ABSTRACT
In this work a Bacillus strain with capacity to produce biosurfactants was isolated from commercial corn steep water (CSW). The identification of this bacterium was based on phenotypic characteristics. This is a Gram-positive Bacillus, macroscopically catalase-negative, that possesses high mobility and forms terminal endospore. The colonies formed by the isolated Bacillus strain are characterized by a creaming, dull and glistening phenotype with a small point-like elevation in the center. Regarding its antimicrobial activity, the isolated Bacillus strain was effective against Candida albicans and Staphylococcus aureus observing, in the presence of pathogenic strains, a phenotype dissociation, switching the colonies from a whitish to yellowish pigmentation. The isolated microorganism, contrarily to other Bacillus strains, has the ability to produce extracellular and cell-bound biosurfactants. The analyses revealed that both biosurfactant extracts are similar to the lipopeptide biosurfactant produced by Bacillus subtilis.
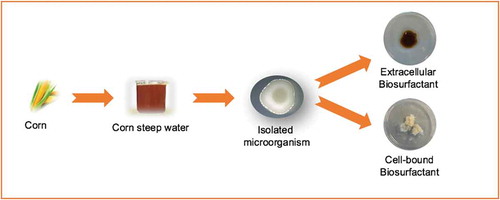
GRAPHICAL ABSTRACT López-Prieto et al., 2019
Resumen
En este trabajo, una cepa de Bacillus con capacidad para producir biosurfactantes fue aislada de los licores de lavado de maíz (Corn Steep Water (CSW)). La identificación de esta bacteria se basó en características fenotípicas. Esta es un Bacillus gram-positivo, catalasa-negativo que, a nivel macroscópico, posee alta movilidad y forma endosporas terminales. Las colonias formadas por la cepa aislada de Bacillus se caracterizan por un fenotipo cremoso, opaco y brillante, con una pequeña elevación en el centro. En cuanto a su actividad antimicrobiana, la cepa aislada de Bacillus fue eficaz contra Candida albicans y Staphylococcus aureus observando, en presencia de cepas patógenas, una disociación en su fenotipo, pasando las colonias de una pigmentación blanquecina a amarillenta. El microorganismo aislado, contrariamente a otras cepas de Bacillus, tiene la capacidad de producir biosurfactantes extracelulares y biosurfactantes ligados a su membrana. Los análisis revelaron que ambos extractos biosurfactantes tienen una composición similar al biosurfactante lipopéptido producido por Bacillus subtilis.
PALABRAS CLAVES:
Introduction
Biosurfactants are biocompatible and biodegradable surface-active compounds produced by microorganisms. In contrast to most surfactants, which are obtained by chemical synthesis, biosurfactants present features such as good efficiency and low levels of toxicity and irritation in addition to the fact that they are environmentally friendly. They can be used to help in solubilisation and stabilization of emulsions and to enhance the sensory and rheological characteristics of mixtures including food formulations (Santos, Rufino, Luna, Santos, & Sarubbo, Citation2016).
On the other hand, it has been demonstrated that some biosurfactants have antimicrobial activities in addition to their surfactant capacity (Das, Mukherjee, & Sen, Citation2008). This characteristic can be an additional advantage for the incorporation of these secondary metabolites in different sectors including pharmaceutical, cosmetic and food industry (López-Prieto, Rodríguez-López, Rincón-Fontán, Moldes, & Cruz, Citation2019; Rodríguez-López, Rincón-Fontán, Vecino, Cruz, & Moldes, Citation2017; Ron & Rosenberg, Citation2001; Souza, Oliveira de Souza de Azevedo, Domínguez, Converti, & Pinheiro de Souza Oliveira, Citation2017; Vecino, Barbosa-Pereira, Devesa-Rey, Cruz, & Moldes, Citation2015a).
In recent years, agro-industrial wastes have gained attention as possible sources for the production and extraction of biosurfactants promoting their use as secondary raw materials (Rodríguez-López et al., Citation2017; Vecino, Barbosa-Pereira, Devesa-Rey, Cruz, & Moldes, Citation2014; Vecino et al., Citation2017). Therefore, Vecino et al. (Citation2014) have proven that corn steep water (CSW), a fermented residual stream of corn-milling industry, is a direct source of biosurfactants due to the spontaneous growth of microorganisms producing biosurfactants. Nevertheless, these microorganisms have not been isolated at the moment.
In the corn wet milling industry, the steeping process of corn is carried out between 45°C and 52°C under acid conditions and in the presence of SO2 (Hull, Yang, Venzke, Kulhavy, & Montgomery, Citation1996), therefore, it can be speculated that the existing microbial biomass in CSW possesses a special mechanism to support these extreme environmental conditions.
On the other hand, López-Prieto et al. (Citation2019) showed that the biosurfactant extract obtained from CSW can be integrated in the dairy industry. These authors proved that the incorporation of this biosurfactant to a drinkable yogurt promoted the growth of Lactobacillus casei, considered a probiotic bacterium. Hence, it can be speculated that the biosurfactant extracted from CSW has prebiotic properties favoring the growth of probiotic bacteria in the intestine.
Regarding the composition of the biosurfactant extracted from CSW, it was characterized as a lipopeptide, composed by C16 and C18 fatty acids, comprising oleic, linoleic and stearic fatty acids which are linked to a small fraction of an organic nitrogen chain composed by amino acids (Rincón-Fontan, Rodríguez-López, Vecino, Cruz, & Moldes, Citation2017; Rodríguez-López, Vecino, Barbosa-Pereira, Moldes, & Cruz, Citation2016; Vecino, Rodríguez-López, Cruz, & Moldes, Citation2015b). Therefore, Rincón-Fontán et al. (Citation2017) reported that this biosurfactant possesses a molecular weight similar to the lipopeptides produced by B.subtillis.
Although most of microbial biosurfactants are produced extracellularly, some microorganisms, including lactic acid bacteria like Lactobacillus pentosus, Lactobacillus paracasei or Lactococcus lactis, can produce cell-bound biosurfactants (Rodrigues, Moldes, Teixeira, & Oliveira, Citation2005; Souza et al., Citation2017; Vecino, Barbosa-Pereira, Devesa-Rey, Cruz, & Moldes, Citation2015c; Vecino et al., Citation2017).
The aim of this work was to isolate and characterize morphologically the microorganism that produces lipopeptide biosurfactants in CSW and compare it with other commercial biosurfactants characterized as lipopeptides, like those produced by B. subtilis. The study also includes the evaluation of the antimicrobial capacity of the isolated microorganism against pathogenic bacteria and fungi.
Materials and methods
Isolation of the microorganism from Corn Steep Water (CSW)
For the isolation of the microorganism, 5 ml of CSW, provided by FeedStimulants (Reg. No. NL214247/Lot NL-2728DK 7) were inoculated in Tryptic Soy Agar (TSA) or Saboraud Dextrose Agar (SDA) medium, in 9 cm Petri dishes, previously autoclaved for 15 min at 121°C. After inoculation, plates were incubated at 37°C during 48–72 h. Following, a loop of the biomass cultivated in the plates was surface-streak and inoculated in the same culture media, under the previous conditions, in order to obtain isolated colonies.
Once the colonies were isolated, a Gram staining and a catalase test were carried out. Catalase test was performed by adding on the colonies a drop of 3% of hydrogen peroxide (H2O2) under sterile conditions as described in previous studies (Reiner, Citation2010).
In addition, isolated colonies were inoculated into 250 ml flasks containing 100 mL of Trypticase Soy Broth (TSB) or Saboraud Dextrose Broth (SDB) medium, previously autoclaved for 15 min at 121°C, in order to evaluate their capacity to produce biosurfactants. The incubation in both media was carried out for 48 h at 37°C and 150 rpm.
Microscopically characterization of the microorganism isolated from CSW
For the visual analysis, Petri dishes of the isolated microorganism in TSA and SDA media were directly evaluated under a Stereoscopic Zoom Microscope Nikon SMZ1500 (Nikon Instruments Europe B.V., Amsterdam, Netherlands) with an Infinity X camera capturing pictures of the isolated colony at the central and side points. Moreover, each colony from TSA and SDA media was re-suspended in sterile physiological water and visualized on a slide covered by a coverslip by scanning electron microscopy (SEM) under a Nikon Eclipse E800 microscope (Nikon Instruments Europe B.V., Amsterdam, Netherlands) with a DS-5 camera.
Antimicrobial activity of the microorganism isolated from CSW
The antimicrobial activity of the microorganism isolated from CSW was assessed by performing an antagonist experiment against three different pathogens obtained from the Spanish Type Culture Collection (CECT) (Valencia, Spain). The pathogenic strains selected were Candida albicans CECT-1392 (ATCC-2091), cultivated in Malt Agar (at 26°C for 48 h), Aspergillus niger CECT-2574 (ATCC-16404), cultivated in Potato Dextrose Agar (PDA) (at 24°C for 48 h) and Staphylococcus aureus CECT-239 (ATCC-6538) which was cultivated in Nutrient Agar (NA) (at 37°C for 24 h), following the indications of CECT.
The antimicrobial experiments were carried out in 9 cm Petri dishes containing TSA or SDA medium autoclaved for 15 min at 121°C. Each pathogen was placed in the middle of the plate and colonies of the isolated microorganism from CSW were placed in the four corners of a square around the pathogens. Once the inoculation was conducted, the plates were incubated for 48–72 h at 37°C. The experiment was carried out in sterile conditions and all plates were performed by duplicate.
Extraction of extracellular and cell-bound biosurfactants
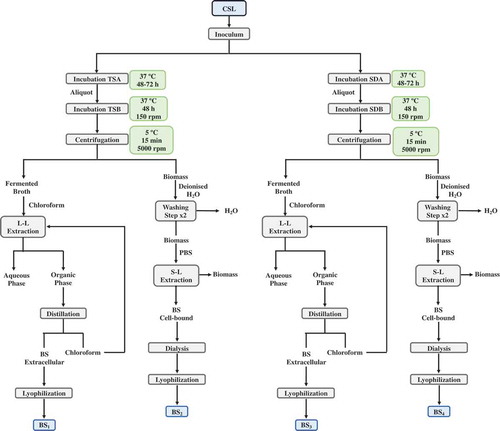
1 mL of the isolated microorganism, growth in TSB or SDB, was re-inoculated again in 100 mL of TSB or SDB medium at 37°C and 150 rpm, in 250 mL Erlenmeyer flasks, for 48 h. Following, the biomasses of fermented media were separated by centrifugation (during 15 min, at 5°C and 5000 rpm), and the supernatants were mixed with chloroform at 56°C for 1 h, in order to extract the extracellular biosurfactants produced in the fermented media, following the methodology described in previous studies (Vecino et al., Citation2015a). The ratio between fermented medium and chloroform was 1:2 v/v. Next, fermented media were separated from the organic phase into separatory funnels and the biosurfactant extracts were recovered and extracted from the organic phase by vacuum distillation in a rotatory evaporator. Afterwards, the extracts were lyophilized using a lyophilizer Telstar LyoQuest (Spain).
On the other hand, in order to test the production of cell-bound biosurfactants, the biomasses separated by centrifugation from the different fermented media were washed twice with demineralized water and re-suspended in 50 ml of phosphate buffer saline (PBS) (10 mM KH2PO4/K2HPO4 and 150 mM NaCl with pH adjusted to 7.0) as described in previous studies (Vecino et al., Citation2015c). Subsequently, the microbial biomasses were removed by centrifugation (during 15 min, at 5°C and 5000 rpm) and the PBS extracts, containing the biosurfactants were filtered with a 0.22 μm membrane, PVDF Stericup® 150 ml Durapore® (EMD Millipore Corporation, Billerica, MA 01821 USA).
Afterwards, the PBS extracts containing the cell-bound biosurfactant were dialyzed against demineralized water at 4°C in a Cellu-Sep© membrane (molecular weight cut-off 6000–8000 Dalton; Membrane Filtration Products, Inc., USA) for 48 h, and finally the biosurfactants were lyophilized using a lyophilizer Telstar LyoQuest (Spain).
As it is shown in , four different biosurfactants extracts were obtained from two different media (TSB and SDB), using two different extraction methods, to obtain both extracellular and cell-bound biosurfactants. These extracts were named as follows: BS1, as the extracellular biosurfactant produced in TSB medium; BS2, as the cell-bound biosurfactant produced in TSB medium; BS3, as the extracellular biosurfactant produced in SDB medium and BS4 as the cell-bound biosurfactant produced in SDB medium.
Figure 1. Flowchart of the methodology used in the isolation of the microorganism from CSW and in the extraction of biosurfactants. BS1: extracellular biosurfactant extract produced in TSB medium; BS2: cell-bound biosurfactant extract produced in TSB medium; BS3: extracellular biosurfactant extract produced in SDB medium and BS4: cell-bound biosurfactant extract produced in SDB medium.
Figura 1. Diagrama de flujo de la metodología utilizada para el aislamiento del microorganismo de CSW y la extracción de biotensoactivos. BS1: extracto de biotensoactivo extracelular producido en el medio TSB; BS2: extracto de biotensoactivo unido a células producido en el medio TSB; BS3: extracto de biotensoactivo extracelular producido en el medio SDB y BS4: extracto de biotensoactivo unido a células producido en el medio SDB.
Evaluation of the surfactant capacity
The surface tension (ST) of extracellular biosurfactants contained in TSB and SDB media and ST of cell-bound biosurfactants contained in PBS was measured using a Krüss K20 EasyDyne tensiometer with a 1.9 cm platinum Willhemly plate at room temperature. All determinations were carried out in triplicate in order to increase the accuracy of the measurements.
Elemental analysis of the biosurfactants extracted from CSW
An elemental analyzer (Fisons Carlo Erba EA-1108 CHNS-O, LabX, Midland, ON, Canada) was used for the determination of C, H, N and S in the biosurfactant extracts obtained from the isolated microorganism after cultivation in TSB or SDB medium.
FTIR characterization of biosurfactants extracted from CSW
1 mg of the biosurfactant extracts obtained from the isolated microorganism after the cultivation in TSB or SDB medium, were ground and pressed with 10 mg potassium bromide (7500 kg for 30 s) to obtain translucent pellets. Infrared absorption spectra of the extracellular and cell-bound biosurfactant extracts were recorded on a Niocolet 6700 FTIR system (Thermo Scientific) with a spectral resolution of 4 cm−1 and wavenumber accuracy between 400 and 4000 cm−1. As background reference, a potassium bromide pellet was used in the evaluation of all measurements consisted in 32 scans. For comparative purposes, FTIR spectrum of commercial fengycin (Lot-SLBS1147V, Sigma Aldrich, Ca, MO, USA), a lipopeptide biosurfactant produced by B. subtilis, was included in the study.
Results and discussion
Isolation and microscopic characterization of biosurfactants produced by the isolated microorganism from CSW
In previous studies it has been demonstrated the presence of biosurfactants in CSW (Rodríguez-López et al., Citation2016; Vecino et al., Citation2014, Citation2015a). This industrial stream is characterized by a high viscosity (it contains 50% of solids), low concentration of sugars and high concentration of lactic acid, which has been employed by many researchers as nutritional supplement in the production of microbial metabolites (Guerra-Rodríguez & Vázquez, Citation2013).
shows a microscopy view of the strain isolated from CSW, which was characterized as a Gram-positive and macroscopically catalase-negative Bacillus strain. During the microscopical observation, it was noticed that this Bacillus possesses high mobility. In it can be observed the vegetative cells as well as the endospore formation of the isolated Bacillus strain, characterized by a terminal endospore.
Figure 2. Images of the microbial strain isolated from CSW, obtained with a scanning electron microscopy (SEM).
Figura 2. Imágenes de la cepa microbiana aislada de CSW, obtenidas usando un microscopio electrónico de barrido (SEM).
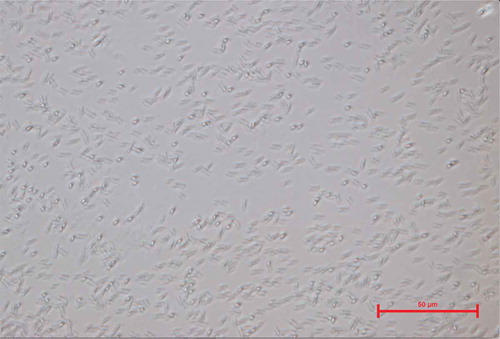
Endospore formation is a distinctive feature of Bacillus spp. as it has been shown also in previous studies (Nitschke & Pastore, Citation2006; Rooney, Price, Ehrhardt, Swezey, & Bannan, Citation2009; Sella, Vandenberghe, & Soccol, Citation2014; Tan & Ramamurthi, Citation2014). Therefore, Sella et al. (Citation2014) have reported that the life cycle of spore-forming Bacillus is divided in three different stages: vegetative growth, sporulation and germination. In turn, sporulation stage can be sub-divided in seven phases (Sella et al., Citation2014): I) first the nuclear material is disposed axially into filaments; II) the completion of DNA segregation and the invagination of the plasmatic membrane in an asymmetric position occurs forming a septum; III) the septum begins to curve, and the smaller forespore becomes entirely contained within the mother cell; IV) the mother cell mediates the development of the fore-spore into the spore; V) the spore coat is synthesized; VI) the mature spores become resistant to heat and organic solvents and VII) lysis of the spore occurs.
Usually, endospores are formed at periods of time of nutritional stress, allowing the organism to persist in the environment until the conditions become favorable (Sella et al., Citation2014), like those conditions produced during the steeping process of corn. The process of endospore formation has profound morphological and physiological consequences, for instance, a radical post-replicative remodeling of two progeny cells can result in a symmetrical division by binary fission and, in some cases, this binary fission can be accompanied eventually by cessation of metabolic activity in one daughter cell (the spore) and death by lysis of the other (the ‘mother cell’).
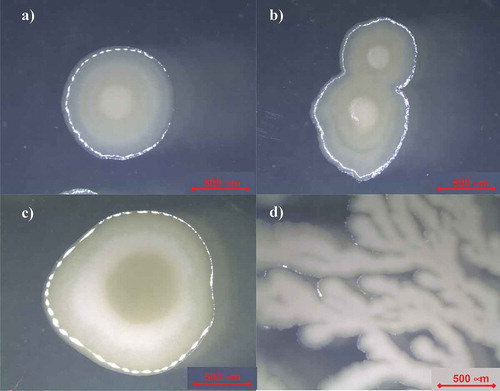
On the other hand, shows pictures of colonies produced by the isolated Bacillus at different stages. These colonies are characterized by a soft, creaming, dull and glistening phenotype defined by a small point-like elevation in the center with a whitish gray color. No changes in the colony phenotype were noticed between the colonies growth in TSA or SDA (see –)). Moreover, also shows the process of binary fission for the microorganism isolated from CSW and shows a colony of the isolated Bacillus in SDA producing ramifications simulating a tree-like formation. This dissociation of phenotype could be characteristic of the strain under some state of stress or reduction of the space able to grow, resulting in a cotton-like colony growth as it has been reported previously in B.mycoides (Di Franco, Beccari, Santini, Pisaneschi, & Tecce, Citation2002). The media used to grow the isolated microorganism from CSW only contained 2.5 g/l of dextrose as carbon source, thus, it can be speculated, that after several days, the reduced concentration of sugars in the medium acts as a trigger inducing changes in the colony phenotype.
Figure 3. Microscopical images of the microbial strain isolated from CSW obtained with a Stereoscopic Zoom Microscope. (Colonies are shown in sporulation stage). a) Isolated colony on SDA b) Division of the isolated colony in two progeny cells c) Isolated colony on TSA d) Colony tree-like growth.
Figura 3. Imágenes microscópicas de la cepa microbiana aislada de CSW obtenida con un microscopio estereoscópico de zoom. (Las colonias se muestran en su etapa de esporulación.) a) Colonia aislada en SDA; b) División de la colonia aislada en dos células de progenie; c) Colonia aislada en TSA; d) Crecimiento en forma de árbol de colonia.
Characterization of the biosurfactants produced by the isolated strain from CSW
In order to test the characteristics of the biosurfactants produced by the isolated microorganism from CSW, this was growth in TSB and SDB media and, after fermentation it was evaluated the capacity of this microorganism to produce extracellular and cell-bound biosurfactants. It was observed that this microorganism has the capacity to produce both extracellular and cell-bound biosurfactants, which represents a distinction from other Bacillus strains that only produce extracellular biosurfactants.
shows the physico-chemical characteristics of the biosurfactants extracted from the Bacillus isolated from CSW. The biosurfactants extracts showed differences in the surface tension capacity. Hence, cell-bound biosurfactants reduced in lower extend the surface tension of water (up to 52.3–52.7 mN/m) than extracellular biosurfactants (up to 43.2–49.1 mN/m) depending on the fermentation media. Regarding their appearance, extracellular biosurfactants (BS1, BS3) were oily and viscous (see ); whereas cell-bound biosurfactants (BS2, BS4) had a white powder appearance (see ). The different aspect between the extracellular and cell-bound biosurfactants could be linked to the differences in their compositions (). Hence, it was observed that extracellular biosurfactants (BS1 and BS3) content higher amount of C than the cell-bound biosurfactants (BS2 and BS4). Regarding the N, H and S content, these were similar among all the biosurfactants extracts produced by the isolated Bacillus strain from CSW, except for the BS3 biosurfactant extract that presents a lower N content and higher H percentage. Therefore, it can be speculated that BS3 is composed by a higher amount of fatty acids. The percentage of N in the extracts can be correlated with the peptide content multiplying by a factor of 6.25 (Mariotti, Tomé, & Miranda, Citation2008).
Table 1. Physico-chemical characteristics of biosurfactants extracted from the isolated microorganism from CSW. ST (Surface Tension). BS1: extracellular biosurfactant extract produced in TSB medium; BS2: cell-bound biosurfactant extract produced in TSB medium; BS3: extracellular biosurfactant extract produced in SDB medium and BS4: cell-bound biosurfactant extract produced in SDB medium.
Tabla 1. Características fisicoquímicas de los biotensoactivos extraídos del microorganismo aislado de CSW. ST (Tensión superficial). BS1: extracto de biotensoactivo extracelular producido en el medio TSB; BS2: extracto de biotensoactivo unido a células producido en el medio TSB; BS3: extracto de biotensoactivo extracelular producido en el medio SDB; y BS4: extracto de biotensoactivo unido a células producido en el medio SDB.
Figure 4. Biosurfactant extracts from the microorganism isolated from CSW. a) Extracellular biosurfactant b) Cell-bound biosurfactant.
Figura 4. Extractos de biotensoactivo obtenidos del microorganismo aislado de CSW. a) Biotensoactivo extracelular; b) Biotensoactivo unido a células.

In previous works it has been proved that differences in the composition of fermentation media can provoke changes in the composition, as well as in the surface tension capacity of the biosurfactants produced by a specific microorganism (Vecino et al., Citation2017), what would explain the differences in the composition found in the biosurfactant (BS3) produced by the isolated Bacillus strain on SDB medium in comparison with the biosurfactant (BS1) extracted from fermented TSB medium. These differences were less remarked when are compared with the cell-bound bisourfactants.
Comparing both extracellular and cell-bound biosurfactants, it was noticed that cell-bound biosurfactants extracts from the isolated Bacillus strain, reduced in lower extend the surface tension of water in comparison with the extracellular biosurfactants. This condition also was noticed by other authors, observing in general that cell-bound biosurfactants produced a lower reduction of surface tension than extracellular biosurfactants (Rodríguez-López, Rincón-Fontán, Vecino, Cruz, & Moldes, Citation2019; Vecino et al., Citation2017).
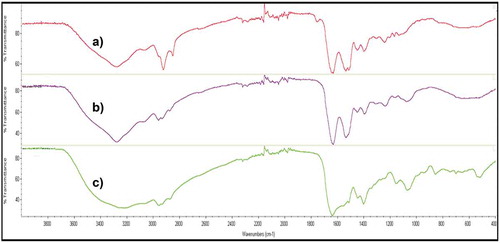
Additionally, Fourier-transform infrared spectroscopy (FTIR) was used to determine the possible similarities of the biosurfactants extracted from the isolated Bacillus strain, in comparison with a commercial biosurfactant, fengycin, which is a lipopeptide biosurfactant produced by B.subtilis that showed in previous works a higher similarity with the biosurfactant extracted from CSW (Rincón-Fontán et al., Citation2017). In this case, it can be observed that the FTIR of fengycin gave a similar response than the biosurfactants extracts obtained from the isolated Bacillus strain (), with a strong band between 3400 and 3200 cm−1 as the result of N-H and O-H stretching indicating the amine and hydroxyl groups. Moreover, the peaks observed between 3000 and 2800 cm−1 indicate the presence of aliphatic chains, C-H, and peaks between 1650 and 1500 cm−1 represent CO-N bonds and the stretching mode of the C-N bonds respectively; whereas the band about 1400 cm−1 indicates the presence of C = O bonds.
Figure 5. FTIR comparison of spectra of a) Fengycin b) Cell-bound biosurfactant extracted from the strain isolated from CSW in TSB medium and c) Extracellular biosurfactant extracted from the strain isolated from CSW in TSB medium.
Figura 5. Comparación FTIR de los espectros de a) Fengicina b) Biotensoactivo unido a células extraído de la cepa aislada de CSW en medio TSB y c) Biotensoactivo extracelular extraído de la cepa aislada de CSW en el medio TSB.
Antagonist effect of the microorganism isolated from CSW on pathogenic microorganisms
The possible antagonistic effect of the strain isolated from CSW was assessed against three pathogenic strains; C.albicans, A.niger and S.aureus on both TSA and SDA media. After incubation, a visual evaluation was carried out to study the ability of the isolated microorganism to inhibit the growth of the pathogens previously mentioned as it is showed in the images included in . In order to compare the capacity of inhibition, Petri dishes controls of the three pathogens in TSA and SDA media were also incubated and photographed. It can be observed in the images included in that in TSA media, the growth of the strain isolated from CSW was not affected by the pathogenic strains used in the experiment. Therefore, it correlates with previous works where Bacillus bacteria shown to have inhibitory abilities against food-borne pathogens (Moore, Globa, Barbaree, Vodyanoy, & Sorokulova, Citation2013), probably due to the production of secondary metabolites such as the biosurfactants like those isolated in the current work. Likewise, in SDA medium, the isolated Bacillus strain was able to grow in the presence of C.albicans and S.aureus though it was not the case of A.niger, where the pathogen covered the isolated Bacillus strain. Benoit et al. (Citation2015) studied the interaction between B.subtilis and A.niger resulting in an attachment of the bacteria on the hyphae of the fungus showing an alteration of both metabolisms, which could suggest why the pathogen covered the isolated strain from CSW completely.
Figure 6. Antagonist effect of Bacillus strain, isolated from CSW, against Candida albicans, Aspergillus niger and Staphylococcus aureus.
Figura 6. Efecto antagonista de la cepa de Bacillus, aislada de CSW, contra Candida albicans, Aspergillus niger y Staphylococcus aureus.
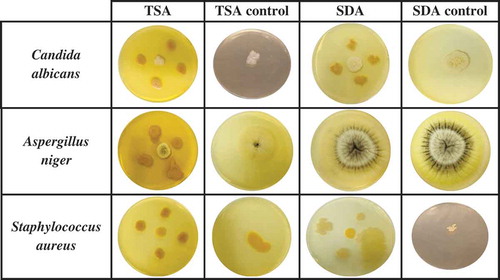
On the other hand, when the isolated colonies are in contact with pathogenic microorganisms (see images of ), it produces a yellowish pigmentation. It can be speculated that these pigments could be also a responsive action of the strain to the presence of other microorganisms. Therefore, various authors have observed in several Bacillus spp such as B.subtilis (Nakamura, Citation1989), B.atrophaeus (Moeller, Horneck, Facius, & Stackebrandt, Citation2005) and B.nakamurai (Dunlap et al., Citation2016) that environmental stresses produced by the presence of pathogenic microorganisms or the presence of ultraviolet (UV) radiation, can induce dissociation of Bacillus phenotype resulting in the production of pigments. Khaneja et al. (Citation2010) also claimed that this photoprotective pigments are related with the presence of melanins and carotenoids, which have been also suggested to play a role as virulence factors in some pathogens like S.aureus (Liu et al., Citation2005).
Conclusions
In this study, the microorganism responsible for the production of biosurfactants in CSW, a residual stream from the corn-milling industry, was isolated and microscopically characterized, observing that is a Bacilllus strain with phenotypic and metabolic characteristics, similar to other Bacilllus strains like B.subtilis, although with the peculiarity that it is macroscopically catalase negative and it has the ability to produce both extracellular and cell-bound biosurfactants. The Bacillus isolated from CSW showed to have ability to produce terminal endospores as well as to exhibit a pigmentation trait that switches from whitish to yellowish in the presence of pathogenic microorganisms. It can be speculated that the presence of endospores allowed to this microorganism to resist the extreme conditions of the corn steeping process and the high viscosity of CSW, which is concentrated up to 50% of solids. Regarding the biosurfactant produced by the isolated Bacillus strain, this possesses a similar composition to other commercial biosurfactants, like fengycin, corroborating that the biosurfactant contained in CSW should be a lipopeptide. Finally, the antimicrobial ability of the isolated Bacillus strain was evaluated against pathogenic bacteria and fungus showing a good antimicrobial activity against C.albicans and S.aureus.
Acknowledgments
This study was supported by the Spanish Ministry of Economy and Competitiveness (MINECO) under the project RTI2018-093610-B-I00 (FEDER funds). L. Rodríguez-López also acknowledges the Spanish Ministry of Education, Culture and Sport, for her pre-doctoral fellowship (FPU15/00205) and Hadassa Martínez-Padrón thanks to the National Council for Science and Technology (CONACYT) for the support of her stay in the University of Vigo. The authors also acknowledge the Center for the Scientific and Technological Support of the University of Vigo (CACTI) for its support in the FTIR analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Benoit, I., van der Esker, M. H., Patyshakuliyeva, A., Mattern, D. J., Blei, F., Zhou, M., … Kovács, Á. T. (2015). Bacillus subtilis attachment to Aspergillus niger hyphae results in mutually altered metabolism. Environmental Microbiology, 17(6), 2099–2113. doi:10.1111/1462-2920.12564
- Das, P., Mukherjee, S., & Sen, R. (2008). Antimicrobial potential of a lipopetide biosurfactant derived from a marine Bacillus circulans. Journal of Applied Microbiology, 104(6), 1675–1684. doi:10.1111/j.1365-2672.2007.03701.x
- Di Franco, C., Beccari, E., Santini, T., Pisaneschi, G., & Tecce, G. (2002). Colony shape as a genetic trait in the pattern-forming Bacillus mycoides. BMC Microbiology, 2, 33. doi:10.1186/1471-2180-2-33
- Dunlap, C. A., Saunders, L. P., Schisler, D. A., Leathers, T. D., Naeem, N., Cohan, F. M., & Rooney, A. P. (2016). Bacillus nakamurai sp.nov., a black-pigment-producing strain. International Journal of Systematic and Evolutionary Microbiology, 66, 2987–2991. doi:10.1099/ijsem.0.001135
- Guerra-Rodríguez, E., & Vázquez, M. (2013). Technical and economical evaluation of microbial transglutaminase production of enzymatic hydrolysates of potato (Solanum tuberosum). CyTA Journal of Food, 11(3), 277–284. doi:10.1080/19476337.2012.736414
- Hull, S. R., Yang, B. Y., Venzke, D., Kulhavy, K., & Montgomery, R. (1996). Composition of corn steep water during steeping. Journal of Agricultural and Food Chemistry, 44(11), 1857–1863. doi:10.1021/jf950353v
- Khaneja, R., Pérez-Fons, L., Fakhry, S., Baccigalupi, L., Steiger, S., To, E., … Cutting, S. M. (2010). Carotenoids found in Bacillus. Journal of Applied Microbiology, 108, 1889–1902. doi:10.1111/j.1365-2672.2009.04590.x
- Liu, G. Y., Essex, A., Buchanan, J. T., Datta, V., Hoffman, H. M., Bastian, J. F., … Nizet, V. (2005). Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. Journal of Experimental Medicine, 202(2), 209–215. doi:10.1084/jem.20050846
- López-Prieto, A., Rodríguez-López, L., Rincón-Fontán, M., Moldes, A. B., & Cruz, J. M. (2019). Effect of biosurfactant extract obtained from the corn-milling industry on probiotic bacteria in drinkable yogurt. Journal of the Science of Food and Agriculture, 99(2), 824–830. doi:10.1002/jsfa.9251
- Mariotti, F., Tomé, D., & Miranda, P. P. (2008). Converting nitrogen into protein-beyond 6.25 and Jones’ factors. Critical Reviews in Food Science and Nutrition, 48(2), 177–184. doi:10.1080/10408390701279749
- Moeller, R., Horneck, G., Facius, R., & Stackebrandt, E. (2005). Role of pigmentation in protecting Bacillus sp. endospores against UV radiation. FEMS Microbiology Ecology, 51, 231–236. doi:10.1016/j.femsec.2004.08.008
- Moore, T., Globa, L., Barbaree, J., Vodyanoy, V., & Sorokulova, I. (2013). Antagonistic activity of Bacillus bacteria against food-borne pathogens. Journal of Probiotics and Health, 1(3). doi:10.4172/2329-8901.1000110
- Nakamura, L. K. (1989). Taxonomic relationship of black-pigmented Bacillus subtilis strains and a proposal for Bacillus atrophaeus sp. nov. International Journal of Systematic Bacteriology, 39, 295–300. doi:10.1099/00207713-39-3-295
- Nitschke, M., & Pastore, G. M. (2006). Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresource Technology, 97, 336–341. doi:10.1016/j.biortech.2005.02.044
- Reiner, K. (2010). Catalase test protocol. American Society for Microbiology, ASMMicrobe Library.
- Rincón-Fontán, M., Rodríguez-López, L., Vecino, X., Cruz, J. M., & Moldes, A. B. (2017). Influence of micelle formation on the adsorption capacity of a biosurfactant extracted from corn on dyed hair. Royal Society of Chemistry (RSC) Advanced, 7, 16444–16452. doi:10.1039/c7ra01351e
- Rodrigues, L., Moldes, A. B., Teixeira, J., & Oliveira, R. (2005). Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochemical Engineering Journal, 28(2), 109–116. doi:10.1016/j.bej.2005.06.001
- Rodríguez-López, L., Rincón-Fontán, M., Vecino, X., Cruz, J. M., & Moldes, A. B. (2017). Ionic behavior assessment of surface-active compounds from corn steep liquor by exchange resins. Journal of Surfactants and Detergents, 20(1), 207–217. doi:10.1007/s11743-016-1897-5
- Rodríguez-López, L., Rincón-Fontán, M., Vecino, X., Cruz, J. M., & Moldes, A. B. (2019). Biological surfactants vs. polysorbates: Comparison of their emulsifier and surfactant properties. Review of the Year 2018. Tenside Surfactants Detergents, 56(1), 4. doi:10.3139/113.110574
- Rodríguez-López, L., Vecino, X., Barbosa-Pereira, L., Moldes, A. B., & Cruz, J. M. (2016). A multifunctional extract from corn steep liquor: Antioxidant and surfactant activities. Food & Function, 7(9), 3724–3732. doi:10.1039/C6FO00979D
- Ron, E. Z., & Rosenberg, E. (2001). Natural roles of biosurfactants. Environmental Microbiology, 3(4), 229–236. doi:10.1046/j.1462-2920.2001.00190.x
- Rooney, A. P., Price, N. P. J., Ehrhardt, C., Swezey, J. L., & Bannan, J. D. (2009). Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. Inaquosorum subsp. nov. International Journal of Systematic and Evolutionary Microbiology, 59, 2429–2436. doi:10.1099/ijs.0.009126-0
- Santos, D. K. F., Rufino, R. D., Luna, J. M., Santos, V. A., & Sarubbo, L. A. (2016). Biosurfactants: Multifunctional biomolecules of the 21st century. International Journal of Molecular Sciences, 17, 1–31. doi:10.3390/ijms17030401
- Sella, S. R. B. R., Vandenberghe, L. P. S., & Soccol, C. R. (2014). Life cycle and spore resistance of spore-forming Bacillus atrophaeus. Microbiological Research, 169, 931–939. doi:10.1016/j.micres.2014.05.001
- Souza, E. C., Oliveira de Souza de Azevedo, P., Domínguez, J. M., Converti, A., & Pinheiro de Souza Oliveira, R. (2017). Influence of temperature and pH on the production of biosurfactant, bacteriocin and lactic acid by Lactococcus lactis CECT-4434. CyTA Journal of Food, 15(4), 525–530. doi:10.1080/19476337.2017.1306806
- Tan, I. S., & Ramamurthi, K. S. (2014). Spore formation in Bacillus subtilis. Environmental Microbiology Reports, 6(3), 212–225. doi:10.1111/1758-2229.12130
- Vecino, X., Barbosa-Pereira, L., Devesa-Rey, R., Cruz, J. M., & Moldes, A. B. (2014). Study of the surfactant properties of aqueous stream from the corn milling industry. Journal of Agricultural and Food Chemistry, 62(24), 5451–5457. doi:10.1021/jf501386h
- Vecino, X., Barbosa-Pereira, L., Devesa-Rey, R., Cruz, J. M., & Moldes, A. B. (2015a). Optimization of liquid-liquid extraction of biosurfactants from corn steep liquor. Bioprocess and Biosystems Engineering, 38(9), 1629–1637. doi:10.1007/s00449-015-1404-9
- Vecino, X., Barbosa-Pereira, L., Devesa-Rey, R., Cruz, J. M., & Moldes, A. B. (2015c). Optimization of extraction conditions and fatty acid characterization of Lactobacillus pentosus cell-bound biosurfactant/bioemulsifier. Journal of the Science of Food and Agriculture, 95(2), 313–320. doi:10.1002/jsfa.6720
- Vecino, X., Rodríguez-López, L., Cruz, J. M., & Moldes, A. B. (2015b). Sewage sludge Polycyclic Aromatic Hydrocarbon (PAH) decontamination technique based on the utilization of a lipopeptide biosurfactant extracted from corn steep liquor. Journal of Agricultural and Food Chemistry, 63, 7143–7150. doi:10.1021/acs.jafc.5b02346
- Vecino, X., Rodríguez-López, L., Gudiña, E. J., Cruz, J. M., Moldes, A. B., & Rodrigues, L. R. (2017). Vineyard pruning waste as an alternative carbon source to produce novel biosurfactants by Lactobacillus paracasei. Journal of Industrial and Engineering Chemistry, 55, 40–49. doi:10.1016/j.jiec.2017.06.014
