowed reacting during 1.5 h under refrigeration and darkness.
TAC was quantified using commercial standards of Trolox (Sigma-Aldrich, St. Louis MO, U.S.A). The results were expressed as mg of Trolox equivalents (TE)/kg DW. These measurements were also performed in triplicate.
2.11. Statistical analysis
Data were processed by analysis of variance (ANOVA) and reported as the mean ± standard error (SE). Significant differences between treatments were analyzed using Tukey’s test (p ≤ 0.05) in the Infostat software package, version 2012 (Universidad Nacional de Córdoba, Argentina).
3. Results and discussion
3.1. Dry matter content
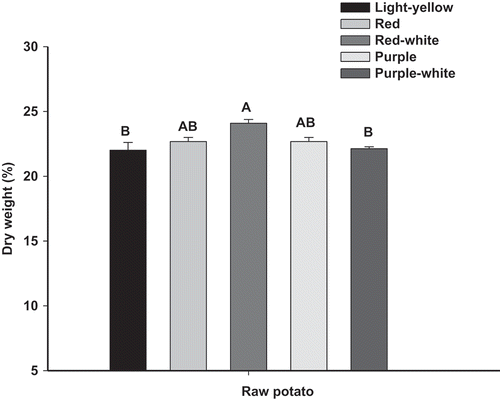
Dry matter contents for raw samples were between 22 and 24% (). Red-white differed from purple-white and light-yellow fleshed potatoes. Cacace et al. (Citation1994), reported that according to dry matter content, potatoes can be categorized into three groups: high dry matter content (˃20.0%), intermediate dry matter content (between 18.0 and 19.9%) and low dry matter content (˂17.9%). Dry matter determines the potential use and quality of the final product. For frying, potatoes should have higher values in order to limit the oil absorption during the process. Potatoes with low dry matter content would be appropriate for boiling while those with medium dry matter content would be suitable for puree and roasted (Feltran, Boges-Lemos, & Lopes-Vieites, Citation2004; Kita, Bąkowska-Barczak, Lisińska, Hamouz, & Kułakowska, Citation2015). In this sense, measured dry matter indicated that all tested varieties are especially suitable for frying.
Figure 1. Dry matter contents of different varieties of raw potato expressed as percentage (%). Vertical bars indicate the standard error of the means (n = 3).
Figura 1. Contenido de materia seca en diferentes variedades de papa cruda expresado como porcentaje (%). Las barras verticales corresponden al error estándar (n = 3).
3.2. Total polyphenols
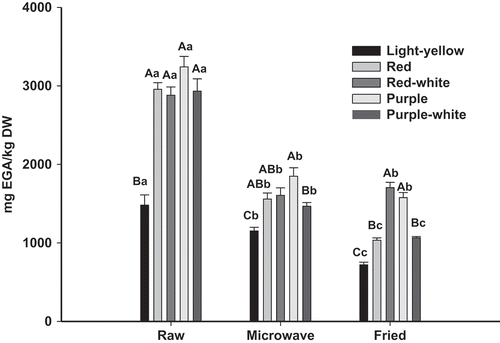
Raw colored potatoes showed similar TP ranges between 2880.5 and 3241.6 mg GAE/kg DW. These values represented among 2–2.5-fold more than light-yellow fleshed (). Other studies have reported similar values as occurred in a characterization of 13 genetic materials with different colored fleshed (Tierno, Hornero-Méndez, Gallardo-Guerrero, López-Pardo, & Ruiz de Galarreta, Citation2015). According to these authors, TP values ranged from 1420 to 3590 mg GAE/kg DW in raw tubers being purple-fleshed potatoes the richest variety with 2500–3590 mg GAE/kg DW.
Figure 2. Total polyphenols of raw, boiled and fried different potatoes varieties. Vertical bars represent standard error of the means (n = 3). Uppercase letters denote significant (Tukey test at p ≤ 0.05) differences among varieties for the same treatment. Lowercase letters denote significant (Tukey test at p ≤ 0.05) differences among treatments for the same variety.
Figura 2. Polifenoles totales en diferentes variedades de papa cruda, cocida y frita. Las barras verticales corresponden al error estándar (n = 3). Letras mayúsculas indican diferencias (Tukey para p ≤ 0,05) entre variedades para el mismo tratamiento. Letras minúsculas indican diferencias (Tukey para p ≤ 0,05) entre tratamientos para la misma variedad.
TP differences between colored fleshed potatoes and non-colored has a strong genetic component. Colored fleshed potatoes have twice to even 10 times TP, compared to white or yellow fleshed, as reported in several studies (Hamouz, Lachman, Dvořák, Jůzl, & Pivec, Citation2006; Lewis, Walker, Lancaster, & Sutton, Citation1998; Mulinacci et al., Citation2008; Rytel et al., Citation2014). Differences can be explained by the fact that the compounds responsible for the color belong to the polyphenols group (anthocyanins and proanthocyanidins), so it is expected that those potatoes with intense colors were richer in these compounds.
Microwave potatoes showed a TP between 1152.2 and 1848.6 mg GAE/kg DW, which represented approximately 22–49% lower content than raw potato.
Although, light-yellow potato showed the lowest value, this variety had a high TP retention after microwave with 78% of the initial TP contents (). These may be due to several factors as the greater thermal stability of specific polyphenols of this variety, the synthesis of new compounds and/or structural modifications at matrix cells for this variety in particular, that protect TP avoiding its release. In this sense, some authors claimed that during cooking it is possible the synthesis of new compounds and the modification on potato matrix cells affecting the stability, bioaccessibility, and extractability of these compounds. The changes observed could explain both the high losses and/or high retention of compounds such as TP, related to cooking processes (Ruiz-Rodríguez, Marín, Ocaña, & Soler-Rivas, Citation2008; Tiam et al., Citation2016). Hence, besides amount and type of compounds, differences in matrix cells must be also considered to analyze TP evolution during cooking process.
On fried potatoes, TP were between 722.2 and 1704.2 mg GAE/kg DW, meaning a reduction of about 41–65% compared to raw ones. Purple along with the red-white fleshed potatoes had the highest retention. On these two varieties, both forms of preparation (microwave and frying) equally affected TP, since no significant differences were registered. Therefore, both cooking methods have the same impact on TP of these varieties, although the temperature reached in frying is far greater than the achieved in microwave.
Contrary to microwave, the frying process affects largely the polyphenols of the light-yellow variety which had a very low content.
According to Dao and Friedman (Citation1994) and Perla et al. (Citation2012), TP are largely affect by cooking condition especially when high temperatures, long cooking time or the combination of both were used. Although in our study, for TP-richest varieties, it was not fulfilled that the most severe cooking method determined greater TP reduction.
3.3. Individual polyphenols
Chlorogenic acid was found in greater proportion either in raw, microwaved or fried potatoes, representing up to 90% of the phenolic compounds (). Additionally, p-coumaric, caffeic, ellagic and ferulic acids were also identified besides the cyanidin 3-rutinoside. These results agreed with other studies with potatoes where chlorogenic acid and its isomers (cryptochlorogenic, neochlorogenic and isochlorogenic acid) represented over 90% of phenolic compounds. Moreover, other minority compounds appeared such as: caffeic, p-coumaric, ferulic, sinapic acid, protocatechuic acid and vanillic acids (Ezekiel et al., Citation2013; Mäder, Rawel, & Kroh, Citation2009; Malmberg & Theander, Citation1985; Rytel et al., Citation2014).
Table 1. Individual polyphenols on different varieties of raw, microwave and fried potatoes (mg/kg DW).
Tabla 1. Polifenoles individuales en diferentes variedades de papa cruda, cocida al microondas y frita (mg/kg PS).
Chlorogenic acid content differed between genetic materials being almost three times higher in raw colored fleshed potatoes with values between 2152.1 and 2541.4 mg/kg DW compared to light-yellow fleshed which showed content around 840.9 mg/kg DW. Variation on caffeic acid between colored fleshed and light-yellow potatoes were also registered. Red fleshed was the richest variety, with 201.4 mg/kg DW, while the light-yellow showed the lowest content (56.3 mg/kg DW).
Navarre et al. (Citation2010) reported that colored potatoes had three to four times more chlorogenic acid, 800–4730 mg/kg DW, than white and yellow fleshed with values ranging from 200 to 760 mg/kg DW. Similar differences were found on the caffeic acid, but in this case, far pronounced variation occurs, since contents ranged from 4.8 to 480 mg/kg DW.
Cyanidin 3-rutinoside and ellagic acid appeared in minor quantity in raw potato, with values between 46.2 and 128.4 mg/kg DW and 61.5 and 178.6 mg/kg DW, respectively. As expected, purple and pink potatoes were the richest in cyanidin 3-rutinoside since this compound is associated with the characteristic color of them. Furthermore, ferulic and p-coumaric acid were only detected on these varieties.
As observed in TP, the cooking process provoked reductions in chlorogenic acid which reached 42–50% in microwave and 75–86% on fried potatoes when compared to raw ones. In microwave potatoes, purple and red fleshed registered the highest values. However, no differences among varieties were registered on fried potatoes. Only in light-yellow-fleshed potato, chlorogenic acid did not show differences between raw and microwave potatoes. This is in accord to the highest TP retention reported on these varieties. Unlike chlorogenic acid, caffeic acid seemed to be more stable during cooking since no differences were observed between the levels found on raw and microwave potatoes, except in red-white fleshed.
Cyanidin 3-rutinoside and ellagic acid were also affected by microwave. The measured amounts were 108.5 and 39.7 mg/kg DW and 56.8 and 9.8 mg/kg DW, respectively, corresponding to a reduction of about 50–76% compared to the raw material. Furthermore, both cyanidin 3-rutinoside and ellagic acid were totally degraded by the frying process.
P-coumaric was found in greater proportion in fried potatoes with content around 94.6 and 208.9 mg/kg DW, representing between 3 and 5 times higher than the content found in raw potato. Even though purple- and red-white-fleshed potatoes did not differ on TP, both microwave as fried, they presented different phenolic profile. Purple-white fleshed showed chlorogenic, caffeic, ferulic acid and p-coumaric acid, the red and purple varieties presented chlorogenic, caffeic and p-coumaric acid.
Therefore, TP did not depend on the same phenolic profile and the cooking method affect not only the quantity but the type of compounds present in a given matrix.
Variation on phenolic compounds in sweet potato, as a result of cooking was also reported by Takenaka, Nanayama, Isobe and Murata (Citation2006) who said it depends on factors such as leaching into water, heat degradation, oxidation by polyphenol oxidase and isomerization process.
The impact of different cooking processes, conventional boiled (boiling water, 600 s) and microwave baking (300 W, 180 s) on phenolic compounds of peeled potato cv. Agria were evaluated by Barba, Calabretti, d´Amore, Piccinelli and Rastrelli (Citation2008). These authors reported that the losses depended on how aggressive was the cooking method. The conventional boiled determined the highest losses varying between 85.6% and 94.8% while microwave baking is considered less aggressive, varying between 49.6 and 77.3.
More recently, Tiam et al. (Citation2016) reported a reduction of 33.8 and 21% of the chlorogenic acid content in purple-fleshed potato after frying at 191°C for 2 min and microwaving at 1000 W for 6 min, respectively. As observed in our work, the authors mentioned that each phenolic acid had different thermal stability which determines that they were differentially affected by the cooking method, depending on temperature and exposure time. However, contrary to our finding, they reported that caffeic acid was more easily degraded when compared to ferulic acid.
3.4. Vitamin C content
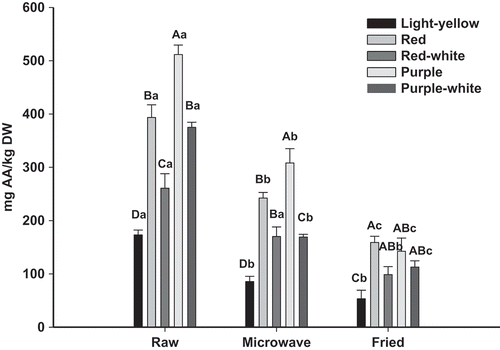
The vitamin C content showed on , corresponds to AA contents since DHA values were very low or not detected (data not shown). Noticeable differences between the analyzed genetic materials were found. Vitamin C contents range from 173.2 to 511.6 mg/kg DW.
Figure 3. Vitamin C contents of raw, boiled and fried different potatoes varieties. Vertical bars represent standard error of the means (n = 3). Uppercase letters denote significant (Tukey test at p ≤ 0.05) differences among varieties for the same treatment. Lowercase letters denote significant (Tukey test at p ≤ 0.05) differences among treatments for the same variety.
Figura 3. Vitamina C en diferentes variedades de papa cruda, cocida y frita. Las barras verticales corresponden al error estándar (n = 3). Letras mayúsculas indican diferencias (Tukey para p ≤ 0,05) entre variedades para el mismo tratamiento. Letras minúsculas indican diferencias (Tukey para p ≤ 0,05) entre tratamientos para la misma variedad.
Especially in the case of native potato, a great variability was recorded on previous works. In this sense, in a characterization study of raw peeled tubers from 25 Andean potatoes, vitamin C contents varied from 223.2 to 1214.3 mg/kg DW (Burgos, Auqui, Amoros, Salas, & Bonierbale, Citation2009). Therefore, the values measured in our work are in the range of the reported by these authors.
Vitamin C on colored fleshed potatoes was about 1.5–3-folds higher than light-yellow potato. Highest content corresponded to purple-fleshed potatoes followed by red- and purple-white-fleshed varieties.
Microwave and specially frying process caused a substantial reduction on vitamin C. Accordingly, frying induced vitamin C losses 60–72% while microwave only led 35–54%. In both cases, the lowest impact was registered on purple- and red-fleshed varieties which exhibit the highest retention.
Despite degrading after cooking, retained on colored potatoes after microwave (169.1–308.1 mg AA/kg DW), turn it into an interesting source of vitamin C to the diet. Kapusta-Duch and Leszczyńska (Citation2013) reported that vitamin C contents of different cruciferous vegetables varied between 4300 and 4600 mg/kg DW, therefore, colored potatoes would provide by 8–11% of vitamin C provided by these species.
Colored potatoes continued having higher vitamin C content than light-yellow fleshed ones. Therefore, it should be preferred colored fleshed for cooking, since vitamin C remained higher after the process.
Vitamin C is easily degraded by various factors including the temperature. Therefore, it was expectable that it was largely affected after cooking, especially in frying process where potatoes were exposed to 180°C for 5 min. Tiam et al. (Citation2016) reported the effects of different cooking treatments on the vitamin C contents in purple-fleshed potatoes. These authors found that the greatest vitamin C losses were observed after air-frying, frying and stir-frying (90.4%, 83.4% and 55.5%, respectively), followed by baking, boiling, steaming and microwaving (71.6%, 40.8%, 23.6% and 7.5%, respectively). The temperature and cooking time are determining factors on the magnitude of vitamin C reduction. This is due to the heat-sensitive ascorbic acid with the intensity and time of the thermal treatment applied. The decrease in vitamin C content in the thermal treatment can be explained by the oxidation of ascorbic acid into dehydroascorbic acid, which is irreversibly converted into 2,3-diketogulonic acid (Davey et al., Citation2000). However, Ikanone and Oyekan (Citation2014) reported that the boiling and frying of Irish potato resulted in a loss of 373 mg/L (63.9%) and 304 mg/L (53.9%) vitamin C, respectively.
3.5. Total antioxidant capacity
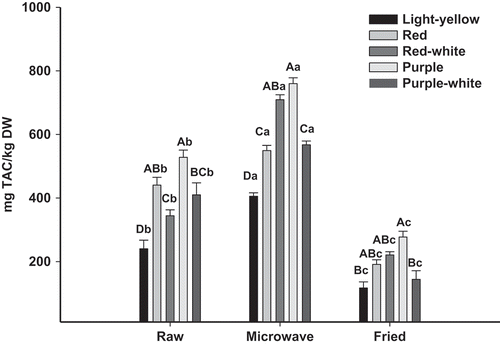
The TAC of the potato varieties studied is depicted on . Raw potato contents were about 250.9 and 527.8 mg/kg DW. TAC measured on colored fleshed was 1.32–2.03-folds higher than those obtained in light-yellow potato. Fleshed varieties showed differences between them where the higher TAC was registered on purple fleshed potato and the lowest values corresponded to red-white fleshed one.
Figure 4. Total antioxidant capacity of raw, boiled and fried different potatoes varieties. vertical bars represent standard error of the means (n = 3). Uppercase letters denote significant (Tukey test at p ≤ 0.05) differences among varieties for the same treatment. Lowercase letters denote significant (Tukey test at p ≤ 0.05) differences among treatments for the same variety.
Figura 4. Capacidad antioxidante total en diferentes variedades de papa cruda, cocida y frita. Las barras verticales corresponden al error estándar (n = 3). Letras mayúsculas indican diferencias (Tukey para p ≤ 0,05) entre variedades para el mismo tratamiento. Letras minúsculas indican diferencias (Tukey para p ≤ 0,05) entre tratamientos para la misma variedad.
Differences in TAC values observed on potato varieties analyzed have been previously reported by other authors attached to genetic variability. Genotype is responsible for determining the potential ability to synthesize and accumulate quantities and/or types of TAC responsible compounds (Kita et al., Citation2015; Shahidi & Naczk, Citation1995; Wang, Cao, & Prior, Citation1997). Kita et al. (Citation2015) recently reported that purple-fleshed potatoes are very rich in TAC.
Moreover, it is mentioned that TP are primarily responsible for the TAC of colored potatoes varieties (Lachman et al., Citation2009; Stushnoff et al., Citation2008). Nevertheless, in our study, it seems that the variations found on TAC would be more linked to other compounds such as vitamins and not to TP. This thought is based on the fact that colored fleshed potatoes showed no differences in TP, but they differed on vitamin C content besides vitamin C follows the same trend that the observed on TAC.
Regarding the cooking method, the microwave increased TAC levels ranging between 549.1 and 759.7 mg/kg DW. The highest TAC levels corresponded to purple- and red-fleshed potatoes.
Moreover, fried potato showed TAC values between 116.9 and 277.4 mg/kg DW achieving reductions of up to 58% related to the raw potato. In this case no substantial differences were observed between varieties.
Experimental results obtained by Faller and Fialho (Citation2009) indicated an increase in TAC after cooking linked to the fact that in raw vegetables predominate hydrolyzable polyphenols whereas in cooked ones predominate the soluble polyphenols. Additionally, as a result of cooking, the enzymatic activity is also affected, so the highest TAC values can be due to a less degradation occurrence (Navarre et al., Citation2010).
4. Conclusions
All the analyzed varieties showed good aptitude to frying according to dry matter content. Colored-fleshed potatoes presented between 1.5- and 3-fold higher phytochemicals contents related to the light-yellow variety. Within the colored-fleshed group, purple, pink-white varieties were the richest in phytochemicals. Microwave and frying reduced phenolic compounds and vitamin C contents. Chlorogenic acid was the phenolic predominant on raw potatoes while boiled and fried presented different phenolic profile.
Under these cooking methods, colored-fleshed potatoes presented higher contents than light-yellow potato so that varieties could be an alternative to traditional potato varieties for its richness in phytochemicals.
Acknowledgments
The authors are grateful to CONICYT-CHILE (FONDECYT Postdoctoral Project No. 3130363) for financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- André, C.M., Ghislain, M., Bertin, P., Oufir, M., Herrera, M., Del, R. … Evers, D. (2007). Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. Journal of Agricultural and Food Chemistry, 55(2), 366–378. doi:10.1021/jf062740i
- André, C.M., Oufir, M., Hoffmann, L., Hausman, J.-F., Rogez, H., Larondelle, Y., & Evers, D. (2009). Influence of environment and genotype on polyphenol compounds and in vitro antioxidant capacity of native Andean potatoes (Solanum tuberosum L.). Journal of Food Composition and Analysis, 22(6), 517–524. doi:10.1016/j.jfca.2008.11.010
- Barba, A., Calabretti, A., D´Amore, M., Piccinelli, A., & Rastrelli, L. (2008). Phenolic constituents levels in cv. Agria potato under microwave processing. LWT-Food Science and Technology, 41(10), 1919–1926. doi:10.1016/j.lwt.2008.02.004
- Bobo-García, G., Davidov-Pardo, G., Arroqui, C., Vírseda, P., Marín-Arroyo, M.R., & Navarro, M. (2015). Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. Journal of the Science of Food and Agriculture, 95(1), 204–209. doi:10.1002/jsfa.6706
- Brand-Williams, W., Cuveliar, M.E., & Berset, C. (1995). Use of free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30. doi:10.1016/S0023-6438(95)80008-5
- Brown, C.R., Culley, D., Yang, C., Durst, R., & Wrolstad, R. (2005). Variation of anthocyanin and carotenoid contents and associated antioxidant values in potato breeding lines. Journal of the American Society for Horticultural Science, 130(2), 174–180.
- Burgos, G., Auqui, S., Amoros, W., Salas, E., & Bonierbale, M. (2009). Ascorbic acid concentration of native Andean potato varieties as affected by environment, cooking and storage. Journal of Food Composition and Analysis, 22(6), 533–538. doi:10.1016/j.jfca.2008.05.013
- Cacace, J.E., Huarte, M.A., & Monti, M.C. (1994). Evaluation of potato cooking quality in Argentina. American Journal of Potato Research, 71(3), 145–153. doi:10.1007/BF02849049
- Cheynier, V. (2005). Polyphenols in foods are more complex than often thought. American Journal of Clinical Nutrition, 81(1), 223S–229S.
- Chun, O.K., Kim, D.-O., Smith, N., Schroeder, D., Han, J.T., & Lee, C.Y. (2005). Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. Journal of the Science of Food and Agriculture, 85, 1715–1724. doi:10.1002/jsfa.2176
- Dao, L., & Friedman, M. (1994). Chlorophyll, chlorogenic acid, glycoalkaloid and inhibitor content in fresh and green potatoes. Journal of Agriculture and Food Chemistry, 42(3), 2152–2156. doi:10.1021/jf00039a006
- Davey, M.W., Van Montagu, M., Inzé, D., Sanmartin, M., Kanellis, A., Smirnoff, N. … Fletcher, J. (2000). Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture, 80, 825–860. doi:10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6
- Ezekiel, B., Singh, N., Sharma, S., & Kaur, A. (2013). Beneficial phytochemicals in potato. A review. Food Research International, 50(2), 487–496. doi:10.1016/j.foodres.2011.04.025
- Falagán, N., Artés, F., Gómez, P., Artés-Hernández, F., Conejero, W., & Aguayo, E. (2016). Deficit irrigation strategies enhance health-promoting compounds through the intensification of specific enzymes in early peaches. Journal of the Science of Food and Agriculture, 96(5), 1803–1813. doi:10.1002/jsfa.7290
- Faller, A.L.K., & Fialho, E. (2009). The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Research International, 42(1), 210–215. doi:10.1016/j.foodres.2008.10.009
- Feltran, J.C., Boges-Lemos, L., & Lopes-Vieites, R. (2004). Technological quality and utilization of potato tuber. Scientia Agricola, 61(6), 598–603. doi:10.1590/S0103-90162004000600006
- Friedman, M. (1997). Chemistry, biochemistry, and dietary role of potato polyphenols. A review. Journal of Agricultural and Food Chemistry, 45(5), 1523–1540. doi:10.1021/jf960900s
- Hamouz, K., Lachman, J., Dvořák, P., Jůzl, M., & Pivec, V. (2006). The effect of site conditions, variety and fertilization on the content of polyphenols in potato tubers. Plant Soil and Environment, 52, 407–412. doi:10.1007/BF02861591
- Ikanone, C.E.O., & Oyekan, P.O. (2014). Effect of boiling and frying on the total carbohydrate, vitamin C and mineral contents of Irish (Solanun tuberosum) and sweet (Ipomea batatas) potato tubers. Nigerian Food Journal, 32(2), 33–39. doi:10.1016/S0189-7241(15)30115-6
- Jansen, G., Flamme, W., Schüler, K., & Vandrey, M. (2001). Tuber and starch quality of wild and cultivated potato species and cultivars. Potato Research, 44(2), 137–146. doi:10.1007/BF02410100
- Jenkins, P.D., & Nelson, D.G. (1992). Aspects of nitrogen fertilizer rate on tuber dry matter content of potato cv. Record. Potato Research, 35(2), 127–132. doi:10.1007/BF02357605
- Kapusta-Duch, J., & Leszczyńska, T. (2013). Comparison of vitamin C and β-carotene in cruciferous vegetables grown in diversified ecological conditions. Polish Journal of Environmental Studies, 22(1), 167–173.
- Kita, A., Bąkowska-Barczak, A., Lisińska, G., Hamouz, K., & Kułakowska, K. (2015). Antioxidant activity and quality of red and purple flesh potato chips. LWT-Food Science and Technology, 62(1), 525–531. doi:10.1016/j.lwt.2014.03.026
- Lachman, J., & Hamouz, K. (2005). Red and purple coloured potatoes as a significant antioxidant source in human nutrition. A review. Plant Soil and Environment, 51, 477–482.
- Lachman, J., Hamouz, K., Šulc, M., Orsák, M., Pivec, V., Hejtmánková, A. … Čepl, J. (2009). Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chemistry, 114(3), 836–843. doi:10.1016/j.foodchem.2008.10.029
- Lewis, C.E., Walker, J.R.L., Lancaster, J.E., & Sutton, K.H. (1998). Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. Journal of the Science of Food and Agriculture, 77, 45–57. doi:10.1002/(SICI)1097-0010(199805)77:1<45::AID-JSFA1>3.0.CO;2-S
- Mäder, J., Rawel, H., & Kroh, L.W. (2009). Composition of phenolic compounds and glycoalkaoids a-solanine and a-chaconine during commercial potato processing. Journal of Agriculture and Food Chemistry, 57(14), 6292–6297. doi:10.1021/jf901066k
- Malmberg, A.G., & Theander, O. (1985). Determination of chlorogenic acid in potato tubers. Journal of Agricultural and Food Chemistry, 33(3), 549–551. doi:10.1021/jf00063a052
- Mulinacci, N., Ieri, F., Giaccherini, C., Innocenti, M., Andrenelli, L., Canova, G. … Casiraghi, C. (2008). Effect of cooking on the anthocyanins, phenolic acids, glycoalkaloids, and resistant starch content in two pigmented cultivars of Solanum tuberosum L. Journal of Agriculture and Food Chemistry, 56(24), 11830–11837. doi:10.1021/jf801521e
- Navarre, D.A., Pillai, S.S., Shakya, R., & Holden, M.J. (2011). HPLC profiling of phenolics in diverse potato genotypes. Food Chemistry, 127(1), 34–41. doi:10.1016/j.foodchem.2010.12.080
- Navarre, D.A., Shakya, R., Holden, M.J., & Kumar, S. (2010). The effect of different cooking methods on phenolics and vitamin C in developmentally young potato tubers. American Journal of Potato Research, 87(4), 350–359. doi:10.1007/s12230-010-9141-8
- Perla, V., Holm, D.G., & Jayanty, S.S. (2012). Effects of cooking methods on polyphenols, pigments and antioxidant activity in potato tubers. LWT-Food Science and Technology, 45(2), 161–171. doi:10.1016/j.lwt.2011.08.005
- Ruiz-Rodríguez, A., Marín, F.R., Ocaña, A., & Soler-Rivas, C. (2008). Effect of domestic processing on bioactive compounds. Phytochemistry Review, 7(2), 345–384. doi:10.1007/s11101-007-9073-1
- Rytel, E., Tajner-Czopek, A., Kita, A., Aniołowska, M., Kucharska, A.Z., Sokół-Łętowska, A., & Hamouz, K. (2014). Content of polyphenols in coloured and yellow fleshed potatoes during dices processing. Food Chemistry, 161, 224–229. doi:10.1016/j.foodchem.2014.04.002
- Shahidi, F., & Naczk, M. (1995). Antioxidant properties of food phenolics. In F. Shahidi & M. Naczk (Eds.), Food phenolics: Sources, chemistry, effects and applications (pp. 235–277). Lancaster, PA: Technomic Publishing Co.
- Singh, P.P., & Saldana, M.D.A. (2011). Subcritical water extraction of phenolic compounds from potato peel. Food Research International, 44(8), 35–38. doi:10.1016/j.foodres.2011.02.006
- Singleton, V.L., & Rossi, J.A. (1965). Colorimetry of total phenolics with phosphomolybdicephosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
- Stushnoff, C., Holm, D., Thompson, M.D., Jiang, W., Thompson, H.J., Joyce, N.I., & Wilson, P. (2008). Antioxidant properties of cultivars and selections from the Colorado potato breeding program. American Journal of Potato Research, 85, 267–276. doi:10.1007/s12230-008-9032-4
- Takenaka, M., Nanayama, K., Isobe, S., & Murata, M. (2006). Changes in caffeic acid derivatives in sweet potato (Ipomoea batatas L.) during cooking and processing. Bioscience, Biotechnology and Biochemistry, 70(1), 172–177. doi:10.1271/bbb.70.172
- Tiam, J., Che, J., Lv, F., Chen, S., Chen, J., Liu, D., & Ye, X. (2016). Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chemistry, 197, 1264–1270. doi:10.1016/j.foodchem.2015.11.049
- Tierno, R., Hornero-Méndez, D., Gallardo-Guerrero, L., López-Pardo, R., & Ruiz De Galarreta, J.I. (2015). Effect of boiling on the total phenolic, anthocyanin and carotenoid concentrations of potato tubers from selected cultivars and introgressed breeding lines from native potato species. Journal of Food Composition and Analysis, 41, 58–65. doi:10.1016/j.jfca.2015.01.013
- Wang, H., Cao, G., & Prior, R.L. (1997). Oxygen radical absorbing capacity of anthocyanins. Journal of Agriculture and Food Chemistry, 45(2), 304–309. doi:10.1021/jf960421t
- Zapata, S., & Dufour, J.-P.. (1992). Ascorbic, dehydroascorbic and isoascorbic acid simultaneous determinations by reverse phase ion interaction HPLC. Journal of Food Science, 57(2), 506–511. doi:10.1111/j.1365-2621.1992.tb05527.x
