ABSTRACT
The swollen succulent stem of tumorous stem mustard (Brassica juncea var. tumida Tsen et Lee) is the raw material in the processing of Chinese Fuling zhàcài. We found that total polyphenols and flavonoids contents of tumorous stem mustard leaf extracts (TSMLE) were significant higher than that of tumorous stem mustard stem extracts (TSMSE). The percentage of isorhamnetin 5-O-hexoside, methyl quercetin O-hexoside, and luteolin O-hexosyl-O-hexosyl-O-hexoside in TSMLE were 170, 230 and 694 times higher than that in TSMSE. TSMLE presented stronger antioxidant capacity against 2,2'-Azino-bis[3-ethylbenzothiazoline]-6-sulfonic acid cationic free radical (ABTS+) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals, and it also showed higher reducing power and ferric reducing antioxidant power. Both TSMLE and TSMSE inhibited lung carcinoma cell growth in a dose-dependent manner, while TSMLE was more effective against A549 cells than TSMSE. This is the first report indicates that tumorous stem mustard, especially its leaf, is a potential diet source in preventing of oxidative stress and cancer.
RESUMEN
El tallo hinchado y suculento de la mostaza de tallo tumoral (Brassica juncea var. tumida Tsen et Lee) es la materia prima para el procesamiento de la verdura china Fuling zhàcài. Al respecto, se constató un contenido total de polifenoles y flavonoides significativamente más alto en los extractos de hojas de mostaza de tallos tumorales (TSMLE) que en los extractos de tallos de mostaza de tallos tumorales (TSMSE). El porcentaje de isorhamnetina 5-O-hexosa, metil quercetina O-hexosa y luteolina O-hexosil-O-hexosil-O-hexosa presente en TSMLE fue 170, 230 y 694 veces mayor que en TSMSE. Asimismo, los TSMLE mostraron mayor capacidad antioxidante contra los radicales libres ABTS + y DPPH, además de un poder reductor y un poder antioxidante reductor del ion férrico más elevados. Tanto los TSMLE como los TSMSE inhibieron el crecimiento celular del carcinoma de pulmón de manera dependiente de la dosis, mientras que los TSMLE fueron más eficaces contra las células A549 que los TSMSE. Este es el primer informe que indica que la mostaza de tallo tumoral, especialmente su hoja, es una fuente dietética con potencial para prevenir el estrés oxidativo y el cáncer.
1. Introduction
Tumorous stem mustard (Brassica juncea var. tumida Tsen et Lee, )), a native cruciferous vegetable crop in Asia countries, is a very important fresh and processed vegetable in winter and spring (Xie et al., Citation2014). The swollen succulent stem of tumorous stem mustard is the critical raw material in the processing of Chinese Fuling zhàcài, which is as famous as French sour-cucumber and German sweet-sour-cabbage. However, thousands of tons of tumorous stem mustard leaf was discarded in farmland per year since zhàcài become a commodity in China from 1899 (Yuan, Citation2016). This not only causes an environmental problem but also is a great waste of dietary resources. Although previous literature has been reported regarding phenolic compounds of tumorous stem mustard leaf (Xie et al., Citation2014), a large percentage of compounds present remain unknown and need to be identified before the health-promoting properties of tumorous stem mustard is properly elucidated.
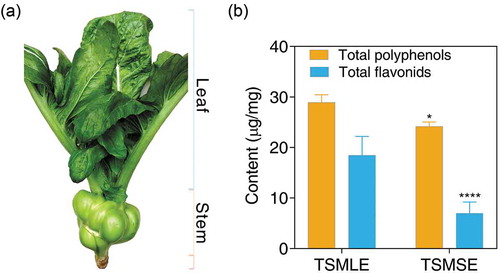
Figure 1. Plant of tumorous stem mustard (Brassica juncea var. tumida Tsen et Lee) (a); and total polyphenols and total flavonoids contents in extracts from tumorous stem mustard leaf and stem, respectively (b). TSMLE, tumorous stem mustard leaf extract; TSMSE, tumorous stem mustard stem extract. *p< 0.05, ***p< 0.001, as compared to the TSMLE.
Figura 1. Planta de mostaza de tallo tumoral (Brassica juncea var. tumida Tsen et Lee) (A); y contenido de polifenoles totales y flavonoides totales en extractos de hoja y tallo de mostaza de tallo tumoral, respectivamente (B). TSMLE, extracto de hoja de mostaza de tallo tumoral; TSMSE, extracto de tallo de mostaza de tallo tumoral. *p< 0.05, ***p<0.001, en comparación con el TSMLE.
Malignant neoplasms have become one of the leading causes of death, only second to heart diseases (Li et al., Citation2013). Among them lung cancer is now the most common cause of cancer-related deaths, which accounts for more than one million worldwide annual deaths (Lee, Kang, Jung, Kim, & Kim, Citation2011). Recently, it is reported that a high intake of vegetables is one of the cornerstones of a healthy diet and has been recommended to the general public to reduce the risk of cancer (Aune et al., Citation2017). The increasing researches also indicate that vegetal foods and nutrients play an important role in the prevention of cancer development (Key et al., Citation2004). In this regard, ingestion of leaf and swollen succulent stem of tumorous stem mustard may be a potential strategy for anticancer. Therefore, the main endeavor of the current study was to investigate the potential anti-proliferation activity of tumorous stem mustard leaf and stem extracts. To further illuminate mechanism and material basis of the anti-proliferation activity, the antioxidant activity was assessed in vitro, and the phytochemistry profiles were determined by ultra-performance liquid chromatography tandem mass spectrometry.
2. Materials and methods
2.1. Materials and reagents
The entire plant of fresh tumorous stem mustard (Brassica juncea var. tumida Tsen et Lee) was obtained from a local agricultural market. Human lung carcinoma cell (A549) was obtained from Cell Bank of Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai,China). Dimethyl sulfo-xide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dihydroethidium (DHE) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) and trichloroacetic acid were provided from AppliChem (Darmstadt, Germany). Tea polyphenols (phenolics >95%) was obtained from Shanghai yuanye biotechnology co. LTD (Shanghai, China). Chromatographic grade acetonitrile and methanol were purchased from Kemiou Chemical Reagent Co., Ltd (Tianjin, China). Deionized water was obtained from Hangzhou Wahaha Group Co., Ltd (Hangzhou, China). All the cell culture reagents were purchased from Sinopharm (Beijing, China). All other chemical reagents were analytical reagents.
2.2. Preparation of tumorous stem mustard extracts
The extraction was performed as described previously (Han, Li, Huang, & Yang, Citation2016). At first, the rootless tumorous stem mustard leaf and swollen succulent stem were separately homogenated. The homogenates were extracted with two-fold (w/v) 80% methanol in 1000 mL conical flask at 70°C for 60 min for three times. After vacuum filtration, filter liquors from each extraction were combined and cooled to room temperature. The methanol extract was concentrated at 45°C with a vacuum rotary evaporator (RE-52AA, Shanghai arong biochemical instrument factory, Shanghai, China). After that, the concentrated solution was further extracted by ethyl acetate in a 1:2 ratio (v/v) for three times to isolate and enrich lipophilic phytochemicals. The extracted solution of ethyl acetate was concentrated 45°C with a vacuum rotary evaporator. The residual aqueous solution (about 50 mL) containing phytochemicals was freeze-dried (RLPHR 1–2 LD, Martin Christ Gefriertrocknungsanlagen, Osterode am Harz Germany). The extracts yielded green and white powder, defined as TSMLE (tumorous stem mustard leaf extracts) and TSMSE (tumorous stem mustard stem extracts) from tumorous stem mustard leaf and swollen succulent stem, respectively.
2.3. Spectrophotometric determination of total polyphenols and total flavonoids
The total polyphenols in the extracts were analyzed using Folin-Ciocalteu’s colorimetric assay as described by Nie, Ren, Lu, Sun, and Yang (Citation2015). The powder of TSMLE and TSMSE were dissolved in 80% methanol (w/v = 2:1). Fifty microliters of the measured solution and 10 μL Folin-Ciocalteu reagent were mixed in 96-well plates. After six minutes, 7% Na2CO3 (100 μL) and deionized water (80 μL) were added and mixed thoroughly. After 90 min of incubation in darkness at room temperature, the absorbance at 760 nm was measured (PT-3502C, Beijing putian new bridge technology co. LTD., Beijing, China). The results were expressed as microgramme of gallic acid equivalent per milligram of leaf or stem extracts (μg GAE/mg, y = 70.421x – 6.0351, R2 = 0.9946). All detections were performed in five repetitions.
The total flavonoids contents of extracts were measured by the method reported by González-Barrio, Periago, Luna-Recio, Javier, and Navarro-González (Citation2018), with some modification. Aliquots of 10 μL measured solution were mixed with 5% NaNO2 (10 μL). After 6 min, 10 μL of 10% AlNO3 was added and the mixture was left for another 6 min before the addition of 4% NaOH (100 μL), and 60% ethanol (120 μL) was added, followed by a thorough mixing and further standing for 15 min. The absorbance of the mixture was determined at 510 nm using a microplate reader (PT-3502C, Beijing putian new bridge technology co. LTD., Beijing, China). The results were expressed as microgramme of rutin equivalent per milligram of leaf or stem extracts (μg RE/mg, y = 3169.5x – 160.42, R2 = 0.9960). All detections were carried out in five repetitions.
2.4. Identification of phytochemicals by UPLC-MS/MS analysis
The measured solution was diluted (v/v = 1:2) with 80% methanol, and then each sample was centrifuged and filtered with a 0.22 μm PVDF membrane for ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) analysis. A 30A liquid chromatography system (Shimadzu, Tokyo, Japan), equipped with a binary pump and coupled to a 4500 QTRAP mass spectrometer detector (AB Sciex Pte. Ltd., CA, USA), was used. UPLC liquid chromatography was performed using a Waters ACQUITY UPLC HSS T3 C18 column (1.8 µm, 2.1 mm × 100 mm), which was maintained at 40°C. Elution with solvent A (water with 0.04% acetic acid, v/v) and solvent B (acetonitrile with 0.04% acetic acid, v/v) in a linear gradient manner was conducted at a flow rate of 0.4 mL/min as: 5%-95% B (0–11 min), 95%-95% B (11–12 min), 95%-5% B (12–15 min). The injection volume was 5 μL, and source conditions were the following: electrospray ionization (550°C), capillary voltage was 5.5 kV and curtain gas was 25 psi.
To facilitate phytochemicals identification, our collaborator (Metware, Wuhan, China) established the Metware database (MWDB). On the basis of accurate mass and obtained migration time of these phytochemicals, peak correction and peak integration method was edited using MultiaQuant (AB Sciex Pte. Ltd., CA, USA). Furthermore, these phytochemicals were also verified based on two main online databases: HMDB and Metlin.
2.5. Determination of antioxidant capacity
2.5.1. DPPH radical scavenging activity
DPPH radical scavenging activity was evaluated according to the method reported by Bannour et al. (Citation2017) with slight modification. Briefly, 150 μL of measured solutions at various concentrations (0, 0.4, 0.8, 1.2, 1.6, and 2.0 mg/mL) were mixed with 150 μL DPPH· working solution (1 mmo1/L) in a 96-well plate and allowed to react at room temperature for 30 min in dark before the absorbance was recorded at 517 nm. The scavenging activity against DPPH· (%) = [1-(Ai-Aj)/Ac] × 100%, where Ac was the absorbance without the sample, Ai was the absorbance with the sample, and Aj was the absorbance of ground color.
2.5.2. ABTS radical scavenging activity
ABTS assay was carried out by using a modified method reported earlier (Peng, Li, Li, Deng, & Zhang, Citation2017). Briefly, the ABTS·+ solution was prepared by adding 100 mL of ABTS solution (7 mmol/L) to 100 mL of K2S2O8 (2.45 mmol/L) and the mixture was kept in the dark at room temperature for 24 h before analysis. The ABTS·+ working solution was diluted with 80% ethanol to the absorbance 0.70 ± 0.05 at 734 nm. Ten microliters of samples were reacted with 200 μL of ABTS·+ working solution in the dark at room temperature for 5 min in a 96-well plate and the absorbance was measured at 734 nm. The scavenging activity against ABTS+ (%) = [1-(A1-A2)/A0]×100%, where A0 was the absorbance without the sample, A1 was the absorbance with the sample, and A2 was the absorbance of ground color.
2.5.3. Ferric reducing antioxidant power (FRAP assay)
FRAP assay followed the method in the previous report with some modifications (Bannour et al., Citation2017). Fifty microliters of samples were reacted with 100 μL of ferric-TPTZ reagent (prepared by mixing 0.3 mol·L−1 acetate buffer, pH = 3.6, 10 mmol·L−1 TPTZ, 20 mmol·L−1 FeCl3 at ratio of 10:1:1 (v/v/v)) and 200 μL of distilled water, and the mixed-solution was warmed to 37°C before use. The absorbance reading was taken after 30 min at 593 nm. The increased absorbance of the reaction mixture suggested its enhanced reducing power (Nie et al., Citation2015).
2.5.4. Measurement of total reducing power
The total reducing power (TRP) of the two extracts was determined as reported by Ren, Zhao, Nie, Yang, and Yang (Citation2014). Two hundred microliters of extracts were combined with 250 μL of 0.2 mol/L phosphate buffer saline (pH = 6.6) and 250 μL of 1% K3Fe(CN)6 solution (w/v). The mixture was incubated at 50°C for 20 min, and then 250 μL of 10% TCA solution was added, and the mixture was centrifuged at 1000 rpm/min for 5 min. The supernatant liquor (100 μL) was mixed with 80 μL deionized water and 0.1% FeCl3 solution (w/v), and the absorbance at 700 nm was detected by a microplate reader (PT-3502C, Beijing putian new bridge technology co. LTD., Beijing, China). A high absorbance of the reaction mixture indicated strong reducing power (Nie et al., Citation2015).
2.6. Cell proliferation assessment
The anti-proliferation of lung cancer cell assay was carried out by our previous method (Li, He, Tian, Shi, & Yang, Citation2016). A549 cells (2 × 105 cells/well) were seeded in 96-well plates and treated with different doses of TSMLE or TSMSE at a concentration of 0, 30, 60, 120, 240, and 480 µg/mL at 37°C for 48 h. Moreover, the A549 cells (2 × 105 cells/well) were also treated with TSMLE at a concentration of 0, 30, 60, 120, 240 and 480 µg/mL at 37°C for 12, 24, and 48 h. After the exposure time, 10 μL of MTT solution (5 mg/mL) in phosphate-buffered saline was added to each well at a final concentration of 0.5 mg/mL and then the plate was further incubated for 4 h. The formed crystal formazan was dissolved in 150 μL of a solution containing 10% SDS, 0.01 mol/L hydrochloric acid and 5% isobutyl alcohol. Subsequently, the absorbance was measured at 570 nm, and the percentage of cell survival was expressed as follows: Cell viability = (absorbance values in treated cells/absorbance of control cells) × 100%.
2.7. Reactive oxygen species (ROS) assay in lung carcinoma cells
Intracellular production of ROS, namely, hydrogen peroxide and superoxide anion were measured by the method of Li et al. (Citation2013) with minor modifications. A549 cells (1 × 105 cells/well) were seeded in six-well plates in triplicate and then exposed to TSMLE at two concentration of 60 μg/mL and 120 μg/mL at 37 °C for 12 h. After incubation, cells were detached with trypsin-EDTA and washed twice with phosphate-buffered saline. Then, the cells were exposed to 10 μmol/L DHE probes at 37°C for 30 min. The morphological changes of cells and ROS levels were visualized by fluorescence microscopy (Leica DMIL LED; Leica, Solms, Germany) (Li et al., Citation2016).
2.8. Statistical analysis
Results are expressed as the mean ± standard deviations (SD). Significant differences (p-value <0.05) were assessed through the analysis of ANOVA with Sidak’s multiple comparisons test using the GraphPad Prism 7.0 (GraphPad Software, Inc.).
3. Results and discussion
3.1. Total polyphenols and total flavonoids contents
We found that the extraction yield of TSMLE and TSMSE from leaf and stem of tumorous stem mustard, which was extracted with 80% methanol, was 0.15% and 0.1%, respectively. It is well known that polyphenols are important phytochemicals present in all vegetal tissues, and it plays a key role in preventing diseases such as cancers, cardiovascular diseases, and chronic inflammatory diseases (Díaz-García, Obón, Castellar, Collado, & Alacid, Citation2013). In this work, the total polyphenols contents of TSMLE and TSMSE analyzed using the Folin-Ciocalteu method were 28.92 µg of gallic acid equivalent per milligram of leaf extracts and 24.15 µg of gallic acid equivalent per milligram of stem extracts, respectively ()). Generally, the antioxidant activity of flavonoids was stronger than that of phenolic acids. Thus, the flavonoids levels of the two extracts were assessed in current study. As shown in ), the contents of total flavonoids in TSMLE and TSMSE were 18.44 microgramme of rutin equivalent per milligram of leaf extracts and 6.95 µg of rutin equivalent per milligram of stem extracts, respectively. These quantitative assays indicated that TSMLE contained a significantly higher number of total polyphenols and total flavonoids than TSMSE, suggested that the TSMLE has higher antioxidant activity and anti-tumor activity than TSMSE.
3.2. Phytochemicals profile of tumorous stem mustard leaf and stem extracts
Besides polyphenols, terpenoids, alkaloids, and some vitamins also have strong antioxidant activity and anti-tumor activity (Dillard & German, Citation2000). Taking into consideration the point mentioned above, the targeted-metabolomics analysis was performed to reveal the phytochemicals profile of TSMLE and TSMSE (supplementary Figure S1A, B). The identity of each compound was obtained on the base of the MS2 fragmentation data and retention time by matching them with the characterization data available in our MWDB database and others. The identification of phytochemical compounds by UPLC-MS/MS allowed the characterization of 32 different classes of phytochemicals in the tumorous stem mustard leaf and stem extracts ()). As can been seen in ), amino acids, organic acids, flavonol, nucleotide, and its derivates, hydroxycinnamoyl derivatives, flavanone and flavone accounted for a large proportion in extracts. Of note, the percentages of powerful anti-oxidants and antineoplastics including organic acids, flavonol, coumarins, anthocyanins, and catechin derivatives in TSMLE were higher than that in TSMSE ()), suggested that TSMLE has stronger bioactivity than TSMSE.
Figure 2. Phytochemicals profile of tumorous stem mustard leaf and stem extracts. The percentage of phytochemicals in tumorous stem mustard leaf and stem extracts (a). The main individual organic acids, polyphenols and other compounds (b); and vitamins and nucleotide derivates (c) in tumorous stem mustard leaf and stem extracts. blue: organic acids; orange: others; purple: polyphenols; green: vitamins; black: nucleotide and its derivates.
Figura 2. Perfil de fitoquímicos de extractos de hoja y tallo de mostaza de tallo tumoral. Porcentaje de fitoquímicos en extractos de hojas y tallos de mostaza de tallo tumoral (A). Principales ácidos orgánicos individuales, polifenoles y otros compuestos (B); y vitaminas y derivados de nucleótidos (C) en extractos de hojas y tallos de mostaza de tallo tumoral. Azul: ácidos orgánicos; naranja: otros; morado: polifenoles; verde: vitaminas; negro: nucleótido y sus derivados.
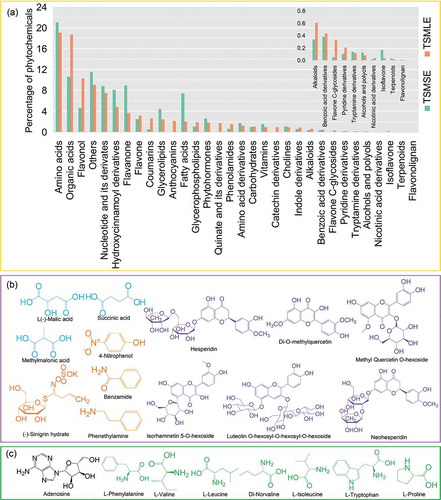
Tumorous stem mustard was a native cruciferous vegetable crop in Asia countries (Xie et al., Citation2014). It may have a high level of glucosinolates like other cruciferous vegetables (Brown & Morra, Citation1995).We found that the proportion of sinigrin hydrate ()), a potent anticancer glucosinolate (Awasthi & Saraswathi, Citation2015), was higher in TSMLE than TSMSE (supplementary Table S1). Among the main phytochemicals of TSMLE and TSMSE, the levels of isorhamnetin 5-O-hexoside, methyl quercetin O-hexoside, and luteolin O-hexosyl-O-hexosyl-O-hexoside were 170, 230, and 694 times in TSMLE compared with TSMSE (). As can be seen from their molecular structures ()), we confirm they are polyphenol compounds. Interestingly, a large number of previous researches have reported that polyphenols are important antioxidants and anticancer nutrients of human diet (Díaz-García et al., Citation2013). Thus, these results strongly suggest that the TSMLE possess stronger antioxidant and anticancer activities than TSMSE. Although L-(-)-malic acid, methylmalonic acid, and succinic acid have no direct antioxidant and anticancer activities, these organic acids can stabilize polyphenols to maintain antioxidant and anticancer activities (Dillard & German, Citation2000). Our results showed that the levels of three main organic acids (L-(-)-malic acid, methylmalonic acid, and succinic acid) in TSMLE were slightly higher than that in TSMSE. Apart from these identified main antioxidants and anticancer compounds, we also identified 11 other compounds including 7 amino acids, one nucleotide and 3 other compounds in the TSMLE and TSMSE (). Furthermore, we also found that these extracts of tumorous stem mustard contained vitamin C, alkaloids, flavone C-glycosides, isoflavone, terpenoids, and other important antioxidants and/or anticancer compounds (supplementary Table S1). It is worth noting that, TSMLE had higher contents of potential antioxidants including caffeic acid O-glucoside, 4-hydroxy-3,5-diisopropylbenzaldehyde, gallocatechin-gallocatechin, 7 anthocyanins, 4 coumarins, 2 flavanone, 5 flavone, 7 flavone C-glycosides, 9 flavonol, 3 organic acids, and 10 quinate derivatives, relative to TSMSE. Totally, the present results lend support to previous findings (Xie et al., Citation2014) and expand the horizon of phytochemicals profile research findings of tumorous stem mustard to a new high. In addition, the results of phytochemical analysis also suggested that tumorous stem mustard, especially its leaf, was a potential diet source with strong antioxidant and anticancer activities.
Table 1. The phytochemicals that differently existed between tumorous stem mustard stem extracts (TSMSE) and tumorous stem mustard leaf extracts (TSMLE).
Tabla 1. Fitoquímicos presentes de manera diferente en los extractos de tallos de mostaza de tallo tumoral (TSMSE) y los extractos de hojas de mostaza de tallo tumoral (TSMLE).
3.3. Antioxidant activity of TSMLE and TSMSE
Polyphenols and some other phytochemicals act at different levels as antioxidants due to their ability to transfer hydrogen atoms and/or electrons (Bannour et al., Citation2017). The antioxidant capacity of plant extracts is affected by various factors and largely depends on both the composition of the extract and the analytical test system (González-Barrio et al., Citation2018). Regrettably, there is not a standardized method for antioxidant capacity evaluation; thus, it is necessary to perform more than one type of antioxidant capacity measurement to take into account the various mechanisms of antioxidant action (González-Barrio et al., Citation2018). In our work, we used four methods, ABTS+, DPPH, TRP and FRAP based on the radical scavenging activity or ferric reducing power. As can be seen in ), TSMLE and TSMSE were found to have the ability to scavenge ABTS+ at a tested concentration range of 0–2 mg/mL. The ABTS+-scavenging effects for TSMLE at all tested concentrations were slightly higher than that of TSMSE. The assay for scavenging DPPH· showed that the antioxidant effect of the polyphenol-enriched extract of tumorous stem mustard leaf was more potently than that of tumorous stem mustard stem, where the DPPH·-scavenging ability of TSMLE was from 25% to 50% at concentrations ranging from 0.4 mg/mL to 2 mg/mL, where the effect of TSMSE was only from 13% to 32% following the treatment ()). The reducing capability was determined by monitoring the transformation of Fe3+ to Fe2+ in the presence of the extracts (Nie et al., Citation2015). As shown in ), the reducing power of TSMLE at 2 mg/mL was as high as 0.12 (absorbance), which was almost 50% higher than that of the same concentration of TSMSE. The FRAP analysis was also performed with these extracts, since the ferric reducing ability is frequently used as an indicator of electron-donating activity, which is an important mechanism of antioxidant activity (Khaled-Khodja, Boulekbache-Makhlouf, & Madani, Citation2014). TSMLE showed obviously higher total antioxidant activity than TSMSE ()). These results indicated that 80%-CH3OH-extracts from tumorous stem mustard leaf and stem possess antioxidant property, and the antioxidant effect of the extract from stem mustard leaf was observably superior to that from the stem. This difference of antioxidant activity might be caused by the fact that the contents of antioxidants in stem mustard leaf were higher than that its stem.
Figure 3. In vitro antioxidant effects of tumorous stem mustard leaf and stem extracts. (a) ABTS+-scavenging assay; (b) DPPH·-scavenging assay; (c) total reducing power; (d) ferric reducing antioxidant power (FRAP). Tea polyphenols extract (pure >97%) was used as a positive control. Results were expressed as the mean ± SD (n = 3).
Figura 3. Efectos antioxidantes in vitro de extractos de hojas y tallos de mostaza de tallo tumoral. (A) ensayo de eliminación de ABTS +; (B) ensayo de eliminación de DPPH ·; (C) poder reductor total; (D) Poder de reducción antioxidante del [ion] férrico (FRAP). Se utilizó el extracto de polifenoles de té (puro> 97%) como control positivo. Los resultados se expresan como la media ± desviación estándar (n = 3).
![Figure 3. In vitro antioxidant effects of tumorous stem mustard leaf and stem extracts. (a) ABTS+-scavenging assay; (b) DPPH·-scavenging assay; (c) total reducing power; (d) ferric reducing antioxidant power (FRAP). Tea polyphenols extract (pure >97%) was used as a positive control. Results were expressed as the mean ± SD (n = 3).Figura 3. Efectos antioxidantes in vitro de extractos de hojas y tallos de mostaza de tallo tumoral. (A) ensayo de eliminación de ABTS +; (B) ensayo de eliminación de DPPH ·; (C) poder reductor total; (D) Poder de reducción antioxidante del [ion] férrico (FRAP). Se utilizó el extracto de polifenoles de té (puro> 97%) como control positivo. Los resultados se expresan como la media ± desviación estándar (n = 3).](/cms/asset/4e395fbb-1517-4c2d-a36a-cdcf5d8930d9/tcyt_a_1577303_f0003_oc.jpg)
3.4. Antitumor activity of TSMLE and TSMSE
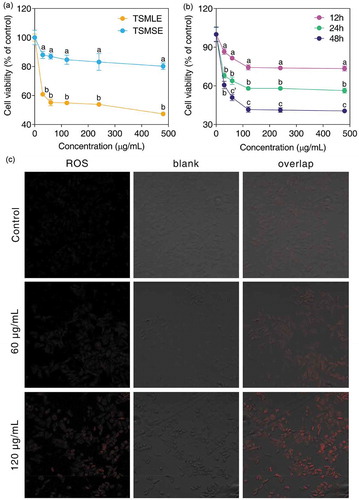
It is well known that the antioxidant activity of phytochemicals was closely related to the anti-tumor activity of phytochemicals (Li et al., Citation2013). Thus, our data of antioxidant assessing suggested that there was an obvious difference in antitumor activity between TSMLE and TSMSE. This is the first time to compare the anti-tumor effects of TSMLE and TSMSE in human lung carcinoma A549 cells. As shown in ), the viability of A549 cells was significantly inhibited by TSMLE and TSMSE in a dose-dependent manner. The inhibitory rate of A549 cells treated with TSMLE at 30, 60 and 120 μg/mL for 24 h was 39%, 45%, and 45%, which was significant higher (p< 0.0001) than that of TSMSE (12%, 13% and 15%), respectively ()). This might be the fact that the antioxidant activity of TSMLE was significantly higher than the antioxidant activity of TSMSE. In addition, the anti-tumor effect of TSMLE was also can be carried out in a time-dependent manner ()). ROS has been implicated as a second messenger in multiple signaling pathways and can also play an important role in apoptosis by regulating the activity of certain enzymes involved in the cell death pathway (Zhang et al., Citation2012). The abnormal increase of ROS level in mitochondria can already push tumor cells to the brink of their toxic threshold, which is implicated in the induction of apoptosis by several phytochemicals (Li et al., Citation2013). To investigate if the mitochondrial dysfunction in TSMLE-treated A549 cells was promoted by ROS production, we analyzed intracellular ROS level in terms of fluorescence by DHE. As depicted in ), as compared with the untreated control cells, the level of ROS in 60 μg/mL TSMLE-treated A549 cells for 24 h was distinctly increased. Furthermore, a further treatment of A549 cells with 120 μg/mL of TSMLE led to a dose-dependent elevation of intracellular ROS level (). This image indicated that the apoptotic effect of TSMLE on A549 cells was concerned with an increased accumulation of intracellular ROS and ROS production might lead to apoptotic cell death via the mitochondrial pathway (Li et al., Citation2016). This anti-tumor activity may be the fact that the extracts form tumorous stem mustard contained polyphenols, sinigrin hydrate and other anti-tumor compounds (supplementary Table S1). Although polyphenols exhibited strong antioxidant capacity in vitro, these antioxidant properties may not be entirely responsible for their chemical prevention effects (Li et al., Citation2016). Yamashita et al. (Citation1998) reported that polyphenols as antioxidants are also considered to exhibit pro-oxidant properties, which may account for their anticancer properties since chromatin-bound endogenous transitional metal ions were regulated by polyphenols to produce ROS during apoptosis processing of cancer cells (Sarwar et al., Citation2015). These results indicated that the extracts from tumorous stem mustard leaf and stem induced growth inhibition and apoptosis through enhancing intracellular oxidative stress of lung cancer A549 cells.
Figure 4. Effects of tumorous stem mustard leaf and stem extracts on cellular viability and ROS generation in human lung cancer A549 cells. Cells were treated with or without different concentrations of TSMLE and TSMSE (0, 30, 60, 120, 240, and 480 μg/mL) for 24 h (a). Cells were treated with or without different concentrations of TSMLE for 12, 24 and 48 h (b). Data were mean values ± SD of 10 duplications. a-c Mean values with different alphabetical letters indicate significant difference (p< 0.0001, c’ p< 0.001) at the same treatment concentration. The fluorescence intensity of DHE (O2−) was measured using DHE probes (c).
Figura 4. Efectos de extractos de hoja y tallo de mostaza de tallo tumoral en la viabilidad celular y la generación de ROS en células de cáncer de pulmón humano A549. Las células se trataron con o sin concentraciones diferentes de TSMLE y TSMSE (0, 30, 60, 120, 240 y 480 μg/ml) durante 24 h (A). Las células se trataron con o sin concentraciones diferentes de TSMLE durante 12, 24 y 48 h (B). Los datos son valores medios ± DE de 10 duplicaciones. a-c Los valores medios con diferentes letras alfabéticas indican una diferencia significativa (p< 0.0001, c’ p< 0.001) en la misma concentración de tratamiento. La intensidad de fluorescencia de DHE (O2-) se midió utilizando sondas DHE (C).
4. Conclusions
This is the first study with unequivocal evidence that tumorous stem mustard leaf contains more polyphenols and other antioxidant and anti-tumor compounds, and its extracts have higher antioxidant and anti-tumor activities in human A549 cells than the edible stem. Considering the more than 100 history of uses of edible stems from tumorous stem mustard as food, its leaf might be an excellent source of dietary nutrient in the prevention and/or treatment of lung cancer. These findings will promote the comprehensive utilization of tumorous stem mustard as food.
Supplemental Material
Download MS Excel (79 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed on the publisher's website.
Additional information
Funding
References
- Aune, D., Giovannucci, E., Boffetta, P., Fadnes, L. T., Keum, N., Norat, T., … Tonstad, S. (2017). Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. International Journal of Epidemiology, 46, 1029–1056.
- Awasthi, S., & Saraswathi, N. T. (2015). Elucidating the molecular interaction of sinigrin, a potent anticancer glucosinolate from cruciferous vegetables with bovine serum albumin: Effect of methylglyoxal modification. Journal of Biomolecular Structure & Dynamics, 34, 2224–2232.
- Bannour, M., Fellah, B., Rocchetti, G., Ashi-Smiti, S., Lachenmeier, D. W., Lucini, L., & Khadhri, A. (2017). Phenolic profiling and antioxidant capacity of Calligonum azel Maire, a Tunisian desert plant. Food Research International, 101, 148–154.
- Brown, P. D., & Morra, M. J. (1995). Glucosinolate-containing plant tissues as bioherbicides. Journal of Agricultural & Food Chemistry, 43, 3070–3074.
- Díaz-García, M. C., Obón, J. M., Castellar, M. R., Collado, J., & Alacid, M. (2013). Quantification by UHPLC of total individual polyphenols in fruit juices. Food Chemistry, 138, 938–949.
- Dillard, C. J., & German, J. B. (2000). Phytochemicals: Nutraceuticals and human health. Journal of the Science of Food & Agriculture, 80, 1744–1756.
- González-Barrio, R., Periago, M. J., Luna-Recio, C., Javier, G. A. F., & Navarro-González, I. (2018). Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chemistry, 252, 373–380.
- Han, X., Li, W., Huang, D., & Yang, X. (2016). Polyphenols from hawthorn peels and fleshes differently mitigate dyslipidemia, inflammation and oxidative stress in association with modulation of liver injury in high fructose diet-fed mice. Chemico-Biological Interactions, 257, 132–140.
- Key, T. J., Arthur, S., Willett, W. C., Allen, N. E., Spencer, E. A., & Travis, R. C. (2004). Diet, nutrition and the prevention of cancer. Public Health Nutrition, 7, 187–200.
- Khaled-Khodja, N., Boulekbache-Makhlouf, L., & Madani, K. (2014). Phytochemical screening of antioxidant and antibacterial activities of methanolic extracts of some Lamiaceae. Industrial Crops and Products, 61, 41–48.
- Lee, H., Kang, C., Jung, E. S., Kim, J. S., & Kim, E. (2011). Antimetastatic activity of polyphenol-rich extract of ecklonia cava through the inhibition of the akt pathway in A549 human lung cancer cells. Food Chemistry, 127, 1229–1236.
- Li, T., Zhu, J., Guo, L., Shi, X., Liu, Y., & Yang, X. (2013). Differential effects of polyphenols-enriched extracts from hawthorn fruit peels and fleshes on cell cycle and apoptosis in human MCF-7 breast carcinoma cells. Food Chemistry, 141, 1008–1018.
- Li, W., He, N., Tian, L., Shi, X., & Yang, X. (2016). Inhibitory effects of polyphenol-enriched extract from Ziyang tea against human breast cancer MCF-7 cells through reactive oxygen species-dependent mitochondria molecular mechanism. Journal of Food and Drug Analysis, 24, 527–538.
- Nie, Y., Ren, D., Lu, X., Sun, Y., & Yang, X. (2015). Differential protective effects of polyphenol extracts from apple peels and fleshes against acute CCl4-induced liver damage in mice. Food & Function, 6, 513–524.
- Peng, H., Li, W., Li, H., Deng, Z., & Zhang, B. (2017). Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycinemax (L.) merr). Journal of Functional Foods, 32, 296–312.
- Ren, D., Zhao, Y., Nie, Y., Yang, N., & Yang, X. (2014). Hypoglycemic and hepatoprotective effects of polysaccharides from Artemisia sphaerocephala Krasch seeds. International Journal of Biological Macromolecules, 69, 296–306.
- Sarwar, T., Zafaryab, M., Husain, M. A., Ishqi, H. M., Rehman, S. U., Rizvi, M. M., & Tabish, M. (2015). Redox cycling of endogenous copper by ferulic acid leads to cellular DNA breakage and consequent cell death: A putative cancer chemotherapy mechanism. Toxicology & Applied Pharmacology, 289, 251–261.
- Xie, Q., Hu, Z., Zhang, Y., Tian, S., Wang, Z., Zhao, Z., … Chen, G. (2014). Accumulation and molecular regulation of anthocyanin in purple tumorous stem mustard (Brassica juncea var. tumida Tsen et Lee). Journal of Agricultural and Food Chemistry, 62, 7813–7821.
- Yamashita, N., Murata, M., Inoue, S., Burkitt, M. J., Milne, L., & Kawanishi, S. (1998). Alphatocopherol induces oxidative damage to DNA in the presence of copper(II) ions. Chemical Research in Toxicology, 289, 855–862.
- Yuan, Q. H. (2016). Features Fuling district agricultural marketing innovation-a case study of Fuling mustard. MD Thesis, Central South University of Forestry & Technology, China. ( in Chinese)
- Zhang, H., Zhang, M., Yu, L., Zhao, Y., He, N., & Yang, X. (2012). Antitumor activities of quercetin and quercetin-5ʹ,8-disulfonate in human colon and breast cancer cell lines. Food and Chemical Toxicology, 50, 1589–1599.
