ABSTRACT
The amount of volatile bioactive compounds of garlic (Allium sativum L.) can be modified by techniques that involve disintegration of cells or/and heat transfer. In this study, after crushing of garlic, the amount of organic sulfides increased in the headspace in the function of time (P < 0.05), while the amount of allicin was not influenced (P = 0.249). Increment of heating temperature enhanced the formation of vinyldithiins, diallyl sulfide (DAMS) and methyl propenyl disulfide (MPDS) but had no significant effect (P ≥ 0.05) on the rate of allicin formation. Heat treatment of garlic at 80°C for 10 min hampered the post-treatment formation of most organic sulfides at room temperature, while heating at 60°C for 10 min did not hamper completely their formation during storage at room temperature. Cooking intact garlic cloves resulted in much less organosulfur compounds than crushed garlic of the same weight (P < 0.001).
RESUMEN
La cantidad de compuestos bioactivos volátiles presentes en el ajo (Allium sativum L.) puede modificarse empleando técnicas que implican la desintegración de las células y/o la transferencia de calor. En el presente estudio, una vez triturado el ajo, aumentó la cantidad de sulfuros orgánicos en el espacio de cabeza en función del tiempo (P < 0.05), no detectándose variaciones en la cantidad de alicina ocasionadas por este procedimiento (P = 0.249). El aumento de la temperatura de calentamiento mejoró la formación de vinilditiinas, sulfuro de dialilo (DAMS) y disulfuro de metil propenilo (MPDS), pero no tuvo un efecto significativo (P ≥ 0.05) sobre la tasa de formación de alicina. El tratamiento térmico del ajo a 80°C durante 10 minutos obstaculizó la formación de la mayoría de los sulfuros orgánicos a temperatura ambiente posteriormente al tratamiento, mientras que el calentamiento a 60°C durante 10 minutos no obstaculizó por completo su formación durante el almacenamiento a temperatura ambiente. La cocción de dientes enteros de ajo resultó en mucho menos compuestos de organoazufre que la cocción del mismo peso de ajo triturado (P < 0.001).
PALABRAS CLAVE:
1. Introduction
Garlic (Allium sativum L.) is a horticultural plant grown for thousands of years. It is originated. from Asia and a part of the Amaryllidaceae family. Knowledge of its broad range of healing capacity dates back to ancient times, but it is also in use in preventive medicine nowadays as well. Garlic consumption can contribute to the prevention of cancerous diseases and type-2 diabetes, helps to maintain an adequate level of immune function, improves the condition of the circulatory/vascular system, and may have anti-inflammatory effects (Martins, Petropoulos, & Ferreira, Citation2016; Putnik et al., Citation2019). Garlic can be also an effective tool against bacterial and fungal infections (Marchese et al., Citation2016).
Among the volatile bioactive compounds of garlic, the group of organic sulfur-containing compounds is of great importance. The precursors of this diverse group of compounds are special non-protein, sulfur-containing amino acids, S-alk(en)yl-1-cysteine-S-oxides, of which alliin (S-allyl-L-cysteine sulfoxide) is the most important precursor in garlic. Alliin, with the help of alliinase enzyme, is first converted to allylsulfenic acid and then condensed to a two allyl groups’ containing thiosulfinate, named allicin (allyl 2-propenethiosulfinate) (Lanzotti, Citation2006; Tocmo, Wu, Liang, Fogliano, & Huang, Citation2017). In further steps, di- and trisulfides, cyclic disulfides (vinyldithiins) and ajoenes (Lanzotti, Citation2006) are formed from thiosulfinates. Among thiosulfinates, allicin, which is the most characteristic of garlic, is chemically or enzymatically decomposed into a number of sulfide compounds such as diallyl sulfide, diallyl disulfide, diallyl trisulfide, diallyl tetrasulfide, or dipropyl disulfide (Martins et al., Citation2016; Putnik et al., Citation2019).
Due to its amino and carboxyl groups, alliin is not volatile, as opposed to its derivatives. When garlic cloves are damaged, alliinase is activated by the disruption of the plant tissue (Marchese et al., Citation2016), and volatile substances produced by the enzymatic processes are released and inhibit the growth of certain pests and pathogenic microbes. The thiosulfinates generated from alliin and organic sulfides formed by the decomposition of thiosulfinates are also biologically active compounds. Among these sulfur-containing compounds, allyl sulfides and vinyldithiins have been shown to be natural antioxidants and antimicrobials as well as their prophylactic effects on thrombosis were detected. Anti-cancer effect was also attributed to allyl sulfides (Martins et al., Citation2016).
The amount of non-volatile amino acid precursors and the concentration of the volatile bioactive compounds formed from them are influenced by genotype, agrotechnics, storage, and processing. The number and amount of bioactive compounds may mostly be modified by processing operations involving heat transfer or crushing (Martins et al., Citation2016; Putnik et al., Citation2019). Blanching is widely used in food or drug production containing garlic (Szymanek, Citation2011). Kinalski and Noreña (Citation2014) observed that blanching decreased the thiosulfinate content and antioxidant activity of garlic. Similarly to blanching, other cooking techniques associated with heating also affect the bioactive material content of garlic. Their loss was particularly significant when the temperature used in the process was at least 100°C and the duration of the heat treatment exceeded 20 minutes (Gorinstein et al., Citation2009).
In previous studies, the effects of different heat treatments and their duration on the level of organosulfur compounds was monitored (Cavagnaro, Camargo, Galmarini, & Simon, Citation2007; Kinalski & Noreña, Citation2014; Putnik et al., Citation2019; Tocmo et al., Citation2017). However, according to the knowledge of the authors, there is scarce information regarding the formation of volatile sulfur-containing compounds at room temperature after crushing as a function of time and how they change during the storage of the cooked material. In Hungary garlic is consumed not only in cooked, but also in raw form beside certain meals, therefore the study was focused on how volatile organosulfur substances develop after crushing of raw garlic at room temperature. Another important consideration is that meals are often kept at room temperature for a shorter or longer period before consumption, which may also affect the level of volatile sulfur compounds, thus the formation of volatile sulfur compounds during storage after heat treatment was also in the scope of the study. In addition, the levels of volatile organosulfur compounds released during the heat treatment of crushed and intact garlic of the same weight were also compared. According to the studied literature, measurements with the same experimental setup have not been conducted yet.
2. Materials and methods
2.1. The garlic samples and their treatments
The investigations were carried out on garlic grown in loose-bound Chernozem soil from the end of February and harvested at the end of July. The bulbs were picked up before complete yellowing and were left on the area for few days for postharvest purposes and the dried-out garlic were tied into wreaths and kept in a well-ventilated place.
First, the time-course release of substances from raw and crushed garlic was investigated at room temperature (25°C). Before the tests, the garlic was cleaned so that the total weight of the cleaned cloves became about 5 grams. Then the cloves were crushed through a garlic press and mixed. The crushing surface of 2.5 cm × 3 cm of the garlic press, were punctuated with 30 holes; each with a diameter of 3 mm. Subsequently, 0.25 g of crushed garlic was weighed into each of five 20 cm3 flanged headspace vials and sealed with caps made of PTFE-butyl seal in the 23rd minute after crushing. The analysis of the first sample started in the 30th minute after crushing, and the analysis of subsequent samples were performed every 70 minutes until all of the samples were measured. There was only one sampling and measurement from the headspace of each vial. The experiment was repeated three times on different days.
Secondly, in the next study, the processing conditions of garlic were the same as above, but following a 30-minute duration at room temperature the vials were immersed in a Julabo 5 water bath for 10 minutes, at 60°C or 80°C or 100°C, respectively. After the heat treatment, the vials were immersed in water at 25°C for 5 minutes. Five samples were analyzed from each heat treatment in every 70 minutes, which enabled the investigation of the effect of storage at room temperature, in addition to the heat treatment, as well. The experiment was repeated three times on different days.
In order to evaluate the effect of disintegration on the release of volatiles during heating, intact and crushed garlic “pairs” were prepared. One member of the pair was an intact clove, with the weight between 0.30 and 0.40 g, while the other member was crushed garlic, with the same weight. There were five pairs in ten headspace vials. The crushed garlic was stored at room temperature for 30 minutes. After that, the sample pairs were heat treated by immersing in water bath at 100°C for ten minutes, then cooled in a water bath at 25°C for 5 minutes then the headspace analysis was conducted.
2.2. Assay of volatile sulfur compounds
Analyses of samples were carried out using a Shimadzu GCMS-QP2010 plus gas chromatograph mass spectrometer coupled with a Perkin Elmer TurboMatrix HS 40 automated headspace sampler. Prior to injection, the samples were thermostated at 35°C; and samples were taken with a needle heated up to 40°C. During the pressure-driven sample input, the pressure was increased to 300 kPa by carrier-gas injection. The one-minute pressurizing period was followed by a 0.2-minute controlled headspace blow-down.
Compounds were separated with a VF-23 ms type WCOT column supplied by Agilent with a stationary phase of cyanopropyl-silicone, length of 20 m, internal diameter 0.15 mm and film thickness 0.15 µm. The carrier gas was high purity helium (5.0) with a column head pressure of 250 kPa. The injector temperature was 45°C. The initial column temperature was 40°C for 10 minutes, and then ramped to 135°C at a rate of 4°C/min and then increased to 200°C at a 10°C/min ramp. The ion source of the mass spectrometer operated in EI mode with 70 eV ionization energy. The mass range was between 35 and 300 m/z, and the spectra were recorded from the 2nd minute after injection. The components were identified by their relative mass spectra using the NIST Mass Spectral Library 08 spectrum database. Peak areas (A) were determined based on total ion chromatograms (TIC), and these values were used to estimate the relative quantity change for a particular compound. The automated headspace sampler was used to provide a snapshot about the condition of the sample at a given time after treatment. After the crushing, heat-treatment and cooling, a time series test of the same schedule was performed for each component; and the relationship between the peak area and the time elapsed following treatments was established.
2.3. Data analysis
The relationship between the duration of treatment and the measured variables was analyzed by regression analysis. The tightness of the linear fit was performed by correlation analysis. The effect of the treatment temperature on the measured components was analyzed by one-way analysis of variance. In case of significant difference among treatment averages, the pairwise comparison was performed with the Student-Newman-Keuls test. In case of a significant difference in group variance, the Tamhane test was used. When comparing crushed and non-crushed garlic samples, the two-sample t-test for the paired sample elements was used. The observed significance level (P-value) used for statistical decisions was 0.05. Statistical analysis was performed using IBM SPSS Statistics 20.0.
3. Results
3.1. Effect of crushing on the formation of volatile components of garlic in the function of time
Volatile organosulfur compounds that were identified following their gas chromatographic separation can be seen on . Dimethyl disulfide (DMDS), methyl propenyl disulfide (MPDS), methyl allyl disulfide (MADS), diallyl disulfide (DADS) and diallyl trisulfide (DATS) are linear polysulfides; the only monosulfide was the diallyl sulfide (DAMS). The presence of all of these compounds had been verified in raw garlic or garlic oil (Kimbaris et al., Citation2006; Lanzotti, Citation2006; Tocmo et al., Citation2017). These organic sulfides are mainly formed by the decomposition of allicin in garlic (Lanzotti, Citation2006).
Figure 1. Total ion chromatogram (TIC) of the headspace of crushed garlic obtained by static headspace GC-MS analysis. DMDS: dimethyl disulfide, DAMS: diallyl sulfide, MPDS: methyl propenyl disulfide, MADS: methyl allyl disulfide, DADS: diallyl disulfide, DATS: diallyl trisulfide
. Cromatograma de iones totales (TIC) del espacio de cabeza del ajo machacado obtenido por análisis de espacio de cabeza estático GC-MS. DMDS: disulfuro de dimetilo, DAMS: sulfuro de dialilo, MPDS: disulfuro de metilpropenilo, MADS: disulfuro de metil alilo, DADS: disulfuro de dialilo, DATS: trisulfuro de dialilo.
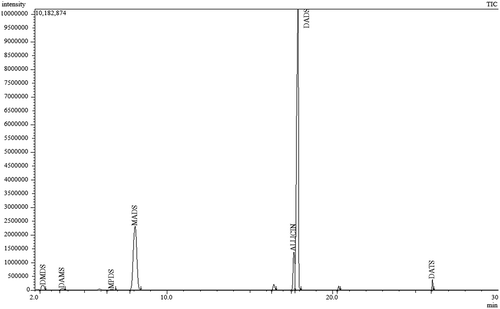
The formation of vinyldithiins as by-products can occur at elevated temperature during gas chromatographic measurements (Tocmo et al., Citation2017). In present work, when samples were kept at room temperature, there were no vinyldithiins detected during the measurement. These compounds are typically formed as a result of heat treatment in the Diels-Alder reaction (Rose, Whiteman, Moore, & Zhu, Citation2005). In the used method, sample injection and elution of the target components were carried out at relatively low temperatures. This was possible, because there was no solvent to be evaporated, as injections were carried out from the headspace.
Most components increased in the headspace between 30 and 240 minutes after crushing. The F-test of regression analyses showed () that a significant part of the variance of all measured components except for allicin (P = 0.249) can be explained by a linear correlation (P < 0.05) in accordance with the correlation analysis (). It suggests that the peak area of the tested volatile organosulfur compounds increased over time, except for allicin. The reason of this might be that allicin is an intermediate in the sequence of the reactions, because it is released from aliine by aliinase and then decomposed into sulfide-compounds.
Table 1. The degree of linear relationship between the amount of sulfur-containing volatile components of crushed, non-heat-treated garlic in the headspace (Y) and time (X). The observed significance levels (P) of the F-test of the regression analysis, and the correlation coefficients (r).
Tabla 1. Grado de relación lineal entre la cantidad de componentes volátiles que contienen azufre del ajo triturado, no tratado térmicamente en el espacio de cabeza (Y) y el tiempo (X). Niveles de significancia observados (P) de la prueba F del análisis de regresión y coeficientes de correlación (r).
3.2. Effect of heat treatment on the formation of volatile components of crushed garlic
Compared to the previous study, two more compounds appeared on the chromatograms besides linear sulfides and allicin. Based on the identification with the spectral library, they were cyclic organic sulfides, vinyldithiin isomers. The first eluted cyclic disulfide was designated as VDT1 and the second one was designated as VDT2 in the followings.
Samples were kept at room temperature after crushing for 30 minutes prior to heat treatment, similar to the work of Tocmo et al. (Citation2017), in order to provide enough allicin precursor for the heat-generated formation of volatile sulfide compounds. presents the average peak area (A) of organosulfur compounds of heat-treated samples at different temperatures. In cooked samples (100°C), the peak area of DAMS and MPDS was higher (P < 0.05) than in samples of lower heat load (60°C). In the case of vinyldithiins (VDT1 and VDT2), variance analysis could not be used due to the non-normal distribution of data. The figure shows that after the 60°C heat treatment they were practically non-detected in the headspace, whereas they were already present to a considerable amount after treatment at 80 and 100°C. The vinyldithiins were neither detectable in the headspace above the crushed garlic samples kept at room temperature, so they were more likely to be attributed to non-enzymatic processes. Contrary, for DAMS and MPDS, there was also a significant positive correlation between the peak area and the duration of treatment in samples kept in room temperature after crushing ().
Figure 2. The average peak area (A) of sulfur-containing volatile components of crushed garlic heated at different temperatures during the same period of time (10 min).
DMDS: dimethyl disulfide, DAMS: diallyl sulfide, MPDS: methyl propenyl disulfide, VDT1 and VDT2: vinyl dithiin isomers. Different letters indicate significant difference (P < 0.05) of means by heat treatment.
. El área pico promedio (A) de los componentes volátiles que contienen azufre del ajo machacado calentado a diferentes temperaturas durante el mismo período de tiempo (10 min).DMDS: disulfuro de dimetilo, DAMS: sulfuro de dialilo, MPDS: disulfuro de metilpropenilo, VDT1 y VDT2: isómeros de vinil ditiina. Diferentes letras indican una diferencia significativa (P < 0.05) de media por tratamiento térmico.
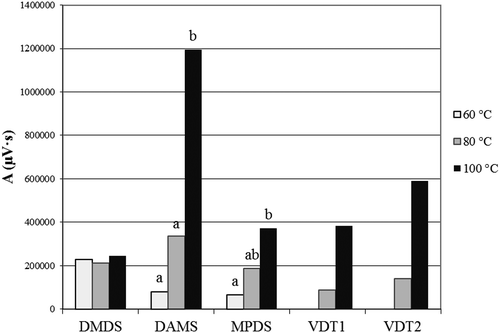
The temperature of the heat treatment had no significant effect (P ≥ 0.05) on the formation of other volatile sulfur compounds, such as DMDS, MADS, DADS, DATS and allicin. Kinalski and Noreña (Citation2014) did not find any difference in the thiosulfinate content of garlic treated for 10 minutes at different temperatures between 80°C and 100°C. Similarly, from this study, no changes in the allicin content of the headspace were observed as a function of the treatment temperature.
3.3. Effect of post-heat storage on volatile components as a function of time
The linear regression and correlation data in refer to samples kept on room temperature after heat treatment.
Table 2. The degree of linear relationship between the amount of sulfur-containing volatile components in the headspace (Y) and time (X) when heat treated crushed garlic is stored at room temperature. The observed significance levels (P) of the F-test of the regression analysis and the correlation coefficients (r).
Tabla 2. Grado de relación lineal entre la cantidad de componentes volátiles que contienen azufre en el espacio de cabeza (Y) y el tiempo (X) cuando el ajo triturado tratado térmicamente se almacena a temperatura ambiente. Niveles de significancia observados (P) de la prueba F del análisis de regresión y coeficientes de correlación (r).
For garlic heat treated at 60°C, except for allicin and DATS, there was a significant positive correlation between the peak area of the components and the duration of storage at 25°C after heat treatment (), similarly to non-heat treated samples ().
In contrast, in the samples treated at 80°C and 100°C, only the amount of DAMS increased over time, with no significant linear relationship between peak area and time for all other components. The reason for this phenomenon may probably be that the lower, 60°C, heat treatment providing the lowest thermal load has not yet fully inactivated the enzymes that promote the formation of sulfur-containing volatile compounds, so the aliin – allicin – organic sulfide transformation continued at 25°C. In contrast, the two other treatments with higher heat loads (80°C and 100°C, 10 minutes) may inactivate the enzymes; moreover their formation without enzymes was not significant, except for DAMS, due to the low temperature (25°C). Therefore, after the heat treatment, most of the compounds, with the exception of DAMS, did not change over time ().
The slopes of regression equations were smaller when samples were heat-treated at 60°C related to that of non-heated samples (, 60°C versus NH). This may also indicate the partial inactivation of the enzymes involved in the formation of the measured compounds at 60°C, for 10 minutes. In the case of DAMS, the higher temperature heat treatment resulted in a higher regression coefficient (), i.e. during storage after treatment more DAMS were released during the same time, in those cases, when the heat effect was higher. It is likely that the higher heat load resulted in the formation of more DAMS precursors that enabled the synthesis of more DAMS at room temperature. The formation of DAMS was facilitated by heat treatment both directly () and indirectly ().
Table 3. Regression coefficients of the models1 (b1; A/min). NH = Non-heat-treated samples stored at room temperature. H = Heat-treated samples stored at room temperature.
Tabla 3. Coeficientes de regresión de los modelos1 (b1; A/min). NH = Muestras no tratadas térmicamente almacenadas a temperatura ambiente. H = Muestras tratadas térmicamente almacenadas a temperatura ambiente.
3.4. Influence of crushed garlic and intact garlic clove cooking on volatile components
During the measurement, the pairs of samples, “whole cloves” and “crushed garlic” of the same weight followed each other, which meant that 70 minutes passed regarding each pair, between their headspace sampling. The reason for this simple measurement arrangement was that in the previous assay it was observed that significant heat load (at least 80°C and 10 min) eliminated the effect of time factor at room temperature for most of the measured compounds. After the heat treatment at 100°C for 10 minutes, with the exception of DAMS, there was no significant correlation between the storage time at room temperature and the peak area of the components (). This observation meant that the investigation of the effect of crushing on heat treatment was enabled independently of time.
In the case of crushed and heat-treated garlic, significantly more DAMS, MADS, allicin, DADS and DATS were released and entered the headspace (P < 0.001) than in the case of the whole and heat-treated garlic cloves (). DMDS and MPDS were not present in detectable amounts in the headspace of the sample bottles containing intact garlic, but were well detectable in the headspace of bottles containing the same weight of crushed garlic. Vinyldithiins were present in the headspace of all two samples, but were significantly higher in the crushed samples. Based on the results of the paired sample t-test, it can be concluded that the disintegration of the cells is indispensable for the formation and release of significant amounts of volatile organosulfur compounds.
Figure 3. Comparison of whole garlic cloves and crushed garlic of the same weight with pairs (1–5) having the same heat treatment (100°C, 10 minutes). The peak area of the components measured in the headspace (A).
DAMS: diallyl sulfide, MADS: methyl allyl disulfide, DADS: diallyl disulfide, DATS: diallyl trisulfide.
. Comparación de dientes de ajo enteros y ajo machacado del mismo peso con pares (1–5) que tienen el mismo tratamiento térmico (100°C, 10 minutos). El área de pico de los componentes medidos en el espacio superior (A).DAMS: dialil sulfuro, MADS: metil alil disulfuro, DADS: dialil disulfuro, DATS: dialil trisulfuro

The destruction of cells not only affects enzymatic processes, but also can lead to the formation of more precursors for the non-enzymatic reactions that occur at high rates during heat treatment. In the crushed garlic, enzymatic processes are more intensive than in the whole garlic clove, so more precursors (e.g. allicin) are available for the non-enzymatic reactions at high temperature heating. This results in the formation of more volatile sulfur-containing compounds in crushed garlic, than in whole garlic cloves.
4. Discussion
Within the same genotype, agrotechnical and storage conditions, the amount of precursor amino acids are considered to be similar in a particular garlic lot and their conversion into volatile compounds depends on the conditions of the processing steps (Martins et al., Citation2016). After disintegration of the cells, alliin decomposition is triggered by the alliinase, and the resulting allicin is further degraded into organic sulfides. However, based on the present observations, the amount of allicin present as an intermediate in the process did not change significantly as a function of time, so its apparent equilibrium was conceivable since the amount of allicin formed from alliin was degraded as well, but further analysis would be needed to confirm or refute this assumption.
In the present study, increasing temperature treatment from 60°C to 100°C increased the amount of DAMS and MPDS and vinyldithiins most, and did not influence the level of allicin (diallyl thiosulfinate). Similarly, Kinalski and Noreña (Citation2014) did not find significant differences in the thiosulfinate level of garlic treated at 80, 90 and 100°C for the same time. Thiosulfinate content decreased at all temperatures as a function of time, but the slopes of the lines were very similar; for the higher temperature treatment was hardly steeper than for the lower temperature heating. The low degree of temperature dependence of allicin decomposition may indicate a low activation energy requirement of the process. During the heat treatment of the crushed garlic, the level of thiosulfinates decreased as a function of time (Kinalski & Noreña, Citation2014), and in parallel the formation of organic sulfides occurred, which are reactive and volatile compounds, so their amount in the food is determined by the formation and decomposition, also by their escape into the headspace. In the experiments of Tocmo et al. (Citation2017), the amount of organic sulfides (diallyl disulfide and diallyl trisulfide) increased over time as a result of the heat treatment, and after reaching a maximum, their concentration decreased, presumably as a result of their chemical decomposition, or leaving the sample matrix. Thiosulfinates and allyl sulfides have antioxidant activity as well as other non-volatile phenolic compounds of garlic (Martins et al., Citation2016), therefore, the combined effect of the quantitative changes in these compounds is associated with a reduction in the antioxidant activity caused by the heat treatment (Kinalski & Noreña, Citation2014). Kinalski and Noreña (Citation2014) detected an immediate, very decisive reduction in antioxidant activity during heat treatment. On the basis of the above, it appears that the decrease in thiosulfinate concentration has a greater effect on antioxidant activity than the initial increase in allyl sulfide levels.
According to the present observations, the examined kitchen techniques contributed to the formation of the various sulfur compounds to different degrees. Short cooking after crushing resulted in the enrichment of vinyldithiins. DAMS has been produced to a significant degree by both processes, crushing and cooking, and in this experiment, it was the only compound that increased after cooking.
The formation of organic sulfides during storage following heating may partly depend on the inactivation of the enzyme system allowing the formation of these compounds at room temperature. The temperature optimum of allinase from Amaryllidaceae family is between 35–40°C (Poojary et al., Citation2017) and half of its activity was lost when garlic was kept at 60°C for 15 minutes (Wang, Cao, Sun, Wang, & Mo, Citation2011). These findings were in accordance with the actual observations, that is, the 60°C for 10-minute treatment, partially inactivated the enzymes, whereas the 80°C and 100°C heat treatment resulted in total inactivation during 10 minutes. During the storage of the heat-treated samples at room temperature, only the peak area of the DAMS increased in the headspace, the other compounds did not show changes or slightly, non-significantly, decreased. This experiment was conducted using closed vessels. In contrast, during cooking of food and its subsequent storage, it should be taken into account that some of the volatile sulfur compounds are released into the environment.
Based on the above, it is likely that allicin supply will not be sufficient to maintain the levels of volatile organic sulfides, when the garlic is cooked. Heat treatment can cause the inactivation of aliinase, and the “allicin pool” is gradually exhausted during baking and cooking. Some part of the organic sulfides produced from allicin are released from the food or decomposed, thus the amount of volatile sulfur containing bioactive substances decrease gradually. Their loss during cooking and baking may be significant if a high temperature is used for a long time in a domestic food preparation. The level of bioactive substances can also be greatly influenced by other factors, such as other compounds besides garlic (matrix effect). The continuous phase of the heterogeneous system may be lipid (e.g. frying) or water based (e.g., cooking) which may affect the dissolution and diffusion rate of the volatile sulfur compounds formed, since the organic sulfides formed in garlic are essentially apolar, oil-soluble compounds.
In this study, the cooking of intact garlic cloves resulted in much less volatile organosulfur compounds than the cooking of crushed garlic of the same weight. The disruption of cell integrity resulted in the formation of volatile sulfur-containing components by catalyzing aliinase. According to other observations (Cavagnaro et al., Citation2007; Song & Milner, Citation2001), crushing the cloves before cooking or baking has improved the effect of garlic on clot and tumor formation. Garlic not only reduced its antioxidant activity, but also reduced its anti-platelet effect on thrombosis as a result of heat treatment over time. However, it seems to be relevant from this point of view that garlic is heated in an intact form or crushed. In an in-vitro experiment (Cavagnaro et al., Citation2007), the unfavorable effect of heating at 200°C was temporarily alleviated by crushing the garlic, since the disappearance of this physiologically beneficial effect in the crushed garlic occurred later than in the whole garlic cloves. Based on the results of the present experiment, it is possible that this protective effect may be associated with a more concentrated presence of organic sulfides.
5. Conclusion
In the assay used in the study, the amount of organic sulfides increased for hours after the raw garlic was crushed, at room temperature. Hence, in order to enrich of the organic sulfides crushing the cloves and keeping the material in a sealed container for hours can be suggested, with or without the addition of other food matrix, and then use it as a meal supplement.
It has been found that, with the exception of DAMS, ten-minute boiling hampered the further post-treatment formation of sulfur compounds, when the heat treated garlic was stored at room temperature. Consequently, it is advisable to consume the prepared meal as soon as possible, because the further formation of organic sulfides is limited. Subsequent studies may include the extent to which these components can be retained by cooling or freezing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cavagnaro, P. F., Camargo, A., Galmarini, C. R., & Simon, P. W. (2007). Effect of cooking on garlic (Allium sativum L.) antiplatelet activity and thiosulfinates content. Journal of Agricultural and Food Chemistry, 55(4), 1280–1288. doi:10.1021/jf062587s
- Gorinstein, S., Jastrzebski, Z., Leontowicz, H., Leontowicz, M., Namiesnik, J., Najman, K., … Bae, J.-H. (2009). Comparative control of the bioactivity of some frequently consumed vegetables subjected to different processing conditions. Food Control, 20(4), 407–413. doi:10.1016/j.foodcont.2008.07.008
- Kimbaris, A. C., Siatis, N. G., Daferera, D. J., Tarantilis, P. A., Pappas, C. S., & Polissiou, M. G. (2006). Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrasonics Sonochemistry, 13(1), 54–60. doi:10.1016/j.ultsonch.2004.12.003
- Kinalski, T., & Noreña, C. P. Z. (2014). Effect of blanching treatments on antioxidant activity and thiosulphinate degradation of garlic (Allium sativum L.). Food and Bioprocess Technology, 7(7), 2152–2157. doi:10.1007/s11947-014-1282-1
- Lanzotti, V. (2006). The analysis of onion and garlic. Journal of Chromatography A, 1112, 3–22. doi:10.1016/j.chroma.2005.12.016
- Marchese, A., Barbieri, R., Sanches-Silva, A., Daglia, M., Nabavi, S. F., Jafari, N. J., … Nabavi, S. M. (2016). Antifungal and antibacterial activities of allicin: A review. Trends in Food Science & Technology, 52, 49–56. doi:10.1016/j.tifs.2016.03.010
- Martins, N., Petropoulos, S., & Ferreira, I. C. F. R. (2016). Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chemistry, 221, 41–50. doi:10.1016/j.foodchem.2016.05.029
- Poojary, M. M., Putnik, P., Kovačević, D. B., Barba, F. J., Lorenzo, J. M., Dias, D. A., & Shpigelman, A. (2017). Stability and extraction of bioactive sulfur compounds from Allium genus processed by traditional and innovative technologies. Journal of Food Composition and Analysis, 61, 28–39. doi:10.1016/j.jfca.2017.04.007
- Putnik, P., Gabrić, D., Roohinejad, S., Barba, F. J., Granato, D., Mallikarjunan, K., … Kovačević, D. B. (2019). An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chemistry, 276, 680–691. doi:10.1016/j.foodchem.2018.10.002
- Rose, P., Whiteman, M., Moore, P. K., & Zhu, Z. Z. (2005). Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Natural Product Reports, 22, 351–368. doi:10.1039/b417639c
- Song, K., & Milner, J. A. (2001). Recent advances on the nutritional effects associated with the use of garlic as a supplement. The influence of heating on the anticancer properties of garlic. Journal of Nutrition, 131, 1054S–1057S. doi:10.1093/jn/131.3.1054S
- Szymanek, M. (2011). Effects of blanching on some physical properties and processing recovery of sweet corn cobs. Food and Bioprocess Technology, 4(7), 1164–1171. doi:10.1007/s11947-009-0246-3
- Tocmo, R., Wu, Y., Liang, D., Fogliano, V., & Huang, D. (2017). Boiling enriches the linear polysulfides and the hydrogen sulfide-releasing activity of garlic. Food Chemistry, 221, 1867–1873. doi:10.1016/j.foodchem.2016.10.076
- Wang, J., Cao, Y., Sun, B., Wang, C., & Mo, Y. (2011). Effect of ultrasound on the activity of alliinase from fresh garlic. Ultrasonics Sonochemistry, 18, 534–540. doi:10.1016/j.ultsonch.2010.09.008
