ABSTRACT
Vanilla juice has been shown empirically to have antifungal activity against some fungal strains; however, there are no activity reported against Alternaria genre. In this work, the chemical profile of vanilla juice was obtained and its antifungal activity against fungal strains from the family Pleosporaceae, isolated from sorghum- and barley-diseased plants, was tested. The strains were identified as Alternaria alternata by their molecular and morphological characteristics. The vanilla juice characterization from Vanilla planifolia pods showed the presence of vanillin, vanillic acid, p-hydroxybenzaldehyde, p-hydroxybenzoic acid, guaiacol, glucovanillin, vanillyl alcohol, and furfural. Vanilla juice showed a fungistatic effect against all A. alternata strains tested in this study and increased the lag time from 50 to 112 h, and no conidia were produced. This result indicates the possible application of vanilla juice as an alternative to control agricultural crops such as barley and sorghum in Mexico.
RESUMEN
El jugo de vainilla ha mostrado tener empíricamente actividad antifúngica contra algunas cepas de hongos; sin embargo, no se tiene reportes contra el género Alternaria. En este trabajo, se obtuvo el perfil químico del jugo de vainilla y se probó su actividad antifúngica contra cepas de hongos de la familia Pleosporaceae, aisladas de plantas enfermas de sorgo y cebada. Las cepas fueron identificadas como Alternaria alternata por sus características morfológicas y moleculares. La caracterización del jugo de vainilla de vainas de Vanilla planifolia mostró la presencia de vainillina, ácido vainillico, p-hidroxibenzaldehído, ácido p-hidroxibenzoico, guaiacol, glucovainillina, alcohol vainillico y furfural. El jugo de vainilla tuvo un efecto fungistático contra todas las cepas de A. alternata probadas en este estudio, aumentó el tiempo de latencia de 50 a 112 h, y no se produjeron conidios. Este resultado indica la posible aplicación del jugo de vainilla como una alternativa para controlar cultivos agrícolas como la cebada y el sorgo en México.
1. Introduction
Vanilla extraction is an artisanal process, consisting of four stages: killing, sweating, drying, and conditioning the vanilla pods (Dignum, Kerler, & Verpoorte, Citation2001a). Vanilla juice is obtained during the sweating process and it is composed mainly of four aromatic compounds: vanillin, vanillic acid, p-hydroxybenzaldehyde, and p-hydroxybenzoic acid (Havkin-Frenkel, French, Graft, Pak, & Frenkel, Citation2004; Rao & Ravishankar, Citation2000). Each sweating cycle lasts between 36 to 48 h (De la Cruz et al., Citation2009) and from 4 to 13 cycles can be undertaken, depending on the vanilla pods maturity (Havkin-Frenkel et al., Citation2004; Purseglova, Drown, Green, & Robins, Citation1981; Rosado, Citation2006). After sweating, vanilla pods are exposed to the sun or are placed on wooden pallets in the conditioning area, depending on weather conditions (Rosado, Citation2006). During water removal, the pressure exerted by the vanilla pods weight facilitates juice collecting (Rosado, Citation2006). The vanillin molecule is recognized as safe and is used as an antimicrobial (Cerruti & Alzamora, Citation1996). Vanillin inhibits bacteria (Cava, Taboada, Valverde, & Marín, Citation2012; Delaquis, Stanich, & Toivonen, Citation2005; Fitzgerald et al., Citation2004) and fungi growth (Lopez-Malo Cerruti & Alzamora, Citation1996; Kim et al., Citation2014; Rivera-Carriles, Argaiz, Palou, & López-Malo, Citation2004). Vanillin changes community structures of Fusarium and decreases the number of bands as confirmed by polymerase chain reaction (PCR)-denaturing gradient gel electrophoresis, and quantitative PCR (Zhou, Jia, Ge, & Wu, Citation2018).
Therefore, vanilla juice and vanillin can be considered agrochemicals for the control of pathogens resistant to different synthetic fungicides (Diaz et al., Citation2011). Alternaria alternata is the causal agent of diseases, like barley black point (Zare, Citation2013) and gray leaf spot in sorghum (Thomas, Citation1991). These diseases reduce the commercial value of cereals, causing farmers to lose between 15 and 90% of untreated field-grown seeds (Lipps, Citation1998; Mathre, Citation1997; Zare, Citation2013). In this study, we characterized the vanilla juice from Vanilla planifolia pods and studied its antifungal effect against A. alternata strains isolated from sorghum and barley plants.
2. Materials and methods
2.1. Fungal cultures
A. alternata fungal strains used in this study were isolated from diseased plants of sorghum (Sorghum sp.) and barley (Hordeum vulgare), collected at different times (). To isolate fungi, some infected sorghum fragments and barley plants were placed in a moist chamber and incubated in darkness at 25°C for 3 days. After that, each fungus was isolated and subcultured on potato dextrose agar, PDA (Sigma, St. Louis, MO), at room temperature for 7 days. Fungal strains were maintained in PDA medium at 4°C and the spore suspension at −87°C.
Table 1. Culture collection data of the Alternaria alternata strains used in this study.
Tabla 1. Datos de recolección de las cepas de Alternaria alternata utilizadas en este estudio.
2.2. Molecular and morphological characterization
Strains isolated (JCP13, JCP25, JCP31, JCP32, JCP49, JCP56) were characterized molecularly and the sequences were deposited in the GenBank. Strain ITV3 was donated and characterized in the genetics laboratory from the Instituto Tecnológico de Veracruz (ITVer). Morphological descriptions are based on comparisons of A. alternata (Ariyawansa et al., Citation2015; Lawrence, Rodonto, & Gannibal, Citation2016) augmented by new observations, as noted. The standard medium used to assess morphology and growth rate was PDA. The morphology, colony color, and diffusing pigment in the agar were recorded. Twenty-five measurements of conidia and conidiophores were made. Measurements of the characters were taken from images using the software IOS 7, 8-megapixel iSight camera, iPhone 5S version Microsystems (Hollyhill, Cork, Republic of Ireland). Scanning electron microscope micrographs were taken with the Cryo Transfer System (Jeol, model IT300) at the Escuela Superior de Apan (ESAp-UAEH).
2.3. Vanilla juice extraction
Vanilla juice was obtained from the sweating stage during the curing process of vanilla in Gutierrez Zamora, stated of Veracruz, Mexico. Vanilla juice was collected in sterile bottles 2 days after initiating the sweating cycles from a batch containing 1000 kg of vanilla pods. Samples were kept on ice during their transport to the laboratory.
2.4. pH measurements
The pH measurements were performed in the vanilla juice using a potentiometer (pH 510 Series Benchtop Meter Oakton model 00702–93) with a magnetic agitator. The measurements were performed in triplicate.
2.5. Chromatographic profile
The chromatographic profile was performed according to the technique proposed by Pérez-Silva et al. (Citation2006), using an HPLC (Varian ProStar model 240) equipped with a UV detector (Waters model 2487), 230 nm wavelength, and a C18 column (Microsorb TM-MV). The mobile phase was a mixture of methanol-acidified water that was prepared as follows: the HPLC grade water (J.T. Baker) was acidified with 0.1 mol L−1 phosphoric acid (HYCEL), filtered through a 0.45-μm membrane, stirred for 5 min, and then sonicated (West prime Systems) during 30 min. HPLC grade methanol (J.T. Baker) was subjected to the same treatment. For the whole test, a flow of 0.7 mL min−1 was used.
Vanilla juice samples were diluted with the mobile phase and filtered through a membrane (0.22-μm) before being injected into the HPLC. To determine glucovanillin, vanillyl alcohol, p-hydroxybenzoic acid, p-hydroxybenzaldehyde, and vanillic acid a column was used, with temperature at 35°C, a ratio phase (methanol-acidified water, 78:22, v/v), and a pressure of 5.269 × 106 Pa. Vanillin and guaiacol were identified using a column temperature at 30°C. A ramp to the mobile phase was applied also, starting with a proportion of 78:22 decreasing 3 mL every 2 min until reaching a proportion of 65:35. The pressure ranged from 7.295 × 106 Pa to 9.727 × 106 Pa. Furfural was determined using a column temperature at 30°C, an initial phase with a ratio of 90:10, and a decreasing ramp of 2 mL every 3 min to reach a ratio 78:22. Pressure ranged from 5.269 × 106 to 5.472 × 106 Pa. Calibration curves were performed with an external control using: vanillic acid (VA; Fluka), vanillyl alcohol (VAL; Fluka), guaiacol (GYL; Fluka), vanillin (V; Sigma), p-hydroxybenzoic acid (BHA; Fluka), p-hydroxybenzaldehyde (PHB; Fluka), and furfural (FUR; Sigma-Aldrich). Glucovanillin (GLU) was extracted with the method reported by Odoux (Citation2000) and was donated by the CIRAD Institute (France).
2.6. In vitro antifungal assays
The antifungal effect of vanilla juice and commercial-grade vanillin was evaluated in vitro on PDA medium. A volume of 2 µL with 2.0 × 106 spores was deposited in the center of each Petri dish, containing PDA medium as a control, PDA with vanilla juice (adjusted to 250 mg L−1 of vanillin), and PDA with commercial-grade vanillin. Experiments were performed at a temperature of 25°C during 7 days. The pure vanilla (Sigma) was used at different concentrations (250, 500, 750, 1000, 1250, and 1500 mg/L). Radial growth (mm) was measured every 24 h during 8 days. The tests were performed in triplicate and the results were analyzed using the STATISTICA software (Hill & Lewicki, Citation2007).
3. Results and discussion
3.1. Isolation and morphological identification
The pleomorphic genus Alternaria (Lawrence, Gannibal, Peever, & Pryor, Citation2013) was named by Lawrence et al. (Citation2013), it comprises around 60 species that have small spores and include A. alternata, A. arborescens, A. gaisen, and A. tenuissima (Lawrence et al., Citation2016).
Alternaria spp. are well known to produce many secondary metabolites-related toxins (Christensen et al., Citation2005; Frisvad, Andersen, & Thrane, Citation2008). These metabolites are responsible for many plant pathogens with or without being host specific (Markham & Hille, Citation2001; Wolpert, Dunkle, & Ciuffetti, Citation2002) and with mycotoxins implicated in food contamination (Fernández-Cruz, Mansilla, & Tadeo, Citation2010; Ostry, Citation2008). In this sense, small-spored organisms, such as A. alternata, also play an important role in inducing and causing some pathologies in humans and plants (Salo et al., Citation2006; Singh, Gupta, & Sharma, Citation2014).
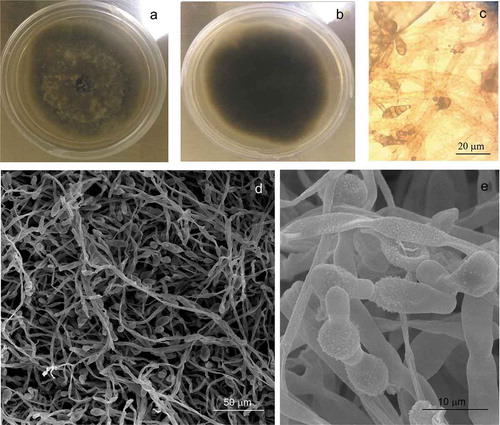
In this study, we isolated seven fungal strains from diseased plants; six strains were isolated from barley (JCP13, JCP25, JCP31, JCP32, JCP49, and JCP56) and one strain from sorghum plants (ITV3). Colonies in PDA medium showed rapid growth, the fungi reached a diameter of 37–40 mm in 7 days, at 25°C ()). The initial growth was hairy, with a gray color that later changed in the center of the colony acquiring a darker tone, with more or less intense tones but still with gray borders. The reverse of the colonies showed a black color ()). Hyphae had a filamentous shape with simple conidiophores, septa, from their end muriform conidia were formed, of a grayish brown color, and transverse and vertical multi-septa in an irregular arrangement ()). The new conidia are formed by gemmation from the apical cell, giving rise to a long chain of more than 10 conidia (,). The primary conidiophores can be curved or straight, from short to very large in size, simple or branched with one or more terminal conidiogenous loci.
Figure 1. Alternaria alternata colony on PDA medium (7 days, 25°C) front plate (a), morphology colony of A. alternata on PDA medium reverse plate (b), Optic microscopy of conidia (c), micrograph of hyphae and conidia (d–e).
Figura 1. Colonia de Alternaria alternata en medio PDA (7 días, 25°C), anverso de la placa (a), morfología de la colonia de A. alternata en medio PDA, reverso de la placa (b), conidios vistos por microscopía óptica (c), micrografías de hifas y conidios (d–e).
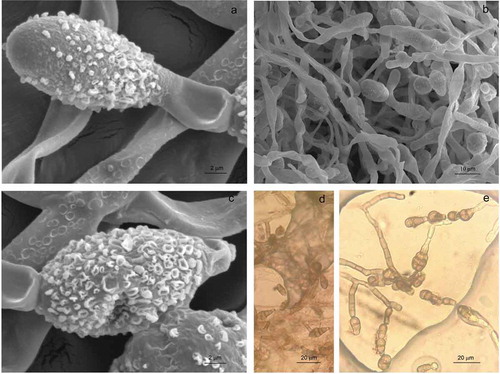
Asexual morphological characteristics on PDA medium showed abundant conidia that were small to moderate in size, (20-)20.68–29.42(−38) × (8-)8.18–9.76(−11) mm (av. = 25.04, SD = 4.37, n = 25; av. = 8.97, SD = 0.79, n = 25), obclavate ()), long ellipsoid or ellipsoid (,), from 3 to 7 transversal septa ()). A slight constriction was observed also in some septa, with 1 or 2 longitudinal septa in one or a few transversal divisions ()). Based on molecular techniques, the phenotype descriptions and the measurements described, the strains were identified as Alternaria alternata. The results observed in this study are within the range of observed values for other A. alternata strains reported by other authors (Ariyawansa et al., Citation2015; Lawrence et al., Citation2016) and lower than those reported by (Nagrale, Gaikwad, & Sharma, Citation2013; Ramjegathesh & Ebenezar, Citation2012) for the maximum length of conidia.
Figure 2. Alternaria alternata colony growth on PDA medium (7 days, 25°C). Micrograph of conidia with obclavate shape (a), long ellipsoid or ellipsoid shape (b–c), Conidia with transversal septa (d), or with longitudinal septa (e) seen with optical microscopy.
Figura 2. Colonias de Alternaria alternata en medio PDA (7 días, 25°C). Micrografía de conidios con forma de basto (a), forma elipsoide o elipsoide larga (b–c), conidios con septos transversales (d), o con septos longitudinales (e).
3.2. Vanilla juice characterization
3.2.1. pH measurement
Vanilla juice had similar organoleptic characteristics to the vanilla extract, with a predominantly acidic pH, in a range between 4.4 and 5.6. The low pH was associated with the organic acids present, such as acetic, propionic, butyric
and isobutyric, propanoic acids, and others (Pérez-Silva et al., Citation2006), the maturity degree of vanilla beans, the sweating cycles (8 to 13), and the harvest time of the juice (Rosado, Citation2006).
This pH result corresponds to the pH reported by Pacheco (Citation2009) for green vanilla extract and vanilla cured extract, 5.12 ± 0.19 and 5.08 ± 0.24, respectively. Vanilla juice is a byproduct obtained from approximately a ton of vanilla beans; therefore, the juice is a concentrated product containing large amounts of the chemical compounds present in vanilla beans. The vanilla juice had an oily or viscous consistency and a brown color. The juice was collected during the first cycles of sweating (18–20 h) as inferred by the amount produced (Tapia et al., Citation2011). At this stage, the vanilla bean cells destruction is accelerated, which leads to the formation of vanillin and the generation of large amounts of chemical compounds (Purseglova et al., Citation1981; Romero, Citation2003; Walton, Mayer, & Narbad, Citation2003). During the sweating phase, temperatures reached up to 65°C, which may facilitate the release of simple aromatic compounds and other polymers such as waxes, resins, gums, and essential oils (Ranadive, Citation1994; Rosado, Citation2006).
3.2.2. Chemical compounds in vanilla juice
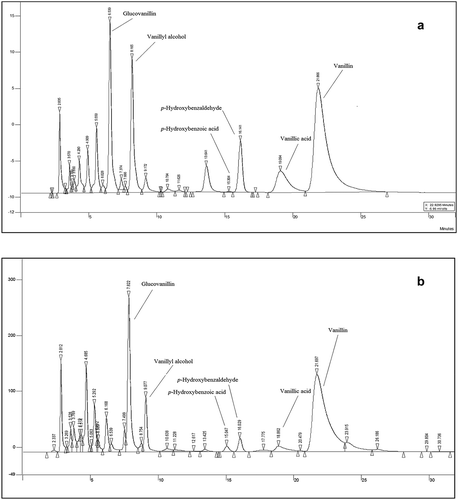
Chromatographic characterization showed the same profile as the cured vanilla bean extract obtained from a traditional curing process ()). The chemical concentration of the compounds in vanilla juice was evident in the chromatograms and was higher than those reported in the cured vanilla ()). Furfural, guaiacol, and vanillyl alcohol compounds have been reported as traces in the cured vanilla extract (Pérez-Silva et al., Citation2006), and their presence in the vanilla juice is due to the amount produced (5 L ton−1; personal communication), therefore, all compounds are concentrated. The juices from two batches (fourth and sixth) showed the highest vanillin concentrations whereas the juices from the other three batches (first, second, and sixth) had the greatest glucovanillin concentration. Moreover, the vanillic acid and p-hydroxybenzoic acid and p-hydroxybenzaldehyde showed no significant differences. Chemical compounds identified in the juice are summarized in .
Table 2. Chemical compounds identified by HPLC in the vanilla juice.
Tabla 2. Compuestos químicos identificados por HPLC en el jugo de vainilla.
Figure 3. Comparison of the chromatographic profile of cured vanilla pods (a) and vanilla juice (b).
Figura 3. Comparación del perfil cromatográfico de vainas de vainilla curadas (a) y jugo de vainilla (b).
The presence of glucovanillin and vanillyl alcohol in the juice was very important as vanillin precursors. The results obtained show that conversion of vanillin does not reach 100% in cured vanilla beans.
The four main aromatic compounds in the juice are due to their formation during the first 24 h after the curing process had started (Havkin-Frenkel et al., Citation2004). Dignum, Kerler, and Verpoorte (Citation2001b) confirmed the presence of vanillin and vanillic acid in the first sweating cycles, but the glucovanillin presence has been reported in the ninth cycle of sweating at concentrations of up to 0.08 g 100 mL−1. Other authors reported concentrations of p-hydroxybenzoic acid between 0.019 g 100 mL−1 and 0.034 g 100 mL−1 (Gassenmeier, Riesen, & Magyar, Citation2008).
Vanillin concentrations ranged from 0.10 g 100 mL−1 to 0.61 g 100 mL−1. Odoux (Citation2000) reported that vanillin concentration increased with each sweating cycle at around 0.6 g kg−1 in dry matter (DM), in the green pod, and at a concentration of 0.2 g kg−1 DM in cured pods. Dignum et al. (Citation2001b) reported the presence of guaiacol in the cured vanilla extract obtained during the killing stage under controlled conditions. Guaiacol showed higher concentration on the first day of the killing stage until reaching a maximum conversion on the fourth day, which explains the changes in concentrations found in this research. Farmers collected the juice during the first sweating cycles because in this step the vanilla beans lose up to 40% water (Havkin-Frenkel et al., Citation2004; Purseglova et al., Citation1981). Unlike the later sweating cycles, where there is less dehydration and the amount of juice produced is minimal (Rosado, Citation2006).
During the sweating stage, the temperature may reach 65°C or higher (Rosado, Citation2006) and can form degraded products such as furfural (Rao & Ravishankar, Citation2000). The vanilla juice showed different concentrations in the five batches studied. In general, the chemical compounds identified in the juice had a higher concentration (except vanillin) than in the cured vanilla beans (Pérez-Silva et al., Citation2006; Rosado, Citation2006). During the sweating and drying stages, the development of aromatic chemical compounds increases and can be dragged with water (Dignum et al., Citation2001a; Rosado, Citation2006).
3.2.3. Antifungal action
The vanilla juice was adjusted to 250 mg/L of vanillin concentration and was compared to commercial-grade vanillin, results did not show antifungal effect at the concentration tested. This can be attributed to the vanillin concentration tested and the difference in the pH value at which the tests were conducted, since the pH of the juice was 4.4; whereas the PDA medium in which vanillin is contained has a pH of 5.0 (Delaquis et al., Citation2005). The decrease in radial growth suggests that the fungus was unable to maintain a positive tannin pressure in the mycelium to counteract the effect of the chemical compounds in the growth medium (Tijerina-Ramírez, Lira-Méndez, Moreno-Medina, González-Prieto, & Mayek-Pérez, Citation2014) by limiting the availability of free water for growth (Harris, Citation1981). A. alternata is a fast-growing fungus of wet environments (Pontón, Moragues, Gené, Guarro, & Quindós, Citation2002). The effect of vanillin depends on the fungal species, solute molecule, and the incubation temperature (Olaya & Abawi, Citation1996). Ramírez, Chulze, and Magan (Citation2004) report that when they exposed a strain of Fusarium graminearum to limited water availability, the strain used more energy to balance the water potential of the cytoplasm with respect to the external environment, altering the physiology of the organism and reflecting the decreased metabolism and growth.
These results indicate the possible application of vanilla juice as an alternative for the control of agricultural crops, such as tomatoes, that are attacked by this phytopathogenic fungus (Sanchez, Bautista, & Castillo, Citation2007). Until now, there are no reports on commercial-grade vanillin or vanilla extract effect against A. alternata, but there are reports on some natural extracts that inhibit the growth of some strains of Alternaria spp. (Mironas, Pokrib, Matescu, Boca, & Jurcoane, Citation2004). Furthermore, other authors report the antimicrobial activity of natural extracts obtained from three kinds of mangoes against A. alternata, reporting a reduction of 89.78% at a concentration of 6.25 mg mL−1 (Vega et al., Citation2013).
In addition, Ravi, Sobita, and Lal (Citation2014) reported an in vitro evaluation of plant extracts, where they demonstrated an effect of different extracts favoring inhibition of A. alternata, among these extracts are the leaf extract of Jatropha curcas (62.9%) and Datura strumarium leaf extract (55.6%). Others plant extracts obtained from Azadirachata indica (51.9%), Moringa oleifera (46.9%), Calotropis gigantean (23.45%), and Morus alba (13.6%) have also been reported.
Other authors (Tapwal, Nisha Garg, Gautam, & Kumar, Citation2011) reported a 50% inhibition of growth by an aqueous leaf extract of Parthenium hysterophorus against Alternaria solani. This effect was explained by the release of some phytotoxic substances such as felulic acid, caffeic acid, vanillin acid, anisic acid, chlorogenic acid, p-coumaric acid, p-hydroxybenzoic acid, parthenin, ambrosin, and coronopilin (Prusti, Mishra, Sahoo, & Mishra, Citation2008). Fitzgerald, Stratford, Gasson, and Narbad (Citation2005) analyzed the structure-function of the vanillin molecule and its antifungal properties and of six analogous chemical compounds. The antifungal activity order was: 3-anisaldehyde (1.97 mM) > benzaldehyde (3.30 mM) > vanillin (5.71 mM) > anisole (6.59 mM) > 4-hydroxybenzaldehyde (9.09 mM) > phenol (10.59 mM) > guaiacol (11.66 mM). According to the latter authors, the aldehyde moiety of vanillin plays a key role in its antifungal activity, but side-group position on the benzene ring also influences this activity.
Other studies have reported that the genus Acalypha has a potential effect against plant pathogenic fungi such as Colletotrichum lagenarium, Aspergillus niger, A. fumigatus, A. flavus, Candida albicans, C. tropicalis, Fusarium oxysporum, F. solani, Microsporum canis, Trichophyton interdistale, and T mentagrophytes (Vargas, Gamboa, Medina, & Pérez, Citation2014). Several Acalypha species showed the presence of metabolites such as vanillic acid, 4–0-neohesperidoside and sakurososaponin (Sanchez-Medina et al., Citation2009). Some of these compounds have been reported in cured vanilla (Pérez-Silva et al., Citation2006), hence, they could be present in the here analyzed vanilla juice.
3.2.4. Antifungal vanillin activity
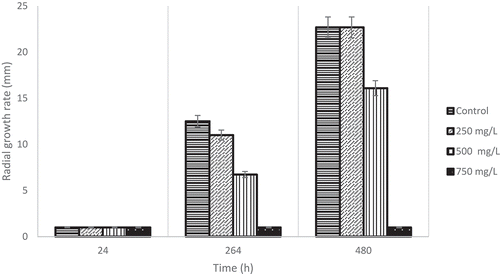
The 250 mg/L vanillin concentration decreased the growth of the Alternaria strains tested to 0.2 mm h−1 (); an increase in the lag time was observed from 50 to 112 h, and a diminished mycelial growth inhibition of up to 37.5%. Moreover, when a vanillin concentration of 500 mg/L at 30°C and pH 5.0 was used, the A. alternata strain showed higher sensitivity by increasing the lag time to 192 h (). At concentrations of 750 mg/L of vanillin, a fungicidal effect against A. alternata was observed. The vanillin concentrations of 1000, 1250, and 1500 mg/L showed the same behavior described at 750 mg/L. This result was very interesting because it is known that A. alternata produces melanin, which is a pigment that gives some resistance to fungal enzyme mixtures, such as chitinases and glucanases (Bloomfield & Alexander, Citation1967).
Figure 4. Commercial-grade vanillin concentration effect on radial growth rate on A. alternata strains at three different times.
Figura 4. Efecto de la concentración de vainillina de grado comercial sobre la tasa de crecimiento radial de la cepas de A. alternata en tres tiempos diferentes.
The vanillin concentration used in this work was less than that reported by Lopez-Malo, Alzadora, and Argaiz (Citation1997), where a vanillin concentration of 350 mg/L did not affect the growth rate of Aspergillus flavus, A. niger, A. ochraceus, and A. parasiticus. On the other hand, Lopez-Malo et al. ((Citation1997), (Citation1998)) reported a fungicidal effect of commercial vanillin against A. ochraceus strain, this effect was achieved at pH 3 and ≤25°C or pH 4 and ≤15°C.
Lopez-Malo, Alzamora, and Argaiz (Citation1998) reported that the germination time and radial growth rate of Aspergillus flavus, A. niger, A. ochraceus, and A. parasiticus was affected significantly when vanillin concentrations of 500, 750, and 1000 mg/L were used at pH 3.0 and 4.0 with aw 0.98. In addition, Delaquis et al. (Citation2005) reported that with the increase of pH, the vanillin effect goes from bacteriostatic to bactericidal. This is because the organic antimicrobial agents, including phenolic acids, are more active in a dissociated state, because the pH is close to the pKa, explaining the bactericidal effect of vanillin (pKa 7.4) against some species of Listeria at pH 7.0.
Vanillin concentrations used in this research were based on the research reported by Lopez-Malo et al. (Citation1997), (Citation1998)). However, the results were not consistent with expectations. These results may be due to the fact that the vanillin concentration was low for the Alternaria strains tested. Minimum inhibitory concentrations reported for certain yeasts have been 2000 and 3000 mg/L (Cerruti & Alzamora, Citation1996; Rivera-Carriles et al., Citation2004).
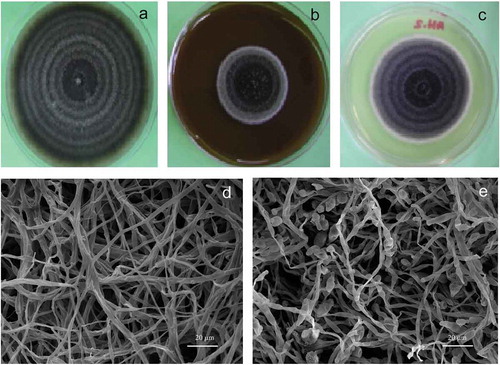
Vanilla juice and commercial-grade vanillin effect on growth parameters are shown in and –), revealing that the juice exerted a fungistatic effect against barley and sorghum A. alternata strains tested in this study and increased the lag time from 50 h to 112 h. Moreover, when A. alternata strains grown on PDA medium were observed with the scanning electron microscope, there was absence of conidia production ()) as compared to the control ()). Likewise, somatic structures showed an atypical morphology (–)). This result corroborates the fungistatic effect of vanilla juice and commercial-grade vanillin observed in the A. alternata strains tested in this study. On the other hand, the juice showed the same chromatographic profile as that of the vanilla extract; therefore, it was inferred that chemical compounds like phenols and acids can contribute with an additive, synergistic or antagonistic effect. Some acids, such as acetic and butyric, were also detected in vanilla juice (data not shown). The vanilla juice effect showed better results than the commercial-grade vanillin against A. alternata (at the concentrations tested); it diminished the mycelial growth to 50%.
Figure 5. Vanilla juice (adjusted to 250 mg/L of vanillin) and commercial-grade vanillin effect on the growth of A. alternata.
Figura 5. Jugo de vainilla (ajustado a 250 mg/L de vainilla) y efecto de la vainillina de grado comercial sobre el crecimiento de A. alternata.
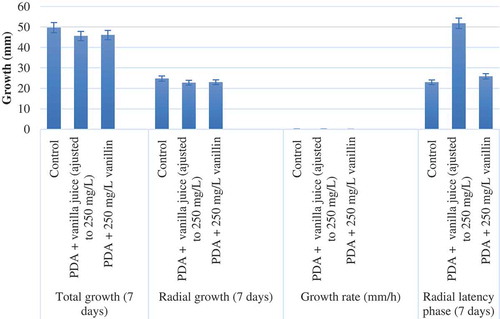
Figure 6. Vanilla juice and comercial-grade vanillin effect on A. alternata. Control (a), vanilla juice (adjusted to 250 mg/L vanillin) (b), comercial-grade vanillin 250 mg/L (c), micrograph of vanilla juice and comercial-grade vanillin effect on A. alternata hyphae (d), and normal morphology of conidia and hyphae (e).
Figura 6. Jugo de vainilla y efecto de la vainillina de grado comercial sobre A. alternata. Control (a), jugo de vainilla (ajustado a 250 mg/L de vainillina) (b), vainillina de grado comercial 250 mg/L (c), micrografía del efecto del jugo de vainilla y la vainillina de grado comercial sobre las hifas (d) y morfología normal de conidios e hifas (e).
4. Conclusion
Vanilla juice shows a fungistatic effect against all A. alternata strains studied and increased the pathogen’s lag time from 50 to 112 h. A synergistic, additive, or antagonistic effect by the other chemical compounds present in vanilla juice was observed as compared to commercial vanillin. This vanilla juice can be concentrated and could be used in programs to evaluate a large number of extracts as natural antifungal products. The information obtained in this research would be useful to obtain the four aromatic compounds reported in the cured vanilla bean (vanillin, vanillic acid, p-hydroxybezaldehyde, and p-hydroxybenzoic acid) and precursors of vanillin (glucovanillin and vanillic acid). The presence of these compounds in the vanilla juice decreases its yield concentration in the cured vanilla beans at the end of the processing. Vanilla juice could be used as an alternative option for the control of fungi responsible for diseases in plants of commercial interest in Mexico.
Acknowledgments
The authors would like to thank Jessica Arlette Porcallo Rojas from ESAp-UAEH for taking SEM micrographs.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ariyawansa, H. A., Thambugala, K. M., Manamgoda, D. S., Jayawardena, R., Camporesi, E., Boonmee, S., … Hyde, K. D. (2015). Towards a natural classification and backbone tree for Pleosporaceae. Fungal Diversity, 71, 85–139.
- Bloomfield, B., & Alexander, M. (1967). Melanins and resistance of fungi to lysis. Journal of Bacteriology, 93, 1276–1280.
- Cava, R. R. M., Taboada, R. A., Valverde, F. M. T., & Marín, I. F. (2012). Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157: H7in milk. Food and Bioprocess Technology, 5, 2120–2131.
- Cerruti, P., & Alzamora, M. E. (1996). Inhibitory effects of vanillin on some food spoilage yeast in laboratory media and fruit purees. International Journal of Food Microbiology, 29, 379–386.
- Christensen, K. B., Van Klink, J. W., Weavers, R. T., Larsen, T. O., Andersen, B., & Phipps, R. K. (2005). Novel chemotaxonomic markers of the Alternaria infectoria species-group. Journal of Agricultural and Food Chemistry, 53, 9431–9435.
- De la Cruz, M. J., Rodríguez, J. G. C., García, H. S., Rosado, Z. T. L., García, A. M. A., & Robles, O. V. J. (2009). Vanilla: Post-harvest operations. FAO, Veracruz, Mexico.
- Delaquis, P., Stanich, K., & Toivonen, P. (2005). Effect of pH on the inhibition of Listeria sp. by vanillin and vanillic acid. Journal of Food Protection, 68, 1472–1476.
- Diaz, D. P., Cabrera, A., Alem, D., Larrañaga, P., Ferreira, F., & Dalla, R. M. (2011). Antifungal activity of medicinal plan extracts against phytopathogenic fungus Alternaria spp. Chilean Journal of Agricultural Research, 71, 231–239.
- Dignum, M. J. W., Kerler, J., & Verpoorte, R. (2001a). β-Glucosidase and peroxidase stability in crude enzyme extracts from green beans of Vanilla planifolia Andrews. Phytochemical Analysis, 12, 174–179.
- Dignum, M. J. W., Kerler, J., & Verpoorte, R. (2001b). Vanilla production: Technological, chemical and biosynthetic aspects. Food Reviews International, 17, 119–120.
- Fernández-Cruz, M. L., Mansilla, M. L., & Tadeo, J. L. (2010). Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. Journal of Advanced Research, 1, 113–122.
- Fitzgerald, D. J., Stratford, M., Gasson, M. J., & Narbad, A. (2005). Structure-function analysis of the vanillin molecule and its antifungal properties. Journal of Agricultural and Food Chemistry, 53, 1769–1775.
- Fitzgerald, D. J., Stratford, M., Gasson, M. J., Ueckert, J., Bos, A., & Narbad, A. (2004). Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. Journal of Applied Microbiology, 97, 104–113.
- Frisvad, J. C., Andersen, B., & Thrane, U. (2008). The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycological Research, 112, 231–240.
- Gassenmeier, K., Riesen, B., & Magyar, B. (2008). Commercial quality and analytical parameters of cured vanilla beans (Vanilla planifolia) from different origins from the 2006-2007 crop. Flavour and Fragrance Journal, 23, 194–201.
- Harris, R. F. (1981). Effect of water potential on microbial growth and activity. In J. F. Par, W. R. Gardner, & L. F. Elliot (Eds.), Water potential relations in soil microbiology (pp. 23–25). Madison: Special Publication No. 9. Soil Science Society of America.
- Havkin-Frenkel, D., French, J. C., Graft, M. N., Pak, F. E., & Frenkel, C. (2004). Interrelation of curing and botany in vanilla (Vanilla planifolia) bean. Future for medicinal and aromatic plants. Acta horticulturae, 629, 93–102.
- Hill, T., & Lewicki, P. (2007). STATISTICS: Methods and applications. Tulsa, OK: StatSoft, (Printed Version).
- Kim, J. H., Lee, H., Cho, Y. J., Kim, J., Chun, J., Choi, J., … Jung, W. H. (2014). A vanillin derivative causes mitochondrial dysfunction and triggers oxidative stress in Cryptococcus neoformans. PloS one, 9(2), e89122.
- Lawrence, D. P., Gannibal, P. B., Peever, T. L., & Pryor, B. M. (2013). The sections of Alternaria: Formalizing species-group concepts. Mycologia, 105, 530–546.
- Lawrence, D. P., Rodonto, F., & Gannibal, P. B. (2016). Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycological Progress, 15(3). doi:10.1007/s11557-015-1144-x
- Lipps, P. E. (1998). Gray leaf sport: A gobal threat to corn production. APSnet Features. Online. doi:10.1094/APSnetFeature-1998-0598
- Lopez-Malo, A., Alzadora, S. M., & Argaiz, A. (1997). Effect of vanillin concentration, pH and incubation temperature on Aspergillus flavus, Aspergillus niger, Aspergillus ocrhaceus and Aspergillus parasiticus growth. Food Microbiology, 14, 117–124.
- Lopez-Malo, A., Alzamora, S. M., & Argaiz, A. (1998). Vanillin and pH synergistic effects on mold growth. Journal of Food Science, 63, 143–146.
- Markham, J. E., & Hille, J. (2001). Host-selective toxins as agents of cell death in plant-fungus interactions. Molecular Plant Pathology, 2, 229–239.
- Mathre, D. E. (1997). Compendium of barley diseases. USA: American Phytopathological Society, AGRIS.
- Mironas, O., Pokrib, C., Matescu, R., Boca, E., & Jurcoane, S. (2004). The inhibitor effect of some vegetal extracts on the fungal strains of the Alternaria sp. and Fusarium sp. Roumanian Biotechnol Letters, 9, 1523–1532.
- Nagrale, D. T., Gaikwad, A. P., & Sharma, L. (2013). Morphological and cultural characterization of Alternaria alternata (Fr.) Keissler blight of gerbera (Gerbera jamesonii H. Bolus ex J.D. Hook). Journal of Applied and Natural Science, 5, 171–178.
- Odoux, E. (2000). Changes in vanillin and glucovanillin concentrations during the various stages of the process traditionally used for curing Vanilla fragrans beans in Réunion. Fruits, 55, 119–125.
- Olaya, G., & Abawi, G. S. (1996). Effect of water potential on mycelial growth and on production and germination of sclerotia of Macrophomina phaseolina. Plant Disease, 80, 1347–1350.
- Ostry, V. (2008). Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin Journal, 1, 175–188.
- Pacheco, R. I. (2009). Evaluación del efecto de sonicación-microondas en el beneficio de la vainilla (Vanilla planifolia Andrews) (Master thesis). Instituto Politecnico Nacional, Oaxaca, Mexico. Retrieved from tesis.ipn.mx
- Pérez-Silva, A., Odoux, E., Brat, P., Ribeyre, F., Rodriguez-Jimenes, G., Robles-Olvera, V., … Günata, Z. (2006). GC–MS and GC–Olfactometry analysis of aroma compounds in a representative organic aroma extract from cured vanilla (Vanilla planifolia G. Jackson) beans. Food Chemistry, 99, 728–735.
- Pontón, J, Moragues, M. D, Gené, J, Guarro, J, & Quindós, G. (2002). Hongos y actinomicetos alergénicos. In Revista iberoamericana de micología españa (pp. 65pp).
- Prusti, A., Mishra, S. R., Sahoo, S., & Mishra, S. K. (2008). Antibacterial activity of some Indian medicinal plants. Ethnobotanical Leaflets, 12, 227–230.
- Purseglova, J. W., Drown, E. G., Green, C. L., & Robins, S. R. (1981). Vanilla (2nd ed.). New York, USA: Logran publishing.
- Ramírez, M. L, Chulze, S. N, & Magan, N. (2004). Impact of osmotic and matric water stress on germination, growth, mycelial water potentials and endogenous accumulation of sugars and sugar alcohols in fusarium graminearum. Mycologia, 96, 470–478. doi:10.1080/15572536.2005.11832946
- Ramjegathesh, R., & Ebenezar, E. G. (2012). Morphological and physiological characters of Alternaria alternata causing leaf blight disease of onion. International Journal of Plant Pathology, 3, 34–44.
- Ranadive, A. S. (1994). Vanilla-cultivation, curing, chemistry, technology and commercial products (34th ed.). Amsterdam, Netherlands: Elsevier Science Publishers.
- Rao, S. R., & Ravishankar, G. A. (2000). Vanilla flavour: Production by conventional and biotechnological routes. Journal of the Science of Food and Agriculture, 80, 289–304.
- Ravi, J. R. R., Sobita, L. S., & Lal, A. A. (2014). In vitro evaluation of some plant extracts against Alternaria alternata causing leaf spot of Aloe vera. ARPN Journal of Agricultural and Biological Science, 9, 323–325.
- Rivera-Carriles, K., Argaiz, A., Palou, E., & López-Malo, A. (2004). Synergistic inhibitory effect of citral with selected phenolics against Zygosaccharomyces bailii. Journal of Food Protection, 68, 602–606.
- Romero, F. (2003). Cultivo: Vainilla (Vanilla planifolia). Manual de Fitoprotección y Análisis de Plaguicidas, 1, 4–14.
- Rosado, Z. T. L. (2006). Efecto de las variables del proceso del beneficiado de la vainilla (Vanilla planifolia) sobre los parámetros de calidad (PhD Thesis). Instituto Tecnológico de Veracruz, Veracruz, Mex.
- Salo, P. M., Arbes, S. J., Sever, M., Jaramillo, R., Cohn, R. D., London, S. J., & Zeldin, D. C. (2006). Exposure to Alternaria alternata in US homes is associated with ashma symptoms. The Journal of Allergy and Clinical Immunology, 118, 892–898.
- Sanchez, D. D., Bautista, B. S., & Castillo, O. P. (2007). Efecto del quitosano en el desarrollo y morfologia de Alternaria alternata (Fr.) Keissl. Anuales de Biologia, 29, 23–32.
- Sanchez-Medina, A., Peña-Rodríguez, L. M., May-Pat, F., Karagianis, G., Waterman, P. G., Mallet, A. I., & Habtemariam, S. (2009). Identification of sakurasosaponin as a cytotoxic principle from Jacquinia flammea. Natural Product Comunications, 4, 1–6.
- Singh, G., Gupta, S., & Sharma, N. (2014). In vitro screening of selected plant extracts against Alternaria alternata. Journal of Experimental Biology and Agricultural Sciences, 2, 344–351.
- Tapia, O. A. P., Camacho, D. B. H., Perea, F. M. J., Ordoñez, R. L. M., Gutierrez, G. F., & Davila, O. G. (2011). Morphometric changes during the traditional curing process of vanilla pods (Vanilla planifolia; Orchidaceae) in Mexico. Revista Mexicana de Ingeniería Química, 10, 105–115.
- Tapwal, A., Nisha Garg, S., Gautam, N., & Kumar, R. (2011). In vitro antifungal potency pf plant extracts against five phytopathogens. Brazilian Archives of Biology Ans Technology, 54, 1093–1098.
- Thomas, M. D. (1991). Development of gray leaf spot on sorghum in Burkina Faso. Plant Disease, 75, 45–47.
- Tijerina-Ramírez, N., Lira-Méndez, K., Moreno-Medina, V. R., González-Prieto, J. M., & Mayek-Pérez, N. (2014). Efecto del estrés osmótico in vitro en el crecimiento, patogenicidad y producción de osmolitos en Macrophomina phaseolina. Revista Mexicana de Micología, 39, 31–39.
- Vargas, D. A. A., Gamboa, A. M., Medina, B. I. L., & Pérez, B. D. (2014). Evaluation of native Yucatecan plant extracts against Alternaria chrysanthemi and antifungal activity spectrum of Acalypha gaumeri. Revista mexicana de fitopatología, 32, 1–11.
- Vega, V. V., Silva, E. B. A., Cruz, V. M. R., Bernal, M. A. T., Gonzalez, A. G. A., Ruiz, C. S., … Ayala, Z. J. F. (2013). Antimicrobial and antioxidant properties of byproduct extracts of mango fruit. The Journal of Applied Botany and Food Quality, 86, 205–211.
- Walton, N. J., Mayer, M. J., & Narbad, A. (2003). Vanillin. Phytochemistry, 63, 505–515.
- Wolpert, T. J., Dunkle, L. D., & Ciuffetti, L. M. (2002). Host-selective toxins and avirulence determinants: What´s in a name? Annual Review of Phytopathology, 40, 251–285.
- Zare, L. (2013). The causal agent of barley black point disease in certified seed loads in Iran. The International Journal of Agriculture and Crop Sciences, 5, 332–335.
- Zhou, X., Jia, H., Ge, X., & Wu, F. (2018). Effects of vanillin on the community structures and abundances of Fusarium and Trichoderma spp. in cucumber seedling rhizosphere. Journal of Plant Interactions, 13(1), 45–50.
