ABSTRACT
We compared the stereo-selective growth inhibition effects of purified enantiomeric pairs of ginsenoside Rh2 (20(S)-ginsenoside Rh2 and 20(R)-ginsenoside Rh2) on human colon cancer cells in vitro with non-invasive real-time cellular analysis. When 50 μM, respectively, of 20(S)-ginsenoside Rh2 and 20(R)-ginsenoside Rh2 were administered to the colon cancer cell line HT-29, only the S-type ginsenoside Rh2 lowered cancer cell viability. Similarly, when the same experiment was conducted on cancer cells with different levels of ginsenoside treatments (20, 40, 60, and 80 μM), cancer cell viability was decreased in a dose-dependent manner only in the 20(S)-ginsenoside Rh2 treatment groups. Cytotoxicity of cancer cells was observed only in the S form-treated group when cellular response and growth pattern were analyzed via real-time cellular analysis. These findings suggest that the anti-tumor effect of ginsenoside Rh2 on colon cancer cells is S-form selective.
RESUMEN
En el presente estudio se compararon los efectos de inhibición del crecimiento estereoselectivo de pares enantioméricos purificados de ginsenósido Rh2 (20 (S)-ginsenósido Rh2 y 20 (R)-ginsenósido Rh2) en células de cáncer de colon humano in vitro, empleando para ello un análisis celular no invasivo en tiempo real. Cuando se administraron 50 μM de 20 (S) -ginsenósido Rh2 y de 20 (R) -ginsenósido Rh2 a la línea celular de cáncer de colon HT-29, se constató que solo el ginsenósido Rh2 de tipo S disminuyó la viabilidad de las células cancerosas. De manera similar, al realizar el mismo experimento en células cancerosas expuestas a diferentes niveles de tratamientos con ginsenósidos (20, 40, 60 y 80 µM), se comprobó que la viabilidad de las células cancerosas se redujo de manera dependiente de la dosis solo en los grupos tratados con 20 (S) -ginsenósido Rh2. Asimismo, cuando se examinó la respuesta celular y el patrón de crecimiento a través de análisis celular en tiempo real se observó que las células cancerosas mostraron citotoxicidad solo en el grupo tratado con la forma S. Estos hallazgos sugieren que el efecto antitumoral del ginsenósido Rh2 en células de cáncer de colon es selectivo en su forma S.
PALABRAS CLAVE:
1. Introduction
Ginseng (the root of Panax ginseng Meyer, family Araliaceae) has long been used as a traditional medicine in East Asia (China, Korea, and Japan) (Xu, Choi, & Huang, Citation2017). Recently, ginseng has attracted attention not only in the Asian market but also in many Western countries as a medicinal herb (Arring, Millstine, Marks, & Nail, Citation2018). Ginseng contains multiple substances that act as immune, stress and cholesterol modulators, such as acidic polysaccharides, ginsenosides, polyacetylenes, sesquiterpene and polyphenolic compounds (Cho et al., Citation2013). Among these, ginsenosides, belonging to the dammarane triterpene saponin group, are regarded as key functional components of the health benefits of ginseng (Ku, You, Park, & Ji, Citation2015; Lee & Kim, Citation2014). Ginsenosides have been reported to have significant in vivo hypoglycemic, hepatoprotective, anti-allergic, and anti-carcinogenic effects (Chen et al., Citation2015; Li et al., Citation2018; Ning et al., Citation2018; Yang et al., Citation2017; Zhou et al., Citation2018). To date, >100 kinds of ginsenosides have been isolated from ginseng (Kim, Yi, Kim, & Cho, Citation2017). Since these ginsenoside molecules have different structures, their biofunctional effects in the human body differ (Ku, Citation2016). Generally, the structure-specific effects of ginsenosides have been investigated in two major ways: (i) level of sugar and (ii) OH side chains attached to the dammarane skeleton (17 carbons in a four-ring structure). First, many researchers have studied how the numbers and positions of sugar moieties attached to ginsenosides can affect the physiological changes of ginsenosides, and it has been reported that ginsenoside aglycones are more potent than glycoside forms. Several groups reported that ginsenosides with fewer sugar moieties, such as Rh2, Compound K, and PPD, had stronger anti-tumor effects than those with more sugar residues such as Rb1, Rb2, and Rc (Dong et al., Citation2011; Popovich & Kitts, Citation2002). Recently, various food and cosmetic companies have released ginseng products containing ginsenoside aglycones and used their inclusion as a marketing tool. Second, ginsenosides can be divided into two groups based on the number of hydroxyl groups attached to the ginsenoside skeleton: protopanaxadiols (PD), which contain two hydroxyl groups at positions C-3 and C-20 (e.g., ginsenosides Rb1, Rb2, Rc, Rd, Rg3, and Rh2), and protopanaxatriols (PT), which contain three hydroxyl groups at positions C-3, C-6, and C-20 (e.g., ginsenosides Re, Rg1, and Rh1, and notoginsenoside R1). Among these ginsenoside molecules, PD types such as Rh2, Compound K, and Rg3 have been reported to show potent apoptotic effects on various cancer cell lines and in in vivo models (Dong et al., Citation2011; Kim et al., Citation2009; Li et al., Citation2018; Liu, Bu, Yan, & Jia, Citation2007; Zheng, Jeong, Song, & Ji, Citation2011; Zou, Wang, Gao, Han, & Fang, Citation2018).
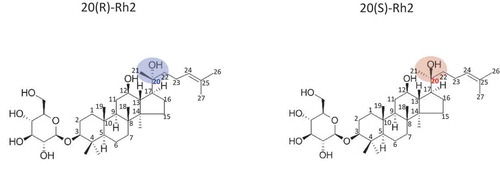
In addition to sugar levels and numbers of hydroxyl groups, recent scholarship is increasingly interested in studying the functional changes of ginsenoside molecules by stereoisomers. Specifically, 20(S) and 20(R) ginsenoside Rh2s are stereoisomers that have the same molecular (C36H62O8) formula and sequence of bonded atoms. However, these two ginsenoside Rh2s have different three-dimensional shapes due to the dissimilar orientation of the C-20 OH group in ginsenoside Rh2. The OH group of 20(S) is three-dimensionally closer to the C-12 OH group of ginsenosides. However, the OH group in 20(R) is relatively far away from the C-12 OH side chain compared to 20(S). This difference in the three-dimensional orientations of the hydroxyl groups of ginsenoside Rh2s potentially results in dissimilar biofunctionalities ().
Figure 1. Chemical structures of the ginsenosides 20 (S)-Rh2 and 20 (R)-Rh2.
Figura 1. Estructuras químicas de los ginsenósidos 20 (S)-Rh2 y 20 (R)-Rh2.
The colon cancer is a malignant tumor consisting of cancer cells that develop in the large intestine. Though early detection and treatment of colon cancer can improve the therapeutic effect, many patients find colon cancer after progression due to the lack of early-stage symptoms (Alonso-Abreu et al., Citation2017). When colon cancer is diagnosed, surgery and medicinal therapy with 5-fluorouracil (5-FU), bevacizumab, and cetuximab are generally performed (Kuipers et al., Citation2015). However, the chemotherapy and targeted therapy showed diverse side effects such as alopecia and hypertension, so supplement with traditional non-toxic medicinal substance could be beneficial for prevention and treatment (Kuipers et al., Citation2015; Scheithauer et al., Citation2003).
Recently, ginsenosides have been in the spotlight as anti-cancer agents in the nutraceutical market. However, the literature based on objective evidence regarding the stereo-selective activity and growth pattern of cancer cells in real time is still lacking. Therefore, the present study examined the efficacy of Rh2-dependent cancer cell repression on enantiomer status using two Rh2 stereoisomer forms (i.e. 20(S) and 20(R)). Further, using a non-invasive, real-time cellular analysis (RTCA) method allowed for the understanding of cellular status and growth pattern of the HT-29 colon cancer cells with different concentrations of both types of two Rh2 stereoisomer forms.
1.1. Growth inhibitory effect of 20(S)-Rh2 on a colon cancer cell line
HT-29 colon cancer cells were treated with 50 μM of each form of Rh2 for 72 h, then an MTT assay was performed to assess their growth-inhibitory effects. The growth inhibitory effect on HT-29 colon cancer cells was observed through a cell viability ratio and compared with the vehicle control (0.1% (v/v) DMSO) as 100%. A non-treated group was used to determine whether the 0.1% DMSO used in the vehicle control group had any effect. The results indicated that the DMSO had no impact. As shown in , each substance was pretested in 50 μM of concentration, and the viability of HT-29 cells treated with 20(S)-Rh2 was found to be significantly lower than that of the control (p< .05). Also, as shown in , the growth of HT-29 cells treated with 20(S)-Rh2 was reduced in a dose-dependent manner compared with the vehicle control and the 20(R)-Rh2 ginsenoside-treated group. Cancer cell viability was significantly reduced by 20(S)-Rh2 treatment. However, 20(R)-Rh2 ginsenoside treatment did not show any significant decrease in cancer cell viability (). We also performed a viability assay of fetal human cells, FHC cell line (ATCC, Manassas, VA, USA) as normal colon cells at the concentration of 50 μM of 20(S)-Rh2 for 72 h. The viability of normal cell was 90 ± 4.6% (vehicle: 100%) presenting very low growth inhibitory effect compared to the viability of HT-29 cells at the same concentration, 32.2 ± 4.5%, so we could clarify the anticancer activity of 20(S)-Rh2 was cancer cell-specific effect.
Figure 2. Significant decrease in the viability of human colon cancer cells (HT-29) after treatment of the culture medium with 50 µM of 20 (S)-Rh2 for 72 hours. Treatments with different letters (where a > b) are significantly different at p < .05. Data are expressed as the mean ± SD (n = 3).
Figura 2. Disminución significativa de la viabilidad de las células de cáncer de colon humano (HT-29) después del tratamiento del medio de cultivo con 50 µM de 20 (S)-Rh2 durante 72 horas. Los tratamientos con letras diferentes (donde a > b) son significativamente diferentes en p < .05. Los datos se expresan como la media ± SD (n = 3).
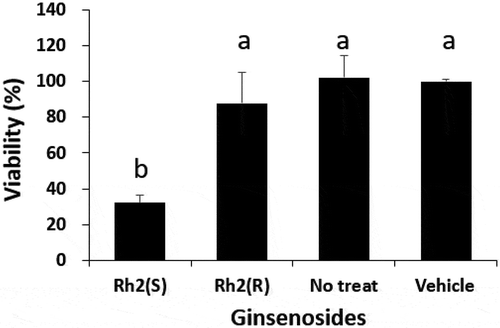
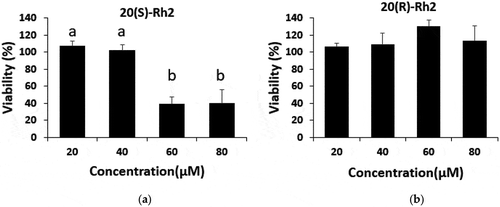
Figure 3. Effect of the treatments 20 (S)-Rh2 (a) and 20 (R)-Rh2 (b) on the viability of HT-29. The viability of the HT-29 cells was assessed by the MTT assay at various concentrations of each molecule 72 hours after the treatment. Treatments with different letters (where a > b) are significantly different at p < 0.05. The data are expressed as the mean ± SD.
Figura 3. Efecto de los tratamientos 20 (S)-Rh2 (a) y 20 (R)-Rh2 (b) sobre la viabilidad de HT-29. La viabilidad de las células HT-29 se valoró mediante el ensayo MTT a diversas concentraciones de cada molécula 72 horas después del tratamiento. Los tratamientos con letras diferentes (donde a > b) son significativamente diferentes en p < 0.05. Los datos se expresan como la media ± DE.
1.2. Real-time growth pattern of colon cancer cells treated with Rh2
To monitor simultaneous cellular status and growth pattern, RTCA was performed in the presence of 2% fetal bovine serum (FBS) with 10, 25, 50, and 75 μM of both types of Rh2s. At 10μM, neither of the Rh2 types caused a cellular profile change, but at higher concentrations, the two types of Rh2 produced different profiles. The treatment with 25 μM of 20(S)-Rh2 showed a cytostatic profile from 18 to 42 h after treatment, followed by a loss of viability (), whereas 20(R)-Rh2 showed no change of profile (). Cells treated with 50 μM of 20(S)-Rh2 maintained growth for 7 h, but after that, a loss of viability was shown in the profile. When the HT-29 cells were treated with 75 μM 20(S)-Rh2, the cells were detached from the surface of the culture well and died immediately after treatment ()).
Figure 4. Analysis of the RTCA profile of HT-29 cells in the presence of ginsenosides (a) 20 (S)-Rh2 and (b) 20 (R)-Rh2. HT-29 cells were seeded in the wells of a 96-well plate and treated with different concentrations of two different types of Rh2 ginsenoside (10, 25, 50 and 75 µM) and the control vehicle (0.1% DMSO [v. / v]). The viability of the cells was monitored continuously using an RTCA system for 72 hours and showed normalized IC values, which were recorded every 30 minutes. The trace for each concentration was obtained as an average of three repetitions.
Figura 4. Análisis del perfil RTCA de las células HT-29 en presencia de ginsenósidos (a) 20 (S)-Rh2 y (b) 20 (R)-Rh2. Se sembraron células HT-29 en los pocillos de una placa de 96 pocillos y se las trató con diferentes concentraciones de dos tipos diferentes de ginsenósido Rh2 (10, 25, 50 y 75 µM) y el vehículo de control (DMSO 0.1% [v/v]). La viabilidad de las células se controló de manera continua empleando para ello un sistema RTCA durante 72 horas y se mostró como valores de CI normalizados, mismos que se registraron cada 30 minutos. La traza para cada concentración se obtuvo como un promedio de tres repeticiones.
![Figure 4. Analysis of the RTCA profile of HT-29 cells in the presence of ginsenosides (a) 20 (S)-Rh2 and (b) 20 (R)-Rh2. HT-29 cells were seeded in the wells of a 96-well plate and treated with different concentrations of two different types of Rh2 ginsenoside (10, 25, 50 and 75 µM) and the control vehicle (0.1% DMSO [v. / v]). The viability of the cells was monitored continuously using an RTCA system for 72 hours and showed normalized IC values, which were recorded every 30 minutes. The trace for each concentration was obtained as an average of three repetitions.Figura 4. Análisis del perfil RTCA de las células HT-29 en presencia de ginsenósidos (a) 20 (S)-Rh2 y (b) 20 (R)-Rh2. Se sembraron células HT-29 en los pocillos de una placa de 96 pocillos y se las trató con diferentes concentraciones de dos tipos diferentes de ginsenósido Rh2 (10, 25, 50 y 75 µM) y el vehículo de control (DMSO 0.1% [v/v]). La viabilidad de las células se controló de manera continua empleando para ello un sistema RTCA durante 72 horas y se mostró como valores de CI normalizados, mismos que se registraron cada 30 minutos. La traza para cada concentración se obtuvo como un promedio de tres repeticiones.](/cms/asset/50dbc548-ae6d-4ff0-ae30-90219d4017c8/tcyt_a_1607562_f0004_oc.jpg)
2. Discussion
According to a number of stereochemistry studies, certain compounds such as ibuprofen and thalidomide can produce different physiological effects depending on their racemic status (Sanganyado, Lu, Fu, Schlenk, & Gan, Citation2017). The pharmaceutical effects of ibuprofen on pain reduction and anti-inflammation are mainly due to S-form ibuprofen (Evans, Citation2001; Kaehler, Phleps, & Hesse, Citation2003). By removing R-form ibuprofen from an existing racemic mixture, the side effects and dosages could be reduced, and medicinal effects could be enhanced (Kaehler et al., Citation2003). Thalidomide, used as a sedative for pregnant women in the 1960s who subsequently gave birth to numerous-deformed babies, is also a racemic mixture. Although R-type thalidomide has a sedative effect, S-type thalidomide has a teratogenicity effect (Blaschke, Kraft, Fickentscher, & Kohler, Citation1979; Eriksson, Bjorkman, Roth, & Hoglund, Citation2000; Heger et al., Citation1994; Hoglund, Eriksson, & Bjorkman, Citation1998). As shown in the example above, when an enantiomer of a useful substance exists under development, it should be verified that there is a difference in activity depending on each form.
Although studies on the anti-cancer activity of ginsenoside Rh2 have been performed on lung, breast, liver, colorectal cancers, and leukemia, there has been no study of the cellular pattern of cancer cells when Rh2 was treated in real-time (Ge, Yan, & Cai, Citation2017; Han et al., Citation2016; Kim & Choi, Citation2016; Li, Li, Dong, Wang, & Li, Citation2017; Ren, Shi, Teng, & Yao, Citation2018; Wan et al., Citation2017). Among the existing studies, there is a study on how Rh2 using HCT-116 cells can act as a specific mechanism of anti-cancer activity for a colorectal cell line (Han et al., Citation2016). This study reported that only 20(S)-Rh2 showed anti-cancer activity when 20(R)-Rh2 or 20(S)-Rh2 were treated with HCT-116 cells. It also demonstrated that 20(S)-Rh2 had the effect of inhibiting the IL-6-mediated tumor invasion process of HCT-116 cells. However, studies on the specific mechanisms that inhibit the growth of colon cancer cells have not yet been conducted. Other groups and researchers have observed that the viability of cancer cells is inhibited by ginsenoside Rh2 through an end-point assay similar to the study method used in this study. However, the end-point assay approach did not use RTCA to determine a timely pattern for how ginsenoside Rh2 inhibited cell growth.
Observation of real-time cellular patterns reveals information that cannot be obtained from an end-point assay (Xing, Zhu, Gabos, & Xie, Citation2006; Yu et al., Citation2006). With RTCA, it is possible to evaluate how soon the cell inhibitory effect is activated after treatment (Jeong, You, & Ji, Citation2012; Lohberger et al., Citation2013). It can also distinguish whether the treatment is a cytotoxic reagent that acts immediately, an apoptosis-inducing substance or substances that delay the growth of cells. In RTCA, the cell index did not increase or decrease during the 72-h treatment with 20(R)-Rh2. This result seems to be due to the fact that the cells in wells had changed to a confluent form. In addition, even though the cells were confluent, it was confirmed that sufficient nutrient in the medium did not lead to cell death. However, no significant difference in cell growth inhibition was observed when 20(S)-Rh2 or 20(R)-Rh2 at 10μM was treated with cancer cells under the same experimental conditions. When the concentration of ginsenoside was increased to 25 μM, a cytostatic pattern from 18 to 42 h was observed in the group treated with 20(S)-Rh2, and 42 h later, the cell index had dramatically decreased. This outcome suggests that HT-29 cells exhibit a pattern similar to that observed in the process of apoptosis after G1 phase cell cycle arrest, as reported in a previous study of ginsenoside compound K, an isomer of ginsenoside Rh2 (Jeong et al., Citation2012). However, further studies that include cell cycle analysis and mechanism-related gene or protein expression are needed in order to clarify the stereo-selective growth inhibitory activity of ginsenoside Rh2 on colon cancer cells
3. Conclusions
Among the two enantiomers of ginsenoside Rh2 tested in this study, 20(S)-Rh2 proved to be an effective reagent for cancer cell inhibition. The patterns of real-time cellular response are either different dependent on the enantiomeric form. Taken together, these results provide novel evidence that the chiral characteristics of ginsenoside Rh2 enantiomeric pairs exhibit stereo-selective growth inhibition effects on cancer cells
4. Materials and methods
4.1. Chemicals and reagents
We purchased commercially available 20(S)-ginsenoside Rh2 (LKT Laboratories, Saint Paul, MN, USA) and 20(R)-ginsenoside Rh2 (LKT Laboratories, Saint Paul, MN, USA). and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). The materials used for cell culture including media, fetal bovine serum (FBS), antibiotic solutions, and related reagents were obtained from GIBCO® (Invitrogen Life Technologies, Carlsbad, CA, USA).
4.2. Cell culture
The human colorectal adenocarcinoma cell line, HT-29(KCLB, Seoul, Korea), was maintained in Dulbecco’s Modified Eagle Medium (DMEM) and contained10%(v/v) of fetal bovine serum(FBS) and 1%(v/v) of antibiotic-antimycotic solution (Invitrogen, Calsbad, CA, USA). The normal colon cell line, FHC was purchased from ATCC (Manassas, VA, USA) and cultured in DMEM/F12 media containing 25 mM HEPES, 10 ng/mL hydrocortisone, 10% (v/v) FBS, and 1% (v/v) antibiotic-antimycotic solution. Cells were incubated at 37℃ in a humidified incubator containing 5% CO2 and subcultured prior to the experiment in order to be confluent in the T-75 flask. After the incubation, cells were harvested and a hemacytometer count was performed using thetrypan blue dye exclusion methods.
4.3. Cell viability
To estimate the viability of both Rh2s on the human cancer cells, MTT assay was performed. The cells were seeded in 96-well plates at 6 × 103 cells per well and attached to the bottom of the plate in a humidified incubator(37℃, 5% CO2) for 24 h. Then, the medium was replaced with an FBS-free medium to adjust the growth phase of each cell equally. After 24 h, the medium was replaced with 2% FBS medium, and test compounds were treated to each well. Then, incubation with test compounds for 72 h, 20μL of the MTT solution(5mg/ml) were added to each well and plates were incubated at 37℃ for 2-4h. The absorbance at 570nm was recorded using a microplate reader(Bio-Rad Laboratories, Philadelphia, PA, USA).
4.4. Real time analysis of cell growth pattern
Monitoring and recording of cellular growth patterns were carried out using real time cell analysis(RTCA) with the xCELLigence RTCA system(ACEA Biosciences, San Diego, CA, USA) and 96-well E-plateTM(ACEA Biosciences, San Diego, CA, USA). The RTCA SP instrument equipped with the E-plateTM and the seeded Rh2 treated cells was placed inside the CO2 incubator and the measured data were transferred to the connected analyzer outside under the control of integrated software (Xing et al., Citation2006; Yu et al., Citation2006, pp. 40–43). One hundred μL of HT-29 cell suspension was added to each well of the E-plateTM and monitored every hour. Approximately 48 h after seeding, the cells were treated with both forms of Rh2 in various concentrations and vehicle controls and were monitored for 72 h.
4.5. Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The student’s t-test was performed for the comparison between two groups and one-way ANOVA with Tukey’s post hoc test was implemented for multiple comparisons. All analyses were done with Prism 7 software.
Conflicts of Interest
The authors declare no conflict of interest.
Author contributions
Y.J. initiated this work in partial fulfillment of her PhD degree at Seoul National University under the supervision of G.E.J. and the mentorship of S.K. and H.J.Y. Y.J.performed cell line experiments under the mentorship of H.J.Y. and G.E.J. Y.J. and S.K. collaboratively wrote the manuscript and performed the literature review. S.K. edited and revised the manuscript based on a non-disclosure research agreement between Middle Tennessee State University and BIFIDO Co., Ltd. All authors discussed the drafts and approved the final manuscript for publication
Acknowledgments
The authors wish to thank Ingrid A. Pierce at Clemson University and Tony V. Johnston at MTSU for their review and feedback.
Additional information
Funding
References
- Alonso-Abreu, I., Alarcon-Fernandez, O., Gimeno-Garcia, A. Z., Romero-Garcia, R., Carrillo-Palau, M., Nicolas-Perez, D., … Quintero, E. (2017). Early Colonoscopy Improves the Outcome of Patients With Symptomatic Colorectal Cancer. Diseases of the Colon and Rectum, 60(8), 837–844. doi:10.1097/DCR.0000000000000863
- Arring, N. M., Millstine, D., Marks, L. A., & Nail, L. M. (2018). Ginseng as a treatment for fatigue: A systematic review. Journal of Alternative and Complementary Medicine (New York, N.Y.), 24(7), 624–633. doi:10.1089/acm.2017.0361
- Blaschke, G., Kraft, H. P., Fickentscher, K., & Kohler, F. (1979). [Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers (author’s transl)]. Arzneimittelforschung, 29(10), 1640–1642.
- Chen, T., Xiao, L., Zhu, L., Ma, S., Yan, T., & Ji, H. (2015). Anti-Asthmatic effects of ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation, 38(5), 1814–1822. doi:10.1007/s10753-015-0159-4
- Cho, C. W., Kim, Y. C., Kang, J. H., Rhee, Y. K., Choi, S. Y., Kim, K. T., … Hong, H. D. (2013). Characteristic study on the chemical components of Korean curved ginseng products. Journal of Ginseng Research, 37(3), 349–354. doi:10.5142/jgr.2013.37.349
- Dong, H., Bai, L. P., Wong, V. K., Zhou, H., Wang, J. R., Liu, Y., … Liu, L. (2011). The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules, 16(12), 10619–10630. doi:10.3390/molecules161210619
- Eriksson, T., Bjorkman, S., Roth, B., & Hoglund, P. (2000). Intravenous formulations of the enantiomers of thalidomide: Pharmacokinetic and initial pharmacodynamic characterization in man. The Journal of Pharmacy and Pharmacology, 52(7), 807–817.
- Evans, A. M. (2001). Comparative pharmacology of S(+)-ibuprofen and (RS)-ibuprofen. Clinical Rheumatology, 20(Suppl 1), S9–14.
- Ge, G., Yan, Y., & Cai, H. (2017). Ginsenoside Rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biological & Pharmaceutical Bulletin, 40(12), 2117–2124. doi:10.1248/bpb.b17-00463
- Han, S., Jeong, A. J., Yang, H., Bin Kang, K., Lee, H., Yi, E. H., … Ye, S. K. (2016). Ginsenoside 20(S)-Rh2 exerts anti-cancer activity through targeting IL-6-induced JAK2/STAT3 pathway in human colorectal cancer cells. Journal of Ethnopharmacology, 194, 83–90. doi:10.1016/j.jep.2016.08.039
- Heger, W., Schmahl, H. J., Klug, S., Felies, A., Nau, H., Merker, H. J., & Neubert, D. (1994). Embryotoxic effects of thalidomide derivatives in the non-human primate callithrix jacchus. IV. Teratogenicity of micrograms/kg doses of the EM12 enantiomers. Teratogenesis, Carcinogenesis, and Mutagenesis, 14(3), 115–122.
- Hoglund, P., Eriksson, T., & Bjorkman, S. (1998). A double-blind study of the sedative effects of the thalidomide enantiomers in humans. Journal of Pharmacokinetics and Biopharmaceutics, 26(4), 363–383.
- Jeong, Y., You, H., & Ji, G. E. (2012). Ginsenoside compound K induces cell cycle arrest and apoptosis in human colon cancer cells. International Journal of Biomedical and Pharmaceutical Sciences, 6((Special Issue 1)), 113–118.
- Kaehler, S. T., Phleps, W., & Hesse, E. (2003). Dexibuprofen: Pharmacology, therapeutic uses and safety. Inflammopharmacology, 11(4), 371–383. doi:10.1163/156856003322699555
- Kim, D. Y., Park, M. W., Yuan, H. D., Lee, H. J., Kim, S. H., & Chung, S. H. (2009). Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. Journal of Agricultural and Food Chemistry, 57(22), 10573–10578. doi:10.1021/jf902700h
- Kim, J. H., & Choi, J. S. (2016). Effect of ginsenoside Rh-2 via activation of caspase-3 and Bcl-2-insensitive pathway in ovarian cancer cells. Physiological Research / Academia Scientiarum Bohemoslovaca, 65(6), 1031–1037.
- Kim, J. H., Yi, Y. S., Kim, M. Y., & Cho, J. Y. (2017). Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. Journal of Ginseng Research, 41(4), 435–443. doi:10.1016/j.jgr.2016.08.004
- Ku, S. (2016). Finding and producing probiotic glycosylases for the biocatalysis of ginsenosides: A mini review. Molecules, 21, 5. doi:10.3390/molecules21050645
- Ku, S., You, H. J., Park, M. S., & Ji, G. E. (2015). Effects of ascorbic acid on alpha-l-arabinofuranosidase and alpha-l-arabinopyranosidase activities from Bifidobacterium longum RD47 and its application to whole cell bioconversion of ginsenoside. Journal of the Korean Society for Applied Biological Chemistry, 58(6), 857–865. doi:10.1007/s13765-015-0113-z
- Kuipers, E. J., Grady, W. M., Lieberman, D., Seufferlein, T., Sung, J. J., Boelens, P. G., … Watanabe, T. (2015). Colorectal cancer. Nature Reviews. Disease Primers, 1, 15065. doi:10.1038/nrdp.2015.65
- Lee, C. H., & Kim, J. H. (2014). A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. Journal of Ginseng Research, 38(3), 161–166. doi:10.1016/j.jgr.2014.03.001
- Li, J., Li, R., Li, N., Zheng, F., Dai, Y., Ge, Y., … Yu, S. (2018). Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. Journal of Pharmaceutical and Biomedical Analysis, 158, 451–460. doi:10.1016/j.jpba.2018.06.024
- Li, K. F., Kang, C. M., Yin, X. F., Li, H. X., Chen, Z. Y., Li, Y., … Qiu, Y. R. (2018). Ginsenoside Rh2 inhibits human A172 glioma cell proliferation and induces cell cycle arrest status via modulating Akt signaling pathway. Molecular Medicine Reports, 17(2), 3062–3068. doi:10.3892/mmr.2017.8193
- Li, Q., Li, B., Dong, C., Wang, Y., & Li, Q. (2017). 20(S)-Ginsenoside Rh2 suppresses proliferation and migration of hepatocellular carcinoma cells by targeting EZH2 to regulate CDKN2A-2B gene cluster transcription. European Journal of Pharmacology, 815, 173–180. doi:10.1016/j.ejphar.2017.09.023
- Liu, G. Y., Bu, X., Yan, H., & Jia, W. W. (2007). 20S-protopanaxadiol-induced programmed cell death in glioma cells through caspase-dependent and -independent pathways. Journal of Natural Products, 70(2), 259–264. doi:10.1021/np060313t
- Lohberger, B., Rinner, B., Stuendl, N., Kaltenegger, H., Steinecker-Frohnwieser, B., Bernhart, E., … Kretschmer, N. (2013). Sesquiterpene lactones downregulate G2/M cell cycle regulator proteins and affect the invasive potential of human soft tissue sarcoma cells. PloS One, 8(6), e66300. doi:10.1371/journal.pone.0066300
- Ning, C., Gao, X., Wang, C., Huo, X., Liu, Z., Sun, H., … Liu, K. (2018). Hepatoprotective effect of ginsenoside Rg1 from Panax ginseng on carbon tetrachloride-induced acute liver injury by activating Nrf2 signaling pathway in mice. Environmental Toxicology, 33(10), 1050–1060. doi:10.1002/tox.22616
- Popovich, D. G., & Kitts, D. D. (2002). Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Archives of Biochemistry and Biophysics, 406(1), 1–8.
- Ren, G., Shi, Z., Teng, C., & Yao, Y. (2018). Antiproliferative activity of combined biochanin a and ginsenoside Rh(2) on MDA-MB-231 and MCF-7 human breast cancer cells. Molecules, 23, 11. doi:10.3390/molecules23112908
- Sanganyado, E., Lu, Z., Fu, Q., Schlenk, D., & Gan, J. (2017). Chiral pharmaceuticals: A review on their environmental occurrence and fate processes. Water Research, 124, 527–542. doi:10.1016/j.watres.2017.08.003
- Scheithauer, W., McKendrick, J., Begbie, S., Borner, M., Burns, W. I., Burris, H. A., … Group, X. A. S. (2003). Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: Safety results of a randomized, phase III trial. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO, 14(12), 1735–1743. doi:10.1093/annonc/mdg500
- Wan, J. Y., Wang, C. Z., Zhang, Q. H., Liu, Z., Musch, M. W., Bissonnette, M., … Yuan, C. S. (2017). Significant difference in active metabolite levels of ginseng in humans consuming Asian or Western diet: The link with enteric microbiota. Biomedical Chromatography: BMC, 31(4). doi:10.1002/bmc.3882
- Xing, J. Z., Zhu, L., Gabos, S., & Xie, L. (2006). Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicology in Vitro : an International Journal Published in Association with BIBRA, 20(6), 995–1004. doi:10.1016/j.tiv.2005.12.008
- Xu, W., Choi, H. K., & Huang, L. (2017). State of panax ginseng research: A global analysis. Molecules, 22, 9. doi:10.3390/molecules22091518
- Yang, X., Zou, J., Cai, H., Huang, X., Yang, X., Guo, D., & Cao, Y. (2017). Ginsenoside Rg3 inhibits colorectal tumor growth via down-regulation of C/EBPbeta/NF-kappaB signaling. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 96, 1240–1245. doi:10.1016/j.biopha.2017.11.092
- Yu, N., Atienza, J. M., Bernard, J., Blanc, S., Zhu, J., Wang, X., … Abassi, Y. A. (2006). Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: An approach to study G protein-coupled receptors. Analytical Chemistry, 78(1), 35–43. doi:10.1021/ac051695v
- Zheng, H., Jeong, Y., Song, J., & Ji, G. E. (2011). Oral administration of ginsenoside Rh1 inhibits the development of atopic dermatitis-like skin lesions induced by oxazolone in hairless mice. International Immunopharmacology, 11(4), 511–518. doi:10.1016/j.intimp.2010.12.022
- Zhou, Y. D., Hou, J. G., Liu, W., Ren, S., Wang, Y. P., Zhang, R., … Li, W. (2018). 20(R)-ginsenoside Rg3, a rare saponin from red ginseng, ameliorates acetaminophen-induced hepatotoxicity by suppressing PI3K/AKT pathway-mediated inflammation and apoptosis. International Immunopharmacology, 59, 21–30. doi:10.1016/j.intimp.2018.03.030
- Zou, M., Wang, J., Gao, J., Han, H., & Fang, Y. (2018). Phosphoproteomic analysis of the antitumor effects of ginsenoside Rg3 in human breast cancer cells. Oncology Letters, 15(3), 2889–2898. doi:10.3892/ol.2017.7654
