ABSTRACT
This study aimed to explore the mechanism whereby the interactions between the endogenous proteins, lipids and starches present in foxtail millet affected the in vitro hydrolysis of starch. Prior proteolysis of the protein matrix significantly increased the enzymatic hydrolysis of foxtail millet starch, suggesting that it is possible that non-surface proteins (i.e. the protein matrix) can retard the rate of starch degradation by acting as a physical barrier between starch and amylase. Furthermore, confocal microscopy demonstrated that the proteins in foxtail millet bind competitively with starch to α-amylase, partially decreasing the activity of α-amylase. In addition, as a result of complexing with the fatty acids, foxtail millet starch had a significantly reduced hydrolysis rate. Therefore, we conclude that the interactions between endogenous proteins and lipids with starch plays a significant role in the hypoglycemic properties of foxtail millet.
RESUMEN
El presente estudio tuvo como objetivo explorar el mecanismo mediante el cual las interacciones entre proteínas, lípidos y almidones endógenos presentes en el mijo afectan la hidrólisis in vitro del almidón. Se constató que la proteólisis previa de la matriz proteica aumentó significativamente la hidrólisis enzimática del almidón presente en el mijo; ello sugiere la posibilidad de que las proteínas no superficiales (es decir, la matriz proteica) retarden la velocidad de degradación del almidón, actuando como una barrera física entre el almidón y la amilasa. Además, la microscopía confocal demostró que las proteínas del mijo compiten con el almidón para unirse a la α-amilasa, disminuyendo parcialmente la actividad de esta enzima. Asimismo, debido a la formación de complejos con los ácidos grasos, el almidón del mijo mostró una tasa de hidrólisis significativamente reducida. Por lo tanto, se concluye que las interacciones de las proteínas y los lípidos endógenos con el almidón desempeñan un papel importante en las propiedades hipoglucemiantes del mijo.
PALABRAS CLAVE:
1. Introduction
Foxtail millet (Setaria italica) is a small-grained cereal that is a staple food consumed by the majority of people in the arid and semiarid tropical regions of the world (Zhang et al., Citation2012). It has been considered as a potential functional food that improves health and helps reduce the risk of illnesses (Anand et al., Citation2008). Epidemiological studies have demonstrated that increasing the consumption of foxtail millet-based foods is related to lowering the risk of chronic diseases such as cholesterol metabolism disorders (Choi et al., Citation2005) and type II diabetes mellitus (Montonen, Knekt, Jarvinen, Aromaa, & Reunanen, Citation2003).
One property of foxtail millet, which can be used for the management of type II diabetes, is its hypoglycemic properties (Annor, Tyl, Marcone, Ragaee, & Marti, Citation2017). Pathak, Grover, and Priyali (Citation2000) reported that the consumption of a foxtail millet-based diet resulted in significantly lower blood glucose levels. Anju and Sarita (Citation2010) demonstrated that refined wheat flour biscuits substituted with 45% foxtail millet flour led to a significant decrease in glycemic index (50.8) compared to biscuits made with 100% refined wheat flour (68.0). Sireesha, Kasetti, Nabi, Swapna, and Apparao (Citation2011) reported that Setaria italica seed aqueous extract (SISAE) had good hypoglycemic effects on streptozotocin-induced diabetic rats. Knowing the factors that contribute to the hypoglycemic properties of foxtail millet will help develop and process healthier foxtail millet-based foods.
It has been reported that various factors control the rate and extent of enzymatic hydrolysis of starches, including starch structural factors, such as amylose/amylopectin ratio, molecular/supra-molecular structure, amylose-lipid complexes, chemical modification, processing, granule/particle size and porosity; barrier factors, such as protein matrices, fibres and intactness of cell walls (Annor et al., Citation2017; Dhital, Dabit, Zhang, Flanagan, & Shrestha, Citation2015a; Dhital, Warren, Butterworth, Ellis, & Gidley, Citation2017). Among these factors, the interactions between major macronutrients such as starches, lipids and proteins can play a fundamental role in the glycemic response caused by starchy foods (Bhattarai, Dhital, & Gidley, Citation2016; Parada & Santos, Citation2016).
To date, knowledge about the effects of interactions between proteins, lipids and starches on enzymatic susceptibility in a variety of cereals is still in its infancy (Bhattarai et al., Citation2016). Yu et al. (Citation2018) found that barley proteins, particularly water-soluble proteins, can hamper starch digestion; moreover, α-amylase may bind to starch granules and to water-insoluble protein, resulting in a decrease in starch hydrolysis. Annor, Marcone, Corredig, Bertoft, and Seetharaman (Citation2015) suggested that the types and contents of fatty acids play an important role in the hypoglycemic properties of millets. Annor, Marcone, Bertoft, and Seetharaman (Citation2013) investigated the starch digestibility of kodo millet flour as affected by starch-protein-lipid interaction and their results showed that starch digestibility and the expected glycemic index increased significantly after removing lipids and/or proteins, especially after removing lipids. Chen et al. (Citation2017) studied the effects of adding corn oil (CO) and soy protein (SP) to corn starch (CS) on the digestive rates of annealed starch complex and mechanisms of interactions between CS, CO and SP. Results indicated that the physical barrier of CO, amylose-lipid complex and protein-starch matrix can provide resistance to starch digestion. However, systematic studies on how endogenous proteins and lipids affect the starch digestibility of foxtail millet are not yet available.
Therefore, this study focused on investigating the effects of proteins and lipids on the enzymatic hydrolysis of starch in a complex matrix (foxtail millet flour) and exploring the mechanisms underlying the inhibition of starch hydrolysis. The interactions between starch granules, proteins and α-amylase were visualized by confocal laser scanning microscopy (CLSM), and the formation of amylose–lipid complexes (ALC) were analyzed using differential scanning calorimetry (DSC).
2. Materials and methods
2.1. Materials
Foxtail millet (Qinghuang-2 variety) was obtained from the Millet Research Institute Shanxi Academy of Agriculture Science, Changzhi, China. The foxtail millet samples were dehulled to remove inedible husk with a millet huller (HM NMJ, Huimin Agricultural Machinery Co., Ltd., Qufu, China). The dehulled grains were then ground into flour using a cyclone mill (CT410, FOSS Scino (Suzhou) Co., Ltd., Suzhou, China) and passed through a 100 mesh sieve. The average particle size of this product was 60.86 μm. The foxtail millet flour contained 9.72% protein, 3.46% crude fat and 68.58% total starch. The nitrogen content of foxtail millet flour sample was determined by standard Kjeldahl methodology. The protein content (%) was then calculated from protein (%) = nitrogen (%) × 6.25. The crude fat content was determined gravimetrically after extraction with ether at 60–70°C for 6–8 h. The total starch was determined by the total starch kit from Megazyme. The particle size distribution of foxtail millet flour was analyzed with a Laser diffraction particle size analyzer (LS13 320, Beckman Coulter, USA) and the median volume-based diameter was used to represent the average particle size.
Porcine pancreatic α-amylase (Sigma, A6255), pepsin from gastric porcine mucosa (Sigma, P-6887), 4-Hydroxybenzhydrazide (PAHBAH, Sigma, H9882), fluorescamine (Sigma, F9015), fluorescein isothiocyanate isomer I (FITC), palmitic acid (PA, Sigma, P0500), oleic acid (OA, Sigma, O1008), and linoleic acid (LA, Sigma, L1376) were purchased from Sigma-Aldrich. All of the other chemicals were of reagent grade.
2.2. Extraction of starch and protein from foxtail millet flour
Foxtail millet starch was extracted from dehulled flour as described in Annor et al. (Citation2013). Foxtail millet flour (100 g) was stirred with sodium borate buffer (12.5 mM, pH 10, containing 0.5% SDS [w/v] and 0.5% Na2S2O5 [w/v]) for 5 min, and the residue was recovered by centrifugation at 900 × g for 5 min to extract the proteins. The extraction step was repeated once again. The residue was washed three times with distilled water and recovered by centrifugation. The residue was then suspended in distilled water and stirred overnight to further release the protein from the starch granules, after which the starch slurry was passed through four layers of cheesecloth and then through 70 μm nylon mesh. The slurry was centrifuged, and the yellow layer that formed on the top of the starch layer was scraped with a spatula. The starch was then suspended again in water and centrifuged in 50 mL centrifuge tubes at 1,600 × g for 10 min. These steps were continued until all the yellow particles were removed from the starch fraction. Thereafter the amylose content of foxtail millet starch was determined according to the international standard, ISO 6647–1: 2007.
Foxtail millet protein was extracted in an alkaline medium by the method of Hassan, Osman, and Babiker (Citation2007). A sample of defatted meal (100 g) was extracted once with 2 L of 0.3 M Tris–HCl buffer (pH 8) containing 10 mM 2-mercaptoethanol (2- ME) at room temperature. After centrifugation (8000 rpm, 5 min, 4°C), the supernatant was acidified to pH 4.8 with 2 M HCl and then centrifuged. The precipitate protein was dissolved in water at 4°C and the pH adjusted to 8. After centrifugation (8000 rpm, 5 min, 4°C), the clear supernatant was dialyzed against distilled water for 24 h at 4°C and then freeze-dried.
2.3. In vitro digestion of foxtail millet starch or foxtail millet flour
To estimate the effects of endogenous proteins, including surface proteins and the protein matrix on starch hydrolysis in foxtail millet, the starch from foxtail millet or foxtail millet flour was incubated with pepsin prior to starch digestion as described in Yu et al. (Citation2018). Foxtail millet starch (100 mg) or foxtail millet flour containing 100 mg of starch was added to 6 mL of deionized water, and the mixture was equilibrated by shaking at 100 rpm for 10 min in a water bath shaker. Then, 5 mL of pepsin (1 mg/mL) in 0.02 M HCl (pH 2.0) was added to the mixture, while 5 mL of 0.02 M HCl (pH 2.0) was added to the control sample. The samples were incubated for 30 min at 37°C, followed by neutralization with 5 mL of 0.02 M NaOH. Subsequently, 1 mL of α-amylase (0.4 U/mg starch) in 0.2M sodium acetate buffer (pH 6.0) was added to start the digestion reaction. At varying time intervals, 0.1 mL of aliquots were taken and mixed with 0.9 mL of 0.3 M sodium carbonate to stop the enzymatic reaction. The reducing sugar content in the samples was determined with a PAHBAH assay (Bhattarai et al., Citation2016).
2.4. Observation of proteins on the surface of foxtail millet starch as well as protein in foxtail millet flour
Proteins on the surface of foxtail millet starch as well as protein in foxtail millet flour, with and without pepsin treatment, were labeled with fluorescamine, and then observed by confocal microscopy. Briefly, foxtail millet starch (100 mg) or foxtail millet flour containing 100 mg starch was hydrolyzed by pepsin according to the procedure described in Section 2.3. The mixture was then centrifuged at 6000 × g for 60 s and the sediment was then re-dissolved in 0.15 mL of PBS buffer (pH 8) followed by addition of 0.1 mL of fluorescamine (2 mg/mL) in DMSO. The samples were stained for 1 h at room temperature and then rinsed three times with distilled water to remove excess dye. Then, 0.5 mL of 50% glycerol was added to the stained sample prior to observation with a confocal microscope at a 405 nm excitation wavelength (Bhattarai et al., Citation2016).
2.5. Investigation of the binding of α-amylase to starch and/or protein by measuring the residual amylase activity
To evaluate the binding interactions between starch, protein and amylase, residual amylase activity was investigated in non-hydrolyzing conditions at 0 °C, based on the method of Dhital, Gidley, and Warren (Citation2015b) with some modifications. In brief, 10 mg of protein and 90 mg of starch in 10 mL of 0.2 M sodium acetate buffer (pH 6.0) were incubated for 10 min with intermittent stirring in an ice water bath. Subsequently, 40 units of α-amylase was added, and the mixture was then incubated for a further 20 min. A 250 μL aliquot was taken from the mixture and centrifuged at 6000 × g for 60 s. The resulting supernatant contained unbound enzyme and 100 μl was transferred to 5 mL of gelatinized soluble potato starch (10 mg/mL). A 100 μL aliquot was withdrawn at 0, 3, 5, 10, 15, and 20 min to determine the reducing sugar content. As a result, linear graphs of the reducing sugar content versus time were generated. The enzyme activity of α-amylase was defined as the amount of reducing sugar released per unit volume of the enzyme solution at 37°C for 1 min.
2.6. Visualization of α-amylase bound to starch granules and/or proteins
Labeling of α-amylase with FITC was following the method from The and Feltkamp (Citation1970). α-amylase at an initial concentration of 25 mg/mL was conjugated to FITC (25 mg/g enzyme)in carbonate buffer (0.1 M, pH 9) for 60 min at room temperature. When the conjugation time was up, unbound FITC was separated by elution with phosphate buffer saline solution using a desalting column (sephadex, pd-10). Then, FITC-labeled enzyme solution was quickly used for subsequent experiments.
The binding of FITC-labeled enzyme to proteins and/or starch granules at 0 °C was observed based on the method of Dhital, Warren, Zhang, and Gidley (Citation2014) with minor modifications. 10 mL of a foxtail millet flour dispersion (10 mg/mL) in 0.2 M sodium acetate buffer (pH 6.0) was cooled to 0 °C for 10 min in an ice water bath. 50 uL of FITC-labeled enzyme solution was then added to the dispersion and left for 30 min at 0°C to allow the binding of the FITC-labeled enzyme, with brief vortexing at 5 min intervals. 100 μL of the mixture was taken and then centrifuged at 6000 × g for 60 s. The unbound enzyme in the supernatant was discarded, and the residue (foxtail millet flour pellets) was recovered. The residue was then observed using CLSM.
2.7. Preparation of cooked millet starch with or without added lipids
Cooked millet starch with or without added lipids was prepared according to a report by Annor et al. (Citation2015). 14 mL of distilled water was added to a sample of 0.7 g of millet starch in a flat-bottomed flask, cooked in a boiling water bath for 10 min and then cooled to 70°C. Palmitic acid, oleic acid, and linoleic acid were added to the starch gel based on the original content in foxtail millet flour (0.01, 0.07, 0.02 mmol/g starch, respectively). The samples were incubated at 70°C for 10 min with vortexing every 2 min to form starch–fatty acid complexes. The samples were then freeze dried, ground into powder, passed through a 100 mesh screen and packaged in plastic bags. A control sample was prepared from cooked foxtail millet starch without any fatty acids added.
2.8. Thermal properties of the cooked foxtail millet starch with or without lipids added
The thermal characteristics of the cooked millet starch with or without added lipids were analyzed with a differential scanning calorimeter (200F3, Netzsch, Germany) using the method described by Ai, Hasjim, and Jane (Citation2013) with minor modifications. The ground sample (~3 mg, dsb) was weighed in an aluminum pan and deionized water (3×, w/w, dsb) was added. The samples were scanned from 20°C to 120°C at a rate of 10°C/min. The gelatinization enthalpy (ΔH), onset temperature (To), peak temperature (Tp), and end temperature (Tc) were derived from the generated curves using Proteus software.
2.9. Enzymatic hydrolysis of the cooked foxtail millet starch with or without lipids added
Enzymatic hydrolysis of the cooked foxtail millet starch with or without lipids added was performed according to the procedure described in Section 2.3.
2.10. Statistical analysis
All of the trials were performed in triplicate. One-way ANOVA by Duncan’s multiple range test (p < .05) were conducted using the SPSS 16.0 Statistical Software Program (SPSS Inc. Chicago, IL, USA).
3. Results and discussion
3.1. Effect of starch granule surface proteins on the enzymatic hydrolysis of starch from foxtail millet
To evaluate the effect of starch granule surface proteins on the enzymatic hydrolysis of starch from foxtail millet, surface proteins from the surface of foxtail millet starch were hydrolyzed with pepsin prior to α-amylase hydrolysis. Using CLSM, we observed that starch granules were surrounded by surface proteins ()), similar to the findings reported by Han, Benmoussa, Gray, BeMiller, and Hamaker (Citation2005). When the surface proteins were effectively removed by pepsin treatment ()), the release of reducing sugars did not show a significant increase during 120 min of starch hydrolysis (). This result is inconsistent with the previous literature (Bhattarai et al., Citation2016), which reported that the depletion of surface proteins from wheat starch increased the release of reducing sugar by almost 10% after 360 min of digestion.
Figure 1. Confocal laser scanning micrographs of foxtail millet starch (a: undigested, b: digested by pepsin) stained with fluorescamine.
Figura 1. Micrografías de escaneo láser confocal de almidón de mijo (a: sin digerir, b: digerido con pepsina) teñidas con fluorescamina.
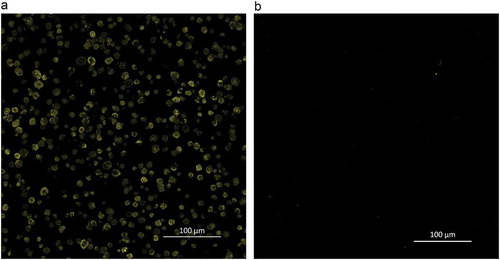
Figure 2. Progress curves of in vitro hydrolysis of foxtail millet starch by α-amylase. FMS is foxtail millet starch and FMS + PD refers to foxtail millet starch that is digested by pepsin for 30 min before α-amylase digestion.
Figura 2. Curvas de progreso de la hidrólisis in vitro del almidón de mijo por α-amilasa. FMS es almidón de mijo y FMS + PD se refiere al almidón de mijo digerido con pepsina durante 30 minutos antes de la digestión con α-amilasa.
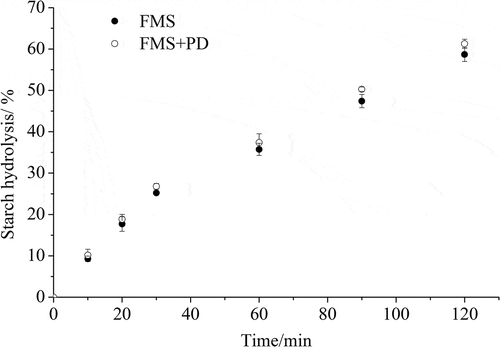
Starch granule surface proteins are derived from storage proteins and some matrix proteins that remain on the surface of starch granules after starch extraction or during starch development. Although the amount of surface protein is considered to be negligible, it has been increasingly recognized that surface proteins significantly affect the properties of a starch. (Hu et al., Citation2017). Surface proteins are assumed to block the access of enzymes to starch granules, hence reducing the susceptibility of starch to enzymatic digestion (Wang et al., Citation2014). In this study, surface proteins were visible by CLSM, but pepsin treatment did not significant increased the starch hydrolysis of foxtail millet, which might be due to the low content of surface proteins. The content of proteins in isolated starch depend on both botanical source and isolation method of starch. Treatments with an alkaline solution or SDS buffer in starch extraction can reduce the protein content of starch granules (Debet & Gidley, Citation2006; Han & Hamaker, Citation2002). In the present research, foxtail millet starch was extracted in an alkaline medium (pH 10, containing 0.5% SDS), thus the obtained starch might contain less surface proteins. As a result, during 120 min of digestion, no significant differences of reducing sugar release were observed between the pepsin-treated starch and the control.
3.2. Effect of protein removal from foxtail millet flour on starch hydrolysis
To investigate the effect of the proteins in foxtail millet on starch hydrolysis, foxtail millet flour was incubated with pepsin to remove proteins before α-amylase digestion. As shown in ), except for starch granule surface proteins, non-surface proteins were found to physically entrap starch granules. When these proteins were removed from foxtail millet flour by pepsin treatment ()), the rate and extent of starch degradation was found to increase as shown in . At 120 min of hydrolysis, 50.4% of starch was hydrolyzed in the case of pepsin-treated millet flour, compared to 42.5% for undigested millet flour. The relatively higher hydrolysis with prior pepsin digestion resulted from the proteolysis of the protein matrix as demonstrated by CLSM. As presented in ), only a small amount of intense fluorescamine signal from still undigested proteins was observed after pepsin digestion.
Figure 3. Confocal laser scanning micrographs of foxtail millet flour (a: undigested, b: digested by pepsin) stained with fluorescamine.
Figura 3. Micrografías de escaneo láser confocal de harina de mijo (a: sin digerir, b: digerida con pepsina) teñidas con fluorescamina.
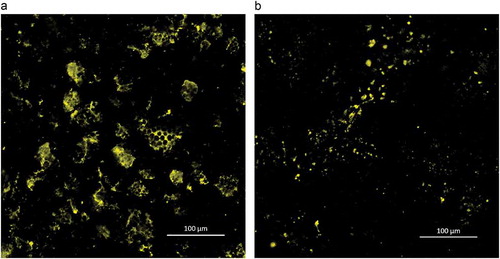
Figure 4. Progress curves of in vitro starch hydrolysis of foxtail millet flour by α-amylase. FMF is foxtail millet flour and FMF + PD refers to foxtail millet flour that is digested by pepsin for 30 min before α-amylase digestion.
Figura 4. Curvas de progreso de la hidrólisis in vitro del almidón de la harina de mijo por α-amilasa. La FMF es harina de mijo y FMF + PD se refiere a la harina de mijo digerida con pepsina durante 30 minutos antes de la digestión con α-amilasa.
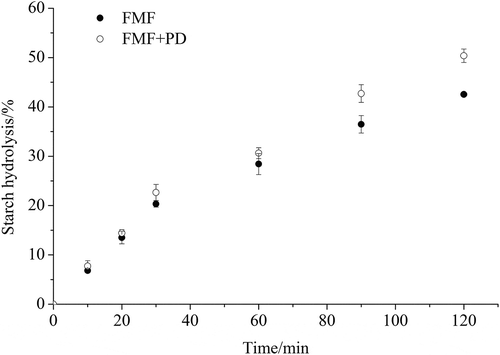
The effect of proteins on starch digestibility is related to the physical barrier that proteins form between starch and degrading enzymes (Annor et al., Citation2017). The continuous protein matrix, which entraps starch granules in food products such as pasta, reduces the availability of starch to α-amylase (Zou, Sissons, Gidley, Gilbert, & Warren, Citation2015). Jenkins et al. (Citation1987) found that removal of gluten from wheat caused more glucose to be absorbed in the small intestine. We show here that a protein network can encapsulate starch granules and form a starch-protein matrix ()). Pepsin treatment of millet flour increased the hydrolysis of starch, which suggests that the degradation of the starch-protein matrix makes α-amylase easier to access starch granules. Protein in some cereals can play an important role in reducing starch digestibility. Wong et al. (Citation2009) reported that the low digestibility of sorghum partly resulted from an abundance of disulfide-bonded proteins present in the sorghum endosperm. Annor et al. (Citation2013) also suggested that hypoglycemic property of Kodo millet was due to the protein encapsulation of starch granules. Therefore, the protein matrix in foxtail millet acting as physical barrier may be a significant factor that reduces its in vitro starch digestibility.
3.3. Effect of foxtail millet protein adhesion to α-amylase on enzyme activity
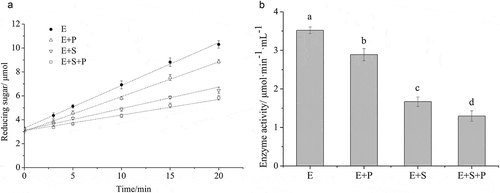
After pre-incubation of α-amylase with foxtail millet proteins and/or starch at 0°C, unbound α-amylase was recovered from these incubation mixtures and the residual amylase activity was then determined. The results of these assays are shown in . We found that the residual α-amylase activity from the mixtures with proteins and/or starch was significantly lower than that of the control (enzyme pre-incubated without starch granules or proteins), indicating that α-amylase can bind with proteins and/or starch. The control (only α-amylase) had the highest enzyme activity (3.52 μmoL·min−1·mL−1), in contrast to the protein (2.89 μmoL·min−1·mL−1), starch (1.67 μmoL·min−1·mL−1) or protein-starch (1.30 μmoL·min−1·mL−1) containing samples. It was clear that the presence of foxtail millet proteins had negative effects on enzyme activity, resulting in a slower rate of starch hydrolysis. Here, our study showed that, in addition to acting as a physical barrier, foxtail millet proteins may inhibit α-amylase activity by binding to amylase, making α-amylase unavailable for starch hydrolysis. This result was consistent with the binding of gluten to α-amylase in wheat flour (Bhattarai et al., Citation2016).
Figure 5. Effects of foxtail millet protein or/and starch binding to α-amylase on enzymatic activity. left: reducing sugar content at different digestion times; right: enzymatic activity. E is control, E + P is enzyme with protein, E + S is enzyme with starch and E + S + P is enzyme with starch and protein. All of the data are obtained from triplicate measurements. Different letters indicate significantly different with p < 0.05.
Figura 5. Efectos de la proteína del mijo y/o de la unión del almidón a la α-amilasa en la actividad enzimática. Izquierda: reduciendo el contenido de azúcar en diferentes tiempos de digestión; derecha: actividad enzimática. E es el control, E + P es la enzima con proteína, E + S es la enzima con almidón y E + S + P es la enzima con almidón y proteína. Todos los datos se obtienen a partir de mediciones por triplicado. Las letras diferentes indican la existencia de diferencias significativas en las mediciones con p < 0.05.
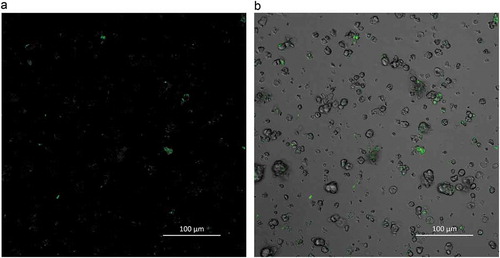
This binding of proteins to α-amylase can be observed as demonstrated by CLSM in . We found that in addition to binding to starch granules, FITC labeled α-amylase (green) also contacted proteins when exposed to millet flour. Starch is the main substrate of α-amylase, so it can easily bind to α-amylase. This enzyme also exhibited adhesion affinity with the proteins found in foxtail millet flour. Bhattarai et al. (Citation2016) observed that labeled α-amylase bound to gluten by CLSM. Zou et al. (Citation2015) reported that the gluten network in pasta probably binds to α-amylase, retarding the diffusion of α-amylase into a gluten network and therefore resulting in reduced starch digestibility. Yu et al. (Citation2018) also found that water-insoluble proteins form barley can reduce the rate of starch hydrolysis by binding to α-amylase. Similarly, our work has shown that α-amylase can bind with the proteins in foxtail millet, decreasing the catalytic activity of α-amylase.
Figure 6. Confocal laser scanning micrographs of FITC-labeled α-amylase binding to starch granules and protein matrix in foxtail millet flour. a: confocal mode and b: reflective mode.
Figura 6. Micrografías de escaneo láser confocal de la unión de α-amilasa marcada con FITC a gránulos de almidón y matriz de proteína en la harina de mijo. a: modo confocal y b: modo reflectivo.
3.4. Effects of lipids present in foxtail millet on starch hydrolysis
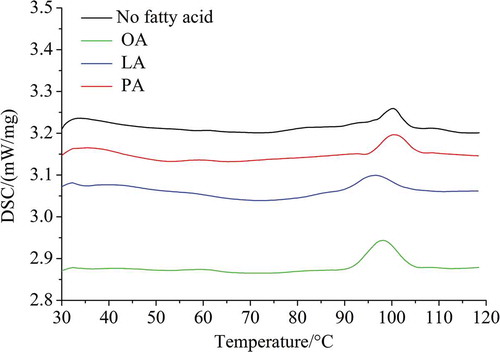
The DSC thermograms of foxtail millet starch and FFAs-added starch samples are shown in . DSC thermograms of starch samples after cooking with FFAs (PA, OA, and LA) did not show a starch gelatinization peak, indicating that the starches were gelatinized completely, but the thermographs did display distinct ALC dissociation peaks. The dissociation of ALC from pre-cooked foxtail millet starch alone (control) and foxtail millet starch pre-cooked with different free fatty acids (FFAs) is shown in . Compared with the control, the △H of the ALC dissociation peak of foxtail millet starch pre-cooked with FFAs was higher, indicating that the ALC in the FFAs-added starch samples increased. Furthermore, foxtail millet starch exhibited different amounts of ALC with different fatty acids. When FFAs were added at the amounts present in foxtail millet (0.01, 0.07, 0.02 mmol/g starch for PA, OA and LA, respectively), foxtail millet starch had a higher △H with OA (0.50 J/g), compared to LA or PA (0.34 and 0.28 J/g, respectively). However, Annor et al. (Citation2015) reported that the ALC formed by foxtail millet starch and LA was significantly higher than that of PA and OA at a concentration of 2 mmol/g starch. These results suggested that the formation of ALC was not only related to the structural properties of the respective FFAs, but also related to the amount of individual FFAs added. In addition, the dissociation temperature of ALC generally increased with an increase in the length of the hydrocarbon chains in the lipid, and decreased with an increase in the number of double bonds in the hydrocarbon chains (Ai et al., Citation2013). In this study, the percentage of amylose in foxtail millet starch was 24.53%. The amylose–PA complexes showed the highest dissociation temperature, which could be attributed to the fact that PA (C16:0) has a straight hydrocarbon chain and therefore could interact most strongly with the hydrophobic cavity of the amylose helix. The lower ALC dissociation temperature of millet starch pre-cooked with OA (18:1) or LA (18:2) may have been due to the weaker interaction of amylose with OA containing one double bond and LA containing two double bonds. A similar trend was observed by Ai et al. (Citation2013) using normal and high-amylose corn starches pre-cooked with lipids.
Table 1. Dissociation of ALC of pre-cooked starch alone (control) and starch pre-cooked with different FFAs.
Tabla 1. Disociación de ALC del almidón precocido solo (control) y del almidón precocido con diferentes FFA.
Figure 7. DSC curves of foxtail millet starch pre-cooked with or without added FFAs. PA is palmitic acid, OA is oleic acid, and LA is linoleic acid.
Figura 7. Curvas DSC de almidón de mijo precocido con o sin FFA añadidos. PA es ácido palmítico, OA es ácido oleico y LA es ácido linoleico.
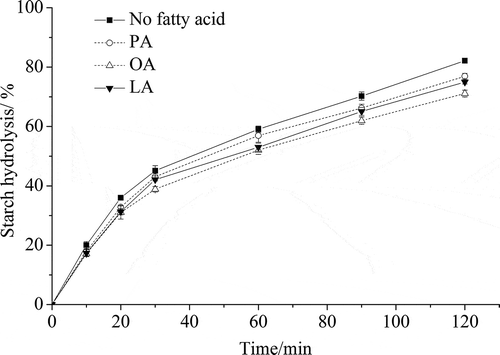
The kinetics of enzymatic hydrolysis of foxtail millet starch pre-cooked with or without added FFAs is shown in . After 120 min incubation of millet starch, the percentage of hydrolysis of starch alone (control) was 84%. With the addition of FFAs, the enzymatic hydrolysis rate of foxtail millet starch decreased significantly at 120 min (p < .05). The percentage of starch hydrolysis was reduced from 82.2% to 71.1% after cooking with OA, followed by LA (75.0%) and PA (76.9%) (). The addition of FFAs reduced the in vitro digestibility of foxtail millet starch, which probably can be attributed to the formation of helical complexes between amylose and the respective lipids. The fact that an amylose–helical complex shows resistance to amylase hydrolysis is well known (Ai et al., Citation2013; Annor et al., Citation2015; Hasjim et al., Citation2010; Kawai, Takato, Sasaki, & Kajiwara, Citation2012). The formation of an ALC also inhibits the swelling of starch granules, further reducing the hydrolysis of starch (Ai et al., Citation2013). Moreover, after pre-cooking with PA, OA and LA, the reductions in the percentages of hydrolysis of foxtail millet starch at 120 min were associated with the amount of fatty acids added. The more of a particular fatty acid added, the more amylose–fatty acid complexes were formed, therefore, the larger the reduction in the percentage of hydrolysis of foxtail millet starch observed. Kawai et al. (Citation2012) also found that the percentage of starch hydrolysis decreased with increasing fatty acid addition in wheat starch. However, when the same amount of fatty acids was added to their respective starches, Ai et al. (Citation2013) found that the ALC with a higher dissociation temperature showed stronger resistance to enzymatic hydrolysis.
Figure 8. Hydrolysis kinetics of foxtail millet starch pre-cooked with or without added FFAs. PA is palmitic acid, OA is oleic acid, and LA is linoleic acid.
Figura 8. Cinética de la hidrólisis del almidón de mijo precocido con o sin FFA añadidos. PA es ácido palmítico, OA es ácido oleico y LA es ácido linoleico.
4. Conclusions
The objective of this study was to determine how endogenous proteins and lipids affect the in vitro starch digestibility of a complex matrix (foxtail millet flour). We found that the protein matrix not only acts as a physical barrier between starch and α-amylase but it also partially sequesters α-amylase, which can retard starch hydrolysis in foxtail millet flour. Starch granule surface proteins do not show a significant effect on starch degradation, which may be due to the low content of surface proteins. Furthermore, foxtail millet starches can form ALC with FFAs, which can reduce the enzymatic-hydrolysis rates of starch. Thus, these findings indicate that the interaction of protein matrix and lipids with starch plays a significant role in the hypoglycemic properties of foxtail millet. Consuming whole grain foods such as foxtail millet flour that have a complex matrix may help individuals achieve nutritional and healthy benefits, due to the unique digestive properties of these foods.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ai, Y., Hasjim, J., & Jane, J. L. (2013). Effects of lipids on enzymatic hydrolysis and physical properties of starch. Carbohydrate Polymers, 92(1), 120–127. doi:10.1016/j.carbpol.2012.08.092
- Anand, P., Kunnumakara, A. B., Sundaram, C., Harikumar, K. B., Tharakan, S. T., Lai, O. S., … Aggarwal, B. B. (2008). Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical Research, 25(9), 2097–2116. doi:10.1007/s11095-008-9690-4
- Anju, T., & Sarita, S. (2010). Suitability of foxtail millet (Setaria italica) and barnyard millet (Echinochloa frumentacea) for development of low glycemic index biscuits. Malaysian Journal of Nutrition, 16(3), 361–368.
- Annor, G. A., Marcone, M., Bertoft, E., & Seetharaman, K. (2013). In vitro starch digestibility and expected glycemic index of Kodo millet (Paspalum scrobiculatum) as affected by starch-protein-lipid interactions. Cereal Chemistry Journal, 90(3), 211–217. doi:10.1094/CCHEM-06-12-0074-R
- Annor, G. A., Marcone, M., Corredig, M., Bertoft, E., & Seetharaman, K. (2015). Effects of the amount and type of fatty acids present in millets on their in vitro starch digestibility and expected glycemic index (eGI). Journal of Cereal Science, 64, 76–81. doi:10.1016/j.jcs.2015.05.004
- Annor, G. A., Tyl, C., Marcone, M., Ragaee, S., & Marti, A. (2017). Why do millets have slower starch and protein digestibility than other cereals? Trends in Food Science & Technology, 66, 73–83. doi:10.1016/j.tifs.2017.05.012
- Bhattarai, R. R., Dhital, S., & Gidley, M. J. (2016). Interactions among macronutrients in wheat flour determine their enzymic susceptibility. Food Hydrocolloids, 61, 415–425. doi:10.1016/j.foodhyd.2016.05.026
- Chen, X., He, X., Zhang, B., Fu, X., Jane, J., & Huang, Q. (2017). Effects of adding corn oil and soy protein to corn starch on the physicochemical and digestive properties of the starch. International Journal of Biological Macromolecules, 104, 481–486. doi:10.1016/j.ijbiomac.2017.06.024
- Choi, Y. Y., Osada, K., Ito, Y., Nagasawa, T., Choi, M. R., & Nishizawa, N. (2005). Effects of dietary protein of Korean foxtail millet on plasma adiponectin, HDL-cholesterol, and insulin levels in genetically type 2 diabetic mice. Bioscience, Biotechnology, and Biochemistry, 69, 31–37. doi:10.1271/bbb.69.31
- Debet, M. R., & Gidley, M. J. (2006). Three classes of starch granule swelling: Influence of surface proteins and lipids. Carbohydrate Polymers, 64, 452−465. doi:10.1016/j.carbpol.2005.12.011
- Dhital, S., Dabit, L., Zhang, B., Flanagan, B., & Shrestha, A. K. (2015a). In vitro digestibility and physicochemical properties of milled rice. Food Chemistry, 172, 757–765. doi:10.1016/j.foodchem.2014.09.138
- Dhital, S., Gidley, M. J., & Warren, F. J. (2015b). Inhibition of a-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydrate Polymers, 123, 305−312. doi:10.1016/j.carbpol.2015.01.039
- Dhital, S., Warren, F. J., Butterworth, P. J., Ellis, P. R., & Gidley, M. J. (2017). Mechanisms of starch digestion by a-amylase-structural basis for kinetic properties. Critical Reviews in Food Science & Nutrition, 57(5), 875−892. doi:10.1080/10408398.2014.922043
- Dhital, S., Warren, F. J., Zhang, B., & Gidley, M. J. (2014). Amylase binding to starch granules under hydrolysing and non-hydrolysing conditions. Carbohydrate Polymers, 113, 97–107. doi:10.1016/j.carbpol.2014.06.063
- Han, X.-Z., Benmoussa, M., Gray, J. A., BeMiller, J. N., & Hamaker, B. R. (2005). Detection of proteins in starch granule channels. Cereal Chemistry Journal, 82(4), 351–355. doi:10.1094/CC-82-0351
- Han, X.-Z., & Hamaker, B. R. (2002). Partial leaching of granule-associated proteins from rice starch during alkaline extraction and subsequent gelatinization. Starch/Staerke, 54, 454−460. doi:10.1002/1521-379X(200210)54:10<454::AID-STAR454>3.0.CO;2-M
- Hasjim, J., Lee, S.-O., Hendrich, S., Setiawan, S., Ai, Y., & Jane, J. (2010). Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chemistry Journal, 87, 257–262. doi:10.1094/CCHEM-87-4-0257
- Hassan, A. B., Osman, G. A., & Babiker, E. E. (2007). Effect of chymotrypsin digestion followed by polysaccharide conjugation or transglutaminase treatment on functional properties of millet proteins. Food Chemistry, 102, 257–262. doi:10.1016/j.foodchem.2006.04.043
- Hu, P., Fan, X., Lin, L., Wang, J., Zhang, L., & Wei, C. (2017). Effects of surface proteins and lipids on molecular structure, thermal properties, and enzymatic hydrolysis of rice starch. Food Science and Technology (campinas), 38, 84–90. doi:10.1590/1678-457x.35016
- Jenkins, D., Thorne, M. J., Wolever, T., Jenkins, A. L., Rao, A. V., & Thompson, L. U. (1987). The effect of starch-protein interaction in wheat on the glycemic response and rate of in vitro digestion. The American Journal of Clinical Nutrition, 45(5), 946–951. doi:10.1093/ajcn/45.5.946
- Kawai, K., Takato, S., Sasaki, T., & Kajiwara, K. (2012). Complex formation, thermal properties, and in-vitro digestibility of gelatinized potato starche-fatty acid mixtures. Food Hydrocolloids, 27(1), 228–234. doi:10.1016/j.foodhyd.2011.07.003
- Montonen, J., Knekt, P., Jarvinen, R., Aromaa, A., & Reunanen, A. (2003). Whole-grain and fiber intake and the incidence of type 2 diabetes. American Journal of Clinical Nutrition, 77, 622–629. doi:10.1093/ajcn/77.3.622
- Parada, J., & Santos, J. L. (2016). Interactions among starch, lipids, and proteins in foods: Microstructure control for glycemic response modulation. Critical Reviews in Food Science & Nutrition, 56(14), 2362–2369. doi:10.1080/10408398.2013.840260
- Pathak, S. S., Grover, S., & Priyali. (2000). Development of food products based on millets, legumes and fenugreek seeds and their suitability in the diabetic diet. International Journal of Food Sciences and Nutrition, 51(5), 409–414. doi:10.1080/096374800427019
- Sireesha, Y., Kasetti, R. B., Nabi, S. A., Swapna, S., & Apparao, C. (2011). Antihyperglycemic and hypolipidemic activities of Setaria italica seeds in STZ diabetic rats. Pathophysiology, 18, 159–164. doi:10.1016/j.pathophys.2010.08.003
- The, T. H., & Feltkamp, T. E. W. (1970). Conjugation of fluorescein isothiocyanate to antibodies: I. Experiments on the Conditions of Conjugation. Immunology, 18(6), 865–873.
- Wang, S., Luo, H., Zhang, J., Zhang, Y., He, Z., & Wang, S. (2014). Alkali-induced changes in functional properties and in vitro digestibility of wheat starch: The role of surface proteins and lipids. Journal of Agricultural and Food Chemistry, 62(16), 3636–3643. doi:10.1021/jf500249w
- Wong, J. H., Lau, T., Cai, N., Singh, J., Pedersen, J. F., Vensel, W. H., … Buchanan, B. B. (2009). Digestibility of protein and starch from sorghum (Sorghum bicolor) is linked to biochemical and structural features of grain endosperm. Journal of Cereal Science, 49(1), 73–82. doi:10.1016/j.jcs.2008.07.013
- Yu, W., Zou, W., Dhital, S., Wu, P., Gidley, M. J., Fox, G. P., & Gilbert, R. G. (2018). The adsorption of α-amylase on barley proteins affects the in vitro digestion of starch in barley flour. Food Chemistry, 241, 493–501. doi:10.1016/j.foodchem.2017.09.021
- Zhang, G. Y., Liu, X., Quan, Z. W., Cheng, S. F., Xu, X., & Pan, S. K. (2012). Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nature Biotechnology, 30(6), 549–556. doi:10.1038/nbt.2195
- Zou, W., Sissons, M., Gidley, M. J., Gilbert, R. G., & Warren, F. J. (2015). Combined techniques for characterising pasta structure reveals how the gluten network slows enzymic digestion rate. Food Chemistry, 188, 559–568. doi:10.1016/j.foodchem.2015.05.032
