ABSTRACT
ϒ-aminobutyric acid (GABA) is a ubiquitous non-proteinaceous amino acid with many health-promoting benefits. GABA in some fruits has been reported previously. However, there have been no previous reports of GABA in jujube fruit. Therefore, the purpose of this study was to investigate the content of GABA in different jujube cultivars and the effect of harvest, drying and storage on GABA of jujube fruit. In six jujube cultivars studied, Z. Jujuba cv. Junzao had the highest GABA content of 333.37 µg.g−1 DW, while Z. Jujuba cv. Dongzao had the lowest GABA content of 150.31 µg.g−1 DW. The GABA content of jujube fruit (Z. jujuba Mill.cv.Junzao) was nearly doubled by delaying harvest time probably due to cold stress, while it was significantly decreased by the treatment of drying and storage. Interestingly, these findings suggest that normal consumption amount of jujube fruit might contain sufficient GABA to produce the effects of improving sleep and lowering blood pressure.
RESUMEN
El ácido ϒ-aminobutírico (GABA) es un aminoácido no proteico ubicuo, que produce muchos beneficios en la salud. En algunas frutas, la presencia de GABA ha sido reportada previamente. Sin embargo, no existen informes anteriores de la presencia de GABA en la fruta de azufaifo. Ante ello, el presente estudio se propuso investigar el contenido de GABA en diferentes cultivares de azufaifo, así como el efecto que conllevan la cosecha, el secado y el almacenamiento en el GABA de esta fruta. Se estudiaron seis cultivares de azufaifo, comprobándose que Z. Jujuba cv. Junzao tuvo el contenido más alto de GABA, 333.37 µg.g-1 DW, mientras que Z. Jujuba cv. Dongzao presentó el contenido de GABA más bajo, 150.31 µg.g-1 DW. Asimismo, se constató que el contenido de GABA de la fruta de azufaifo (ZJZ) casi se duplicó cuando se retrasó el tiempo de cosecha, probablemente debido al estrés por frío, reduciéndose significativamente con el tratamiento de secado y el almacenamiento. Curiosamente, estos hallazgos sugieren que la cantidad consumida normalmente de fruta de azufaifo puede aportar suficiente GABA para mejorar el sueño y disminuir la presión arterial.
Introduction
Jujube (Z. jujuba Mill), also called Chinese date or red date, has been known as a native fruit in China for more than 4,000 years. Nowadays, jujube has flourished and is widely cultivated in the warmer parts of Asia, Africa, Europe, and America (Hernández et al., Citation2016). Although there is no record on the yield in the world, its annual yield in China reached 8.24 million tons (Huang, Citation2017), and its yield still keeps increasing. Jujube is a good source of nutrients, such as sugars, organic acids, vitamins and minerals, as well as phytochemicals such as cyclic adenosine monophosphate, alkaloids, saponins, polyphenols (Xie, Youa, Huang, & Zhang, Citation2017), and polysaccharides (Zhan, Xia, Shao, & Cheng Wang, Citation2018). Jujube is a popular and profitable fruit, and much admired for its exquisite taste, unique flavor and high nutritious value (Rostami & Taghi, Citation2017). It has been commonly consumed in Asian countries as a food and food additive as well as an important folklore medicine for thousands years (Finotti, Bersani, Del Prete, & Friedman, Citation2015).
Traditionally, Shennong Bencao Jing, the earliest documented medical book in China, recorded jujube as an herbal medicine having functions of calming the mind, improving sleep and prolonging life-span (Chen et al., Citation2013). A folk prescription was recorded in Compendium of Materia Medica that the symptoms of dysphoria and insomnia could be alleviated through drinking a bowl of jujube soup, which is made of fourteen jujubes. In addition, the Chinese Pharmacopoeia (Commission, Citation2015) also included it as an important herbal medicine for the treatment of fatigue, anemia, insomnia and others health problems. In recent years, numerous cell and animal studies have revealed that jujube has a variety of biological activities such as anti-tumor, antioxidant, memory modulating, anti-inflammatory, hepatoprotective effect, and sedative effect (Bai et al., Citation2016; Damiano et al., Citation2017; Li et al., Citation2017), which are believed to be attributed by a variety of the phytochemicals found in the jujube including flavonoids, polyphenols, alkaloids, saponins and polysaccharides. Jiang, Huang, Chen, and Lin (Citation2007) found that saponins had more effective sedative and hypnotic functions than that of flavonoids, while polysaccharides didn’t show either sedative or hypnotic effects. Jujubosides were reported to be responsible for the anxiolytic and sedative effects (Cao et al., Citation2010). In a more recent paper, it was reported that jujuboside A, jujuboside B and jujubogenin exhibited significant effects on the expression and activation of γ-amino-butyric acid A (GABA(A)) receptors, which accordingly resulted in sedative effect on Sprague−Dawley (SD) rats (Song et al., Citation2017). It makes us wonder how much fruit should be eaten to achieve a sedative effect. In our previous study, the yield of jujube fraction which mainly contained saponins was less than 0.02% (0.19 mg.g−1.DW) (Pu, Ding, Zhang, Jiang, & Liu, Citation2017). The total content of JuA and JuB was less than 0.8 mg.g−1 in jujube fruit reported by Zhao, Li, Yang, Li, and Wang (Citation2006), and the dosage for sedative effect has been reported to be either 40 mg.kg−1, 25 mg.kg−1 or 9 mg.kg−1 reported by Jiang et al. (Citation2007), Song et al. (Citation2017) and Cao et al. (Citation2010), respectively. If the data of saponins content (0.8 mg.g−1) and the dosage (9 mg.kg−1) were adopted to calculate the suitable amount of jujube consumption, a person (about 80 kg) should eat at least 0.9 kg jujube fruit for achieving a sedative effect. This is far more than the normal consumption amount (about 0.1 kg), so we inferred that the sedative effects of jujube fruit should probably be attributed to other components, rather than saponins. Sedative effects related to the expression and activation of γ-amino-butyric acid A (GABA(A)) receptors (Song et al., Citation2017), and γ-aminobutyric acid (GABA) is well established as the primary inhibitory transmitter acting at ionotropic GABA A receptors (Bowery Citation2006). So, we speculated that the sedative effect of jujube fruit might be related to GABA.
GABA is a non-proteinaceous amino acid, and it is widely distributed in species ranging from microorganisms to vertebrates and plants (Meeploy & Deewatthanawong, Citation2016). It has been recognized as a major inhibitory neurotransmitter in the central nervous system (CNS) of vertebrates, playing a key role in maintaining mental health (Katsuno et al., Citation2015). Furthermore, it exerts a series of physiological functions including lowering blood pressure, improving memory, inhibiting cancer cell proliferation, enhancing immunity, reducing sleeplessness, and others (Suwanmanon & Hsieh, Citation2013; Woraharn et al., Citation2015). These functions of GABA are quite similar to those which have been described following consumption of jujube fruit. The concentrations of GABA in some fruits have been reported previously, including rambutan (Meeploy & Deewatthanawong, Citation2016), strawberry (Deewatthanawong, Nock, & Watkins, Citation2010), grape (Ramesh, Tyerman, Gilliham, & Xu, Citation2017), loquat (Cao, Cai, Yang, & Zheng, Citation2012), logan (Zhou, Ndeurumio, Zhao, & Hu, Citation2016) and litchi (Zi-Chen et al., Citation2016). However, to the best of our knowledge, no studies have been previously reported the presence of GABA in jujube. Therefore, for better understanding the functions of jujube fruit, we chose to investigate whether GABA was present in jujube fruit.
In this study, the GABA contents of six promising cultivars were evaluated, and the effects of harvest, drying and storage on GABA were also investigated. The results might be useful to understand the functional properties of jujube fruit, and also be of benefit to breed jujube cultivars, optimize harvest time and develop strategies for processing and storage.
Materials and methods
Chemicals
ϒ-aminobutyric acid (GABA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade methanol was obtained from Tedia Company, Inc (Fairfield, USA), Ortho-phthalaldehyde (OPA), β-mercaptoethanol (BME), sodium tetraborate, ascorbic acid, formic acid and other analytical grade chemicals were obtained from J&K Chemical Ltd (Shanghai, China).
Materials and pretreatment
For investigating the GABA content of fresh jujubes, the six promising cultivars were collected from the local orchards located in Xinjiang, China. All the samples (Z. jujuba. cv. Qiyuexian (ZQY), Z. jujuba. cv. Zanhuangzao (ZZH), Z. jujuba Mill. cv. Dongzao (ZDZ), Z. jujuba Mill.cv.Huizao (ZHZ), Z. jujuba Mill.cv.Junzao (ZJZ) and Z. jujuba Mill.cv.Junyou (ZJY)) were harvested on October 6th, 2016. The samples (about 3 kg per cultivar) were picked with a full particle, intact appearance and similar morphological properties, randomly divided into three shares, deposited in zipper bags and immediately transferred to the laboratory under ambient temperature. Then, the fruits were washed with tap-water and cut into about 0.3 mm slices, and then freeze-dried and ground into powder using a commercial blender. Finally, all samples were packed in zipper bags and stored at −20°C for analysis. The sample treatments were started less than 4 h after harvest.
For investigating the effect of harvest, drying and storage on GABA, Z. jujuba Mill.cv.Junzao (ZJZ) was chosen in this study, because it is one of the most common widely cultivated cultivars for dried fruit production (Pu et al., Citation2018). A total of five samples, including FF1: fresh jujube fruit was harvested on October 6th, 2016, FF2: fresh jujube fruit was harvested on November 8th, 2016, DF: FF2 was dried by hot air at 55°C for 24 h, SF1: DF was packed in zipper bags and stored at ambient temperature for 6 months, and SF2: DF was packed in zipper bags and stored at ambient temperature for 12 months, were carefully prepared for this purpose. All samples (about 3 kg per sample) were cut in half for removing kernels, and then freeze-dried and ground into powder as described above.
Extraction, derivatization and HPLC analysis
Jujube samples were extracted with deionized water at ambient temperature for 30 min using sonication, and the extract was centrifuged at 3,000 rpm for 15 min. The supernatant was stored at 4°C for less than 3 days before analysis.
The derivatization reagent was prepared according to the method described by Sari and Velioglu (Citation2011) with little modification. Briefly, 150 mg o-phthaldialdehyde was mixed with 3 mL methanol, 27 mL 0.1 M sodium tetraborate (pH 9.5), 500 μL β-mercaptoethanol and 30mg ascorbic acid. The derivatization reagent should be prepared one day before analysis.
For sample analysis, 700 μL sample was mixed with equal volume derivatization reagent, vortex mixed for 10 s, filtered and injected into HPLC system. The chromatographic system consisted of a Waters 2695–2998 system (Waters Corp., Milford, MA, USA). The column was an Inertsil ODS-3 (250 × 4.6 mm, i.d.) column (Kyoto, Japan), and the column was maintained at 30°C. The spectrum of GABA was recorded at 338 nm. The elution solvents were methanol (A) and 0.05% formic acid (B). The flow rate was 0.5 mL.min−1, and the injection volume was 10 μL. The gradient program was as follows: 0-15min, 35%-28% of B; 15-17min, 28%-35% of B; 17-25min, 35% of B.
Statistical analysis
All the samples were measured in triplicate, and the results were expressed as the mean and standard deviation. The data was subjected to one-way variance analysis, and the significant differences were compared by the Duncan’s test (p ≤ 0.05). All data was processed by using the SPSS statistical software (Version: 20.0, USA).
Results and discussion
Identification of GABA in jujube fruit
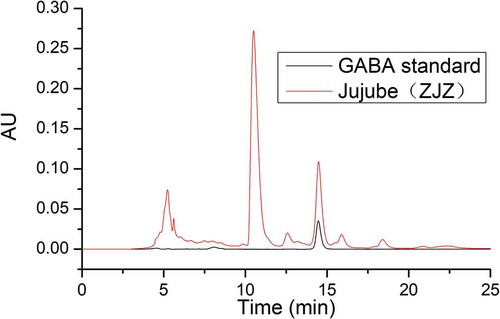
The chromatograms of GABA standard and jujube (ZJZ) were showed in , both of which exhibited very high similarity. In addition, the chromatographic peak of GABA was the second highest peak in the chromatogram of jujube sample. The results show that GABA is present in jujube fruit, in a relatively high amount, as measured by the present method.
GABA of fresh jujubes
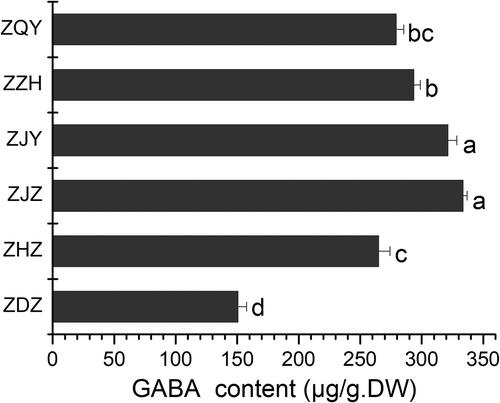
The GABA contents of fresh jujubes from six cultivars are shown in . There were significant differences in concentration between these cultivars (P<0.05). ZJZ had the highest GABA of 140.28 µg.g−1.FW or 333.37 µg.g−1.DW, while ZDZ had the lowest of 51.16 µg.g−1.FW or 150.31 µg.g−1.DW. The concentrations of GABA in some fruits have been reported previously, such as rambutan 710 µg.g−1.FW (Meeploy & Deewatthanawong, Citation2016), strawberry 1560–3640 µg.g−1.FW (Deewatthanawong et al., Citation2010), grape 146 µg.g−1.FW (Ramesh et al., Citation2017), loquat 15.6–36.4 µg.g−1.FW (Cao et al., Citation2012), logan 1998 µg.g−1 (Zhou et al., Citation2016) and litchi 1700–3500 µg.g−1.FW (Zi-Chen et al., Citation2016). Compared with these fruits, jujube fruit contains a relatively low amount of GABA.
Figure 2. Contents of GABA in fresh fruit of six jujube cultivars. ZQY: Z. jujube Mill.cv.Qiyuexian, ZZH: Z. jujube Mill.cv.Zanhuangzao, ZJY: Z. jujuba Mill.cv.Junyou, ZJZ: Z. jujuba Mill.cv.Junzao, ZHZ: Z. jujube Mill.cv.Huizao and ZDZ: Z. jujuba Mill. cv. Dongzao. Different letters in each column indicate significant difference (p ≤ 0.05 and n = 3), according to Tukey’s test.
Figura 2. Contenido de GABA en la fruta fresca de seis cultivares de azufaifo. ZQY: Z. jujube Mill.cv.Qiyuexian, ZZH: Z. jujube Mill.cv.Zanhuangzao, ZJY: Z. jujuba Mill.cv.Junyou, ZJZ: Z. jujuba Mill.cv.Junzao, ZHZ: Z. jujube Mill .cv.Huizao y ZDZ: Z. jujuba Mill. cv. Dongzao. Las diferentes letras en cada columna indican una diferencia significativa (p ≤ 0.05 y n = 3), según la prueba de Tukey.
Okada et al. (Citation2000) reported that when a total amount of 26.4 mg GABA was consumed daily, symptoms such as sleeplessness, somnipathy (sleep disturbance) and depression can be improved in more than 65% of the patients with such symptoms. In a randomized, placebo-controlled trial, a dose of 10–12 mg GABA in 100 mL fermented milk significantly decreased blood pressure within 2 or 4 weeks in a 12 week study in 39 mildly hypertensive patients (16 women and 23 men) aged 28–81 y (mean, 54.2 y) (Inoue et al., Citation2003). A comprehensive review by Diana, Quilez, and Rafecas (Citation2014) summarized that, for humans, an oral administration of food containing GABA ranging from 10–70 mg daily was effective to lower blood pressure. According to these findings, daily consumption of about 200g fresh fruit (their moisture ranged from 56.81%-58.88%), except the ZDZ strain (its moisture,65.96%), would contain enough GABA to achieve sedative effect calculated according to the data of 26.4 mg reported by Okada et al. (Citation2000), as well being of partial benefit as an antihypertensive food according to the data of 10–70 mg summarized by Diana et al. (Citation2014).
Effect of harvest, drying and storage on GABA of jujube fruit
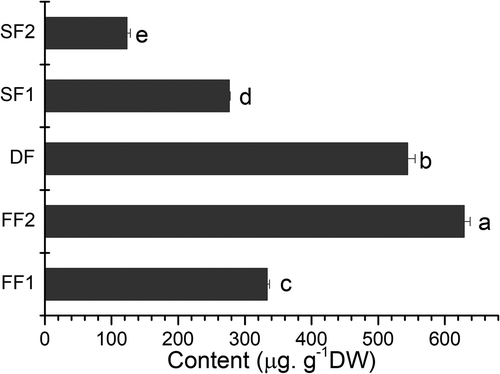
shows the concentration of GABA in jujube fruit. Harvest, drying and storage had significant influence on the levels of GABA. To our surprise, fresh jujube (FF2) showed the highest amount of GABA of 628.74 ± 9.24 µg.g−1.DW, which was nearly twice as high as that of FF1. Actually, there was about one month difference in harvest time between FF1 and FF2, because the fresh fruits for table fruit (FF1) were picked at the beginning of October, while those for drying (FF2) were picked at the beginning of November. In general, the GABA content in plant tissues is very low ranging from 3.12 to 624 µg.g −1.FW, but prone to large and rapid increases (<1000-fold) following exposure to a multitude of biotic and abiotic stresses (Ramesh et al., Citation2015, Citation2017). A number of publications have reported that GABA increased in vegetables and fruits through the treatment of cold stress. Within 5min of mechanical or cold stimulation, the GABA concentration in soybean leaves increased to 1 to 2 µmol.g−1.FW, a 20- to 40- fold increase (Shelp, Bown, & McLean, Citation1997). After precooling treatment, the GABA concentration of longan was increased by 89 percent (Zhou et al., Citation2016). The greenhouse grown-spinach exposed to more than 14 days of cold stress (4–7°C) recorded considerably higher GABA content than the control (Yoona et al., Citation2017). Cao et al. (Citation2012) found that when loquat fruits were stored at 1°C for 35 days, its GABA content nearly doubled. Yang, Cao, Yang, Cai, and Zheng (Citation2011) reported that GABA could induce chilling-tolerance of peach fruit by improving antioxidant enzyme activities and maintaining higher levels of ATP content and energy charge thereby protecting membranes from chilling damage. Moreover, a comprehensive review by Kinnersley and Turano (Citation2010) suggested that environmental stresses increase GABA accumulation through two different mechanisms. Firstly, stresses that caused metabolic and/or mechanical disruptions resulting in cytosolic acidification and also induce an acidic pH-dependent activation of glutamate decarboxylase and GABA synthesis. Secondly, stresses which include cold, heat, salt, and mild or transient environmental factors that increase cellular levels of Ca2+. Increased cytosolic Ca2+ stimulates calmodulin-dependent glutamate decarboxylase activity and GABA synthesis. From the beginning of October to the beginning of November, it is just the late autumn in Xinjiang, and the temperature usually falls below zero at night. This means that jujube fruit has naturally experienced a period of cold stress before harvest. These results suggest that the later jujube fruit is harvested, not only the less energy will be used for drying, but the more GABA will accumulate probably due to cold stress.
Figure 3. Effect of harvest, drying and storage on GABA in jujube fruit (Z. jujuba Mill.cv.Junzao). FF1: fresh jujube fruit was harvested on October 6th, 2016, FF2: fresh jujube fruit was harvested on November 8th, 2016, DF: FF2 was dried by hot air at 55°C for 24 h, SF1: DF was stored at ambient temperature for 6 months, and SF2: DF was stored at ambient temperature for 12 months. Different letters in each column indicate significant difference (p ≤ 0.05 and n = 3), according to Tukey’s test.
Figura 3. Efecto de la cosecha, el secado y el almacenamiento en el GABA de la fruta de azufaifo (Z. jujuba Mill.cv.Junzao). FF1: La fruta fresca de azufaifo se cosechó el 6 de octubre de 2016, FF2: la fruta fresca de azufaifo se cosechó el 8 de noviembre de 2016, DF: FF2 se secó con aire caliente a 55°C durante 24 h, SF1: DF se almacenó a temperatura ambiente durante 6 meses, y SF2: DF se almacenó a temperatura ambiente durante 12 meses. Las diferentes letras en cada columna indican una diferencia significativa (p ≤ 0.05 y n = 3), según la prueba de Tukey.
As can be seen from , the effect of drying and storage had significant and negative influence on the levels of GABA. In comparison with fresh jujube (FF2), the GABA content showed decreases of 13.50% in dried jujube fruit (DF), which was dried by hot air at 55°C for 24 h. This result is in line with that reported by Wang, Liu, Zhao, Qiu, and Zhuang (Citation2015), who found that temperature played a critical role in the loss of GABA during fortified milk production. When compared with dried jujube fruit (DF), the GABA content showed decreases of 49.18% (SF1, stored for 6 months) and 77.44% (SF2, stored for 12 months), respectively (). These results suggested that GABA of dried jujube fruit is prone to decrease during storage at ambient temperature, and suitable storage technologies should be developed for reducing the losses of GABA and other bioactive components in dried jujube fruit.
According to the present results, daily consumption of 66 g fresh fruit (FF’s moisture, 36.44%) or 61 g dried fruit (DF’s moisture, 20.03%) might contain sufficient GABA to produce the effects of improving sleep and lowering blood pressure. However, this suggestion would need to be tested in human trials, perhaps using a dose response strategy to look for graded effects. These amount of jujube consumption comprise nearly three fruits (Z. jujuba cv. Junzao), which happen to coincide with the Chinese proverb “Three jujubes a day keeps the doctor away and keeps you young”.
Conclusion
The present work confirmed that jujube fruit contains GABA. The GABA of fresh jujube can be increased by harvesting later probably due to cold stress, while decreased during drying and storage. According to the amount of jujube fruit consumption, GABA might contribute to the suggested health functions of jujube fruit. Further work should be done on the investigation of more jujube cultivars, optimization of harvest time and development of processing techniques for retaining as much GABA as possible during drying and storage.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bai, L., Zhang, H., Liu, Q., Zhao, Y., Cui, X., Guo, S., … Bai, N. (2016). Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food & Function, 7(6), 2870–2877.
- Bowery, N. G. (2006). GABAB receptor: A site of therapeutic benefit. Current Opinion in Pharmacology, 6, 37–43.
- Cao, J.-X., Zhang, Q.-Y., Cui, S.-Y., Cui, X.-Y., Zhang, J., Zhang, Y.-H., … Zhao, -Y.-Y. (2010). Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. Journal of Ethnopharmacology, 130(1), 163–166.
- Cao, S., Cai, Y., Yang, Z., & Zheng, Y. (2012). MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chemistry, 133(4), 1466–1470.
- Chen, J., Zhonggui, L., Maiwulanjiang, M., Zhang, W. L., Zhan, J. Y. X., Lam, C. T. W., … Tsim, K. W. K. (2013). Chemical and biological assessment of Ziziphus jujuba fruits from China: Different geographical sources and developmental stages. Journal of Agricultural and Food Chemistry, 61(30), 7315–7324.
- Commission, C. P. (2015). The Chinese pharmacopoeia (Vol. 1, pp. 21–22). Beijing: Chemical Industry Press.
- Damiano, S., Forino, M., Arpan, D., Vitali, L. A., Lupidi, G., & Taglialatela-Scafati, O. (2017). Antioxidant and antibiofilm activities of secondary metabolites from Ziziphus jujuba leaves used for infusion preparation. Food Chemistry, 230, 24–29.
- Deewatthanawong, R., Nock, J. F., & Watkins, C. B. (2010). γ-aminobutyric acid (GABA) accumulation in four strawberry cultivars in response to elevated CO2 storage. Postharvest Biology and Technology, 57(2), 92–96.
- Diana, M., Quilez, J., & Rafecas, M. (2014). Gamma-aminobutyric acid as a bioactive compound in foods: A review. Journal of Functional Foods, 10, 407–420.
- Finotti, E., Bersani, E., Del Prete, E., & Friedman, M. (2015). Application of a functional mathematical index (FMI) for predicting effects of the composition of jujube fruit on nutritional quality and health. Journal of Food Composition and Analysis, 42, 164–170.
- Hernández, F., Noguera-Artiaga, L., Burló, F., Wojdyło, A., Áa, C.-B., & Legua, P. (2016). Physico-chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. Journal of the Science of Food and Agriculture, 96(8), 2682–2691.
- Huang, B. (2017). The Chinese Rural Statistc Yearbook. Beijing: China Statistic Press.
- Inoue, K., Shirai, T., Ochiai, H., Kasao, M., Hayakawa, K., Kimura, M., & Sansawa, H. (2003). Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives. European Journal of Clinical Nutrition, 57, 490–495.
- Jiang, J.-G., Huang, X.-J., Chen, J., & Lin, Q.-S. (2007). Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Natural Product Research, 21(4), 310–320.
- Katsuno, N., Sakamoto, C., Yabe, T., Yamauchi, R., Nishizu, T., & Kato, K. (2015). Methods for enrichment of γ-aminobutyric acid in sesame seeds. Food Science and Technology Research, 21(6), 787–791.
- Kinnersley, A. M., & Turano, F. J. (2010). Gamma aminobutyric acid (GABA) and plant responses to stress. Critical Reviews in Plant Sciences, 19(6), 479–509.
- Li, X., Li, X., Zhou, B., Man, S., Gao, W., & Jing, S. (2017). Study on the bioactive constituents and in vitro antioxidant and in vivo anti-inflammatory activities of extracts from the fruits of ziziphus jujuba mill. cv. jinsixiaozao hort. Food Science and Technology Research, 23(3), 417–426.
- Meeploy, M., & Deewatthanawong, R. (2016). Determination of gamma-aminobutyric acid (GABA) in rambutan fruit cv. rongrian by HPLC-ELSD and separation of GABA from rambutan fruit using dowex 50W-X8 column. Journal of Chromatographic Science, 54(3), 445–452.
- Okada, T., Sugishita, T., Murakami, T., Murai, H., Saikusa, T., Horino, T., … Takahashi, T. (2000). Effect of the defatted rice germ enriched with GABA for sleeplessness depression, autonomic disorder by oral administration. Journal of the Japanese Society for Food Science and Technology, 47(8), 596–603.
- Pu, Y., Ding, T., Wang, W., Xiang, Y., Ye, X., Li, M., & Liu, D. (2018). Effect of harvest, drying and storage on the bitterness, moisture, sugars, free amino acids and phenolic compounds of jujube fruit (Zizyphus jujuba cv. Junzao). Journal of the Science of Food and Agriculture, 98(2), 628–634.
- Pu, Y., Ding, T., Zhang, N., Jiang, P., & Liu, D. (2017). Identification of bitter compounds from dried fruit of Ziziphus jujuba cv. Junzao. International Journal of Food Properties, 20(sup1), S26–S35.
- Ramesh, S. A., Tyerman, S. D., Bo, X., Bose, J., Kaur, S., Conn, V., … Gilliham, M. (2015). GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nature Communications, 6, 7879.
- Ramesh, S. A., Tyerman, S. D., Gilliham, M., & Xu B. (2017). gamma-aminobutyric acid (GABA) signalling in plants. Cellular and Molecular Life Sciences, 74(9), 1577–1603.
- Rostami, H. G., & Taghi, S. M. (2017). Mathematical modeling of mucilage extraction kinetic from the waste hydrolysates of fruiting bodies of zizyphus jujuba mill. Journal of Food Processing and Preservation, 41(4), e13064.
- Sari, F., & Velioglu, Y. S. (2011). Effects of particle size, extraction time and temperature, and derivatization time on determination of theanine in tea. Journal of Food Composition and Analysis, 24(8), 1130–1135.
- Shelp, B. J., Bown, A. W., & McLean, M. D. (1997). Metabolism and functions of gamma-aminobutyric acid. Trends in Plant Science, 11(4), 446–452.
- Song, P., Zhang, Y., Ma, G., Zhang, Y., Zhou, A., & Xie, J. (2017). Gastrointestinal absorption and metabolic dynamics of Jujuboside A, A saponin derived from the seed of ziziphus jujuba. Journal of Agricultural and Food Chemistry, 65(38), 8331–8339.
- Suwanmanon, K., & Hsieh, P.-C. (2013). IsolatingBacillus subtilisand optimizing its fermentative medium for GABA and nattokinase production. CyTA - Journal of Food, 12(3), 282–290.
- Wang, Y., Liu, M., Zhao, L., Qiu, Y., & Zhuang, Y. (2015). Influence of processing conditions on reducing γ-aminobutyric acid content during fortified milk production. Food Research International, 72, 215–222.
- Woraharn, S., Lailerd, N., Sivamaruthi, B. S., Wangcharoen, W., Sirisattha, S., Peerajan, S., & Chaiyasut, C. (2015). Evaluation of factors that influence the L-glutamic and γ-aminobutyric acid production duringHericium erinaceusfermentation by lactic acid bacteria. CyTA - Journal of Food, 14(1), 47–54.
- Xie, P.-J., Youa, F., Huang, L.-X., & Zhang, C.-H. (2017). Comprehensive assessment of phenolic compounds and antioxidant performance in the developmental process of jujube (Ziziphus jujuba Mill.). Journal of Functional Foods, 36, 233–242.
- Yang, A., Cao, S., Yang, Z., Cai, Y., & Zheng, Y. (2011). γ-aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chemistry, 129(4), 1619–1622.
- Yoona, Y.-E., Kuppusamy, S., Cho, K. M., Kim, P. J., Kwack, Y.-B., & Lee, Y. B. (2017). Influence of cold stress on contents of soluble sugars, vitamin C and free amino acids including gamma-aminobutyric acid (GABA) in spinach (Spinacia oleracea). Food Chemistry, 215, 185–192.
- Zhan, R., Xia, L., Shao, J., Wang, C., & Chen, D. (2018). Polysaccharide isolated from Chinese jujube fruit (Zizyphus jujuba cv. Junzao) exerts anti-inflammatory effects through MAPK signaling. Journal of Functional Foods, 40, 461–470.
- Zhao, J., Li, S. P., Yang, F. Q., Li, P., & Wang, Y. T. (2006). Simultaneous determination of saponins and fatty acids in Ziziphus jujuba (Suanzaoren) by high performance liquid chromatography-evaporative light scattering detection and pressurized liquid extraction. Journal of Chromatography, A 1108(2), 188–194.
- Zhou, M., Ndeurumio, K. H., Zhao, L., & Hu, Z. (2016). Impact of precooling and controlled-atmosphere storage on γ-aminobutyric acid (GABA) accumulation in longan (Dimocarpus longan Lour.) fruit. Journal of Agricultural and Food Chemistry, 64(33), 6443–6450.
- Zi-Chen, W., Yang, Z.-Y., Jian-Guo, L., Chen, H.-B., Huang, X.-M., & Wang, H.-C. (2016). Methyl-inositol, gamma-aminobutyric acid and other health benefit compounds in the aril of litchi. International Journal of Food Sciences and Nutrition, 67(7), 762–772.