 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, a two-prong strategy was devised to reduce fat-uptake in deep-fat fried fish by developing a low cost, sustainable and product-friendly edible coating using fish processing by-products and replacement of corn starch in batter with sweet potato starch. Edible coatings (protein concentrations of 0, 5%, 10%, and 15%) were prepared from fish protein isolates using isoelectric solubilization and precipitation. Coating with 15% protein and sweet potato starch-based batter resulted in the highest reduction of fat-uptake and moisture loss of fried fish by 85% and 8.21%, respectively. Coated samples had lower L* values. However, samples with sweet potato starch-based batter had better textural properties. The results of this study suggest that edible coating in combination with a batter containing sweet potato starch could be useful in reducing the fat-uptake reduction and improve the physicochemical properties of the fried fish.
RESUMEN
Para la realización del presente estudio se diseñó una estrategia de dos vértices con el objetivo de reducir la absorción de grasa en el pescado frito en abundante grasa, creando un recubrimiento comestible, sostenible y amigable con el producto, de bajo costo. Para ello se utilizaron subproductos provenientes del procesamiento de pescado y en la masa se remplazó el almidón de maíz con almidón de camote [batata]. Así, se prepararon recubrimientos comestibles (concentraciones de proteína de 0, 5%, 10% y 15%) a partir de aislados de proteínas de pescado usando solubilización isoeléctrica y precipitación. El recubrimiento con 15% de proteína y masa de almidón de camote propició la mayor reducción en la absorción de grasa y en la pérdida de humedad del pescado frito, en 85% y 8.21%, respectivamente. Las muestras recubiertas registraron valores más bajos de L*. Sin embargo, las muestras con masa a base de almidón de camote exhibieron mejores propiedades de textura. Los resultados de este estudio dan cuenta de que el recubrimiento comestible en combinación con una masa que contiene almidón de camote puede ser útil para reducir la absorción de grasa y mejorar las propiedades fisicoquímicas del pescado frito.
1. Introduction
Deep-fat frying is a traditional cooking method in which food is submerged in an edible oil and heated to over 100°C. This oil is absorbed into the food and increases the total fat content (Ananey-Obiri et al., Citation2018). Fried fish is one of the popular fried food products around the world and García et al. (Citation2008) showed that the fat content of fish increased from 1.4% in raw fish to 18% in fried fish. Cross-sectional studies have shown that consumption of fried foods positively correlates with several cardiometabolic risk factors including low serum HDL cholesterol (Donfrancesco et al., Citation2008), hypertension (Soriguer et al., Citation2003), and obesity (Donfrancesco et al., Citation2008; Guallar-Castillón et al., Citation2007). Increased risk of type 2 diabetes, due to high consumption of fried foods, has also been documented through prospective studies (Alhazmi, Stojanovski, McEvoy, & Garg, Citation2014). Thus, health experts have sought out means to reduce the fat uptake by foods during deep-fat frying.
In general, there are three main steps in deep-fat frying of foods including battering, breading and then frying. Each of these steps may increase the fat-uptake of the final product. However, reducing the fat content in fried foods is not that simple since it may affect organoleptic properties such as taste and mouthfeel (Parikh & Nelson, Citation2013). Williams and Mittal (Citation1999) indicated that the selection of an appropriate food coating before battering is one possible means of reducing fat-uptake. An edible coating is a thin layer of edible material formed as a coating on a food product. These coatings can act as barriers to moisture loss, which is important commercially, and also reduce fat uptake during frying (Al-Abdullah, Angor, Al-Ismail, & Ajo, Citation2011).
Currently, the food industry is looking for ways to reduce the food processing by-products due to their adverse effects on the environment. On the other hand, they could be used as starting materials for new product development or fabrication of food packaging. Filleting the fish to make fried products generates large quantities of by-products (frames, heads, and meat left over on the bone and skin). The by-products are highly nutritious. However, they are usually discarded without any effort for nutrient recovery (Gehring, Gigliotti, Moritz, Tou, & Jaczynski, Citation2011). These by-products are good sources of myofibrillar proteins that could be recovered and used as coating materials. According to Ananey-Obiri et al. (Citation2018), myofibrillar proteins can form an effective edible coating due to formation of a three-dimentional network. This 3-D network prevents moisture loss during deep-fat frying of foods. Consequently, less fat is taken up by the fried food. Myofibrillar protein coating solutions may be prepared from the washed meat of low-cost fish (Sharaf Eddin & Tahergorabi, Citation2017) or filleting of trimmed meat. Muscle protein edible films increase the nutritional value of the food while performing the function of oil-uptake reduction. Application of myofibrillar proteins as a coating for muscle foods, which are rich in these proteins, is novel and product-friendly.
Batters are usually starch-based with high solid content and low viscosity. The nature of the starch used, and the percent of solids dictate the flow properties of the batter. Starch also contributes to the drying time of the applied batter and the development of a crispy texture during the frying operation. Normally, corn starch is used for battering in deep-fat fried foods. According to Sánchez-Zapata, Viuda-Martos, Fernández-LÓpez, and Pérez-Alvarez (Citation2015), resistant starch helps reduce fat uptake in deep-fried foods, and by the percentage of total starch, sweet potato starch has higher resistant starch concentration than the corn starch. In our previous research, sweet potato starch exhibited promising film-forming ability and good barrier properties (Issa et al., Citation2018). Thus, replacement of corn starch with sweet potato starch in the batter formulation could be more effective in reducing the fat uptake either in combination with the edible coating or without edible coating. The primary objectives of this research were to (1) determine the effectiveness of fish protein as an edible coating on the fat-uptake reduction during deep-fat frying of fish, (2) examine the effect of replacing corn starch with sweet potato starch in battering system on fat-uptake reduction during deep-fat frying, (3) study synergistic impact of edible coating and batter modification on quality attributes of deep-fat fried fish.
2. Materials and methods
2.1. Fish preparation
Fresh caught whiting fish (Merlangius merlangus) were purchased from a local store, packed in ice boxes and transported within 2 h to the laboratory. The heads and frames of the fish were separated and used for protein isolation. The fish fillets were cut in strips (10 ± 1 g) and the narrow tips were discarded to make the pieces as uniform as possible. Then, they were stored in a refrigerator until used.
2.2. Preparation of washed fish muscle
Prior to protein isolation, fish processing by-products (meat left over on bones, skin, and head) were rinsed with tap water and then washed with a diluted salt solution to further reduce the fat content and other impurities. Fish processing by-products were ground using a meat grinder (LEM grinder, Grinder-0.35 HP, West Chester, OH, USA) with a hole diameter of 0.5 cm. Ground fish by-products were homogenized with 5 vol. of cold 0.05 M NaCl (2–4°C) at a speed of 13,000 rpm for 2 min, using a homogenizer (Homogenizer, OMNI International, Kennesaw, GA, USA) followed by centrifuging at 5000 × g for 20 min at 4°C, using a refrigerated centrifuge (Thermo Scientific, Model ST 16, Asheville, NC, USA). The washed fish muscle obtained from the first step underwent the same washing steps for the second time (Tongnuanchan, Benjakul, Prodpran, & Songtipya, Citation2011). The washed fish meat obtained was stored in the refrigerator until used for analysis and protein isolation.
2.3. Protein isolation
Protein isolation was conducted according to Tahergorabi, Beamer, Matak, and Jaczynski (Citation2013). The washed fish meat was homogenized with deionized distilled water at 1:6 ratio (washed fish meat:water, w:v) using a laboratory homogenizer (Homogenizer, OMNI International, Kennesaw, GA, USA) set at speed five. Processing temperature was carefully controlled at 4°C during recovery by placing the container in an ice bucket. The pH of homogenate was adjusted to 11.50 ± 0.05 by using 10 N NaOH and homogenized for 10 min. Then, the homogenate was centrifuged at 10,000 × g at 4°C for 20 min using a laboratory batch centrifuge (Thermo Scientific, Model ST 16, Asheville, NC, USA). The centrifugation resulted in three layers including fish fat (top layer), fish muscle protein solution (middle layer), and insoluble (bones, skin, insoluble proteins, membrane lipids, etc.) (bottom layer). HCl (6 N) was used to adjust the pH of the muscle protein solution to isoelectric point of fish (5.50 ± 0.05) to precipitate the proteins. Homogenization continued for 10 min at this pH followed by centrifugation. The centrifugation resulted in two layers including process water (top layer) and precipitated and de-watered fish muscle proteins (bottom layer). The retentate was further dewatered by centrifugation at 12,000 × g for 20 min at 4°C. The precipitated and de-watered proteins were collected and used in the development of edible coating.
2.4. Edible coating preparation
The edible coating was prepared according to the method described by Cortez-Vega, Pizato, de Souza, and Prentice (Citation2014) with slight modification. The protein isolate (5% or 10% or 15% (w/w)) was added with 3 vol. of distilled water and homogenized at 13,000 rpm for 1 min. Glycerol, as a plasticizer, was added at 0.4% (w/w) of protein. The mixture was stirred gently for 30 min at room temperature. Subsequently, the pH of the mixture was adjusted to 11 using 10 N NaOH. The final pH of the sample was adjusted to pH 7.0 using 6 N HCl. The solution was filtered through two layers of cheesecloth to remove un-dissolved debris. The edible coating was kept refrigerated (4°C) for up to 12 h before use.
2.5. Batter preparation and breading
2.5.1. Commercial batter
The commercial batter (Seafood Batter mix, Hunt Valley, MD, USA) was prepared as described on the product. The ingredients include wheat flour, niacin, iron, thiamine mononitrate, riboflavin, folic acid, yellow corn flour, modified corn starch, and salt.
2.5.2. Preparation of batter containing corn starch and sweet potato starch
The batters were prepared from 48.75% (w/w) wheat flour (All- purpose flour, Bentonville, MD, USA), 48.75% corn starch or sweet potato starch (Wako Pure Chemical Industry, Richmond, VA. USA), 1.0% HPMC (Methocel E15 Premium LV Hydroxypropyl Methylcellulose, Midland, USA), 1.0% salt (Morton salt, Chicago, IL, USA), 0.5% baking powder (Rumford, Terre Haute, IN, USA) and cold deionized water. The batter was standardized based on viscosity, using Stein Cup method. The batter was made on the day it was used and held no longer than 12 h before use.
2.5.3. Breading
The breading was made up of plain bread crumbs (Progresso, bread crumbs, Minneapolis, MN, USA) which were sieved using a 2 mm sieving device to assure a particle size of less than 2 mm.
2.6. Fish preparation for frying
The previously prepared fish strips (10 ± 1 g) individually were dipped into the edible coating solutions, after dipping, gently shaken to remove excess. Then, the samples were pre-dusted, battered, and breaded. Fish samples were fried in 160°C canola oil in a deep fryer (Presto® Dual ProFry ™/1800W, National Presto Industries Inc., WI., U.S.) for 3 min till their central temperature reached to the point that decreased the level of the harmful microorganisms to an acceptable level (63°C). Fried samples were allowed to drip for a few seconds, then weighed and analyzed. To ensure consistent quality, the use of frying oil was limited to 5 h. Fish samples were randomly assigned to six treatments, including (treatment 1, T1: without coating and battering), (treatment 2, T2: commercial battered; without coating), (treatment 3, T3: corn starch or sweet potato starch-based battered; without coating), (treatment 4, T4: corn starch or sweet potato starch-based battered and fish protein-based coating at 5%), (treatment 5, T5: corn starch or sweet potato starch-based battered and fish protein-based coating at 10%), (treatment 6, T6: corn starch or sweet potato starch-based battered and fish protein-based coating at 15%).
2.7. Fat determination
The fat content was determined quantitatively by Soxhlet extraction method. This method has widely been used in fat determination in poultry, meat, etc. A total of 3–4 g from fried fish samples were measured into a disposable aluminum dish. The sample was dried in an oven for 1 ½ h at 125°C. The sample was cooled and transferred into an extraction thimble. The weight of an extraction flask was determined, and about 85 mL of petroleum ether was then added to the flask. The extraction process was done for a minimum of 80 cycles in about 6 h. After the extraction process, the flask was heated over a water bath at 42-62°C for few minutes. The flask was dried in an oven at 100°C −102°C for few minutes and cooled to room temperature. Fat content (FC) was calculated using the equation below:
2.8. Moisture determination
The moisture of fried fish samples was analyzed according to AOAC (Citation1995). The samples were grounded separately into fine particles in a laboratory grinder, and 3 g of each sample was transferred into an empty dish in a vacuum oven. The samples were evenly spread with a spatula in the dish. Samples were left to dry at 105°C for 3 h. After this, the samples were cooled and weighed. The moisture content (MC) of each sample was calculated using the equation below:
2.9. Determination of pH
The measurement of pH was performed by the method described by Alakhrash, Anyanwu, and Tahergorabi (Citation2016) with slight modifications. Fried fish meat (5 g) was homogenized with 20 ml of deionized water for 2 min using a homogenizer (OMNI International, Kenneswa, GA, USA). The homogenate was kept at room temperature for 5 min. The pH was determined using a hand-held pH meter (Oakton, Vernon Hills, IL, USA).
2.10. Texture properties
Texture profile analysis (TPA) was performed for deep-fat fried fish samples. The fish samples were subjected to two-cycle pressure (half crush) utilizing the texture analyzer (Model TA-XT2, Texture Analyzer, Texture Technologies Corp., Scarsdale, NY, USA) furnished with a cycle 70-mm width TPA plate connection moving at a speed of 90 mm/min. The result of power time curve was characterized by Bourne (Citation2002), which incorporates hardness, cohesiveness, springiness, gumminess, chewiness, and resilience.
2.11. Color properties
The color properties of the samples were determined by utilizing a Minolta Chroma Meter CR-400 colorimeter (Minolta Camera Co. Ltd., Japan) (Tahergorabi et al., Citation2013). The tristimulus color values L* (Lightness), a* (red to green) and b* (yellow to blue) values were determined.
2.12. Frying yield
The frying yield was calculated as follows:
2.13. Statistical analysis
Each experiment was conducted three times, independently (n = 3). For every experiment, the mean, as well as ±standard deviation of the results, was recorded; then an analysis of variance utilizing the one-way ANOVA was completed using SAS (version 16.0, SAS Institute, Cary, North Carolina, USA). Finally, the differences in the mean values of the results of triplicated experiments were computed using Tukey’s test, and the criterion for labeling a difference as significant was P < .05.
3. Results and discussion
3.1. Fat content
) depicts the fat-uptake of raw and deep-fried fish samples for cornstarch and sweet potato starch-battered, respectively. Fat accounted for nearly 10.5% of the content for the sample without any coating or battering (T1). One of the mechanisms of fat-uptake in deep-fat fried food is capillary mechanism in which water vapor escapes through the food pores resulting in oil uptake in the food. Similarly, Bakar, Moradi, and Man (Citation2010) observed increased fat uptake in the non-battered and non-breaded fish samples. The commercial batter reduced the level of fat absorption significantly (P < .05) when compared with T1. For samples which were coated with 5%, 10%, and 15% of fish protein-based edible coating and cornstarch-based battered, significant lower fat absorption was recorded as 3.04%, 1.88%, and 0.66%, respectively. The higher fat-uptake reduction (26. 56% versus 24%) was observed when cornstarch batter was replaced with sweet potato starch and protein-based coating at 15%. This could be explained by the difference in amylose and amylopectin content of these two starches. During gelatinization, hydrogen bonds break apart, the starch granules lose their orderliness and swell due to oil entering granules. The amylose content leaches out of the granules, allowing for more oil to be entrapped. Amylose leach more easily from cornstarch than sweet potato starch. The differential higher amount of amylose in cornstarch than the sweet potato starch indicates that more space will be created in the corn starch than the sweet potato starch (Bertoft, Citation2017).
Figure 1. Fat content (%) of raw fish and deep-fat fried fish for samples with edible coating and batter containing (a) corn starch and (b) sweet potato starch. Data are given as mean values ± standard deviation (n = 3). Different letters on the top of data bars indicate significant differences (Tukey’s Test, P < 0.05) between mean values.
Figura 1. Contenido de grasa (%) de muestras de pescado crudo y pescado frito en abundante grasa con recubrimiento comestible y masa que contiene (a) almidón de maíz y (b) almidón de camote. Los datos son valores medios ± desviación estándar (n = 3). Las diferentes letras en la parte superior de las barras de datos indican diferencias significativas (Prueba de Tukey, P < 0.05) entre los valores medios.
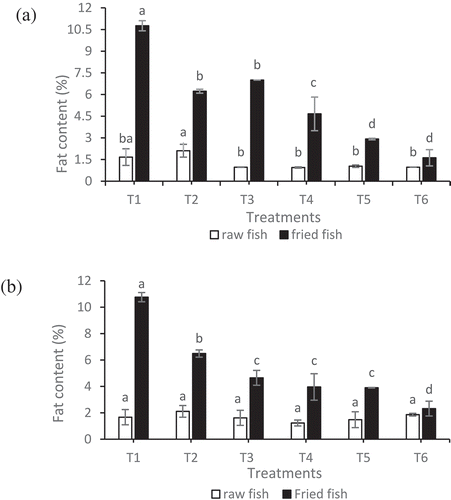
In general, fish protein-based edible coating regardless of the type of starch used in battering system was able to reduce fat uptake. In contrast, when He, Franco, and Zhang (Citation2015) used fish protein hydrolysates (FPH) as an edible coating for deep-fat fried fish, they noticed that fat uptake of the samples with coatings from the enzymatic process and the microwave-intensified enzymatic process was significantly higher than the control (uncoated samples). This could be attributed to the different isolation method used for the recovery of protein and edible coating preparation. The higher oil-binding capacity of FPH is generally associated with higher fat-uptake. The oil permeability of the material depends upon the solubility of the oil in the film, the diffusivity of oil as well as the film thickness.
In a comprehensive study, various edible coatings were evaluated for fat-uptake reduction in deep-fried cereal products. The comparison was conducted on 11 hydrocolloid materials to evaluate fat and water transfer properties. The highest values, in terms of heat stability and fat-uptake effectiveness, were for soy-protein isolate, whey protein isolate ad methylcellulose (Albert & Mittal, Citation2002). Mixed coatings were seen to be even more effective in terms of reduction of moisture loss and fat uptake, however, they did increase the thickness of the coating (Albert & Mittal, Citation2002). They reported an 80.1% fat-uptake reduction when soy-protein isolate coating was used which is lower than the fat-uptake reduction in our study (i.e. 85%) when sweet potato starch batter and protein-based coating at 15% was used.
3.2. Moisture content
) exhibits the moisture content for the samples with edible coating and batter containing cornstarch and sweet potato starch, respectively. The moisture results showed that the application of the protein film coating treatments was successful in reducing moisture loss in the fried samples significantly (P < .05). Myofibrillar proteins (myosin and actin) are the major proteins of the muscle proteins including fish proteins. Myosin can form a three-dimensional network structure that prevents waterscape when gels (Tahergorabi & Jaczynski, Citation2016). This gives the muscle protein a film-forming ability with high mechanical and water barrier properties, coupled with nutritional value. Similarly, Marquez, Di Pierro, Esposito, Mariniello, and Porta (Citation2014) reported soy-protein isolate- and whey protein isolate- based edible coatings reduced moisture loss in doughnuts and French fries since they form a crust which acts as a barrier to moisture loss. It is believed that fat uptake is dramatically affected by the water content in deep-fried food; a higher water reduction is generally associated with higher fat uptake (Mellema, Citation2003). In contrast to this widely known mechanism, the findings of this study could not establish a direct relationship between moisture loss and oil uptake. Treatments that retained a high amount of moisture did not produce a corresponding fat-uptake reduction, as proposed by this mechanism. This finding is congruous with the findings of Ouchon, Aguilera, and Pyle (Citation2003), who could not find a regular pattern between moisture loss and fat absorption during frying. It can partly be explained by the mechanism that oil absorption during frying is a surface phenomenon involving equilibrium oil adhesion and drainage upon removal from the oil (Ufheil & Escher, Citation1996). In general, samples with sweet potato starch batter had consistent moisture content and lower moisture loss when compared with samples battered with corn starch. This could be due to structural differences with corn starch that was discussed earlier.
Figure 2. Moisture content (%) of raw fish and deep-fat fried fish samples with edible coating and batter containing (a) corn starch and (b) sweet potato starch. Data are given as mean values ± standard deviation (n = 3). Different letters on the top of data bars indicate significant differences (Tukey’s Test, P < 0.05) between mean values.
Figura 2. Contenido de humedad (%) de muestras de pescado crudo y pescado frito en abundante grasa con recubrimiento comestible y masa que contiene (a) almidón de maíz y (b) almidón de camote. Los datos son valores medios ± desviación estándar (n = 3). Las diferentes letras en la parte superior de las barras de datos indican diferencias significativas (Prueba de Tukey, P < 0.05) entre los valores medios.
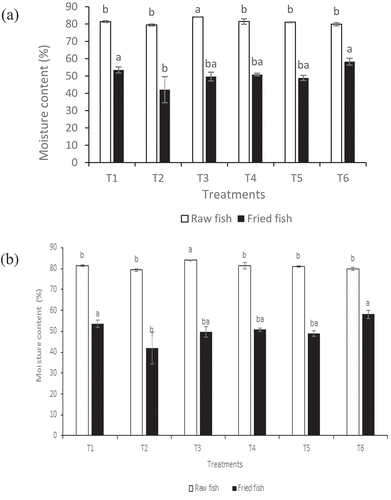
3.3. Changes in pH
) illustrates the changes in pH determined for the cornstarch and sweet potato starch batter preparations, respectively. The pH value for the samples without coating and battering (T1) started as neutral and then proceeded to gradual decline due to the addition of commercial batter (T2). In general, no significant difference (P > .05) between T1 and T2 in both groups was noted. However, the rest of the treatments did not show pH changes due to edible coating or corn batter formulation. Slightly lower pH values were recorded for samples treated with sweet potato starch batter systems. According to the information provided by the manufacturer, the sweet potato starch used in this study contains a higher content of carbohydrate (3%) in comparison with corn starch (2%). Also, sweet potato amylopectin is unique among other starches in having more of phosphate ester groups. The phosphorus content of sweet potato starch has been reported as 600–100 mg/kg. The phosphate ester groups give sweet potato starch amylopectin a slight negative charge which may attract more hydrogen ions. However, cereal starch molecules like corn either do not have phosphate ester groups or have much lower small amounts than do potato starches (Hoover, Citation2001). This might have contributed to slightly lower pH values observed in samples treated with sweet potato starch. Mah and Brannan (Citation2009) showed that lowering the pH of an aqueous whey protein isolate (WPI) dip to pH 2 decreased the oil content of deep-fried chicken patties. These authors speculated that lowering the pH resulted in gel formation that was responsible for inhibiting oil absorption into the product. This confirms our fat-uptake results in which samples contain sweet potato starch batter had lower fat-uptake. Protein dips at lower pH are theorized to produce gels with smaller pores. While increased protein concentration does promote random aggregation due to the close contact between monomers (Mah & Brannan, Citation2009). These factors might have played a role to inhibit oil uptake in the studied deep-fat fried fish.
Figure 3. Changes in pH values of deep-fat fried fish samples with edible coating and batter containing (a) corn starch and (b) sweet potato starch. Data are given as mean values ± standard deviation (n = 3). Different letters on the top of data bars indicate significant differences (Tukey’s Test, P < 0.05) between mean values.
Figura 3. Cambios en los valores de pH de muestras de pescado frito en abundante grasa con recubrimiento comestible y masa que contiene (a) almidón de maíz y (b) almidón de camote. Los datos son valores medios ± desviación estándar (n = 3). Las diferentes letras en la parte superior de las barras de datos indican diferencias significativas (Prueba de Tukey, P < 0.05) entre los valores medios.
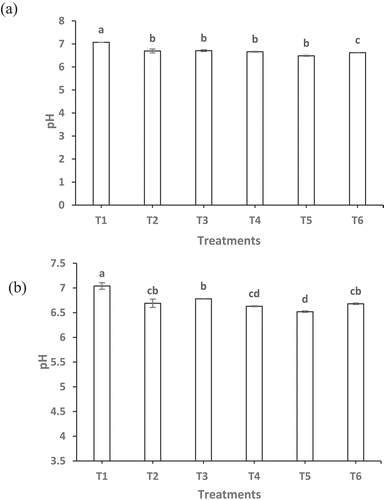
3.4. Texture profile analysis
The results for texture profile analysis (TPA) are shown in and . The TPA has been widely used for the empirical determination of a number of textural attributes of muscle foods. The texture profile quantifies specific characteristics that can be directly related to the overall acceptance or hedonic ratings. The maximum force of the first compression determines hardness, and the ratio of the area under the second cycle compression curve to the area under the first cycle compression curve determines cohesiveness. The hardness and cohesiveness of TPA can be expected to correlate with the sensory texture profile evaluation (Bourne, Citation2002). The hardness scores increased significantly (P < .05) with each increment of protein concentration in the edible coating. However, these results were significantly lower (P < .05) for sweet potato starch batter system than the cornstarch batter system. The highest score exhibited at 15% protein film with cornstarch batter and breading (T6). A previous study found that adding 1% or 3% of WPI to the batter of deep-fried chicken samples significantly increased the hardness of deep-fried chicken samples when compared with samples to which no WPI was added to the batter (Dogan, Sahin, & Sumnu, Citation2005). This is due to the gel network formation of the protein upon heating (Schokker, Singh, Pinder, & Creamer, Citation2000). However, no significant difference (P > .0) was observed in the cohesiveness of deep-fat fried fish samples regardless of the batter system or protein concentration in the edible coating. Springiness for cornstarch batter system was seen to either increase or decrease with treatment, attributable to the extra layer of breading, batter, and coating. The higher protein concentrations in edible coating did not have a significant effect (P > .05) on springiness rating (i.e., 10% and 15%). For sweet potato starch batter system, higher concentrations of protein coating exhibited a drastic decrease in springiness rating (i.e., 10% and 15%) but at a lower score than corn starch treatments. As expected, an incremental pattern of gumminess scores was seen that denoted higher total work of the second chew compression following the first.
Table 1. Texture profile analysis of deep-fat fried fish with batter containing corn starch and edible coating.
Tabla 1. Análisis de perfil de textura de pescado frito en abundante grasa con masa que contiene almidón de maíz y revestimiento comestible.
Table 2. Texture profile analysis of deep-fat fried fish with batter containing sweet potato starch and edible coating.
Tabla 2. Análisis de perfil de textura de pescado frito en abundante grasa con masa que contiene almidón de batata y recubrimiento comestible.
The trend of changes in chewiness was almost the same for both battering systems. Chewiness is the product of gumminess and springiness, the score of chewiness yielded a progressively increasing rate for different treatments. This can be correlated to the factors that supplement gumminess and springiness (i.e., battering, coating and breading). The edible protein coating, together with breading and cornstarch batter, augment the energy required to masticate the fried fish samples.
In general, the TPA attributes of deep-fat fried fish samples for the corn starch batter preparation are significantly higher than that of the sweet potato batter for all of the treatments excluding the control samples; signifying the T6 of corn-starch batter to be harder and requiring much more chewing cycles than the sweet potato batter. This is primarily because of the nature of sweet potato starches to slowly retrograde as opposed to corn starch batter (Chen, Citation2003).
3.5. Color determination
The tristimulus color values (L*, a* and b*) were evaluated and shown in and . For the cornstarch batter system, the obtained L* values showed a darker hue which ranged from 56.09 (untreated sample) to 48.32 (sample with 15% protein coating). The L* value continued to significantly (P < .05) decrease in each consecutive treatment utilizing different concentrations (5%, 10%, and 15%) of edible protein film, while the L* value ranged from 56.09 (untreated samples) to 55.74 (sample with 15% protein coating) for sweet potato starch battering system. This indicates that L* value was not affected by treatments when sweet potato starch batter system used. As previously discussed, the presence of phosphate ester group in sweet potato starch amylopectin provides unique properties including good clarity. Therefore, samples battered with sweet potato starch-based batter tended to have clearer batters in comparison with corn starch. The results of the tristimulus color values are also in agreement with the moisture results since the higher moisture content in samples battered with sweet potato starch-based batter produced brighter colorations than cornstarch. Mah and Brannan (Citation2009) reported the same trend for chicken patties treated with different concentrations of whey protein isolate. Commercial batter (T2) signified the darkest hue. Application of commercial batter resulted in the least moisture content (P < .05). This could be related to the particle size of the commercial batter. The increased particle size might have reduced the water-holding capacity of the fried sample which is evident in the moisture content of T2. For the cornstarch battering system, there was not much difference in a* values of different treatments except for the last treatment (15% protein edible coating) which decreased significantly. However, b* value was declined for all treatments with edible coating and sweet potato starch battering system. These results are in accordance with Adedeji and Ngadi, (Citation2011) for deep-fat fried chicken nuggets. The lowest b* values were recorded for edible coated samples with both batter systems.
Table 3. Color properties of deep-fat fried fish with batter containing corn starch and edible coating.
Tabla 3. Propiedades de color del pescado frito en abundante grasa con masa que contiene almidón de maíz y revestimiento comestible.
Table 4. Color properties of deep-fat fried fish with batter containing sweet potato starch and edible coating.
Tabla 4. Propiedades de color del pescado frito en abundante grasa con masa que contiene almidón de batata y recubrimiento comestible.
3.6. Frying yield
The frying yield calculated for samples with batters containing corn starch, sweet potato starch and edible coating for the various treatments is shown in . The frying yield is analyzed as an important quality in fried food production. Frying yield is influenced by batter pickup as the viscosity of the batter determines how well it acts as a protective coating. A higher viscosity correlates to a higher frying yield and decreased oil uptake. The frying yield among all the corn starch-battered samples and the sweet potato starch-battered samples was significantly (P < .05) higher than the controls. In addition, the samples with sweet potato starch-based batter had a higher frying yield than all the other samples, indicating a more gelatinous coating and increased viscosity. It also confirms the higher moisture content of the samples which battered with sweet potato starch-based batters.
Table 5. Frying yield (%) of deep-fat fried fish with batter containing corn starch\Sweet Potato Starch (SPS) and edible coating.
Tabla 5. Rendimiento de fritura (%) de pescado frito en abundante grasa con masa que contiene almidón de maíz \ almidón de camote (SPS) y recubrimiento comestible.
4. Conclusions
The total fat content of deep-fat fried fish samples was reduced by using a protein-based edible coating. Application of fish protein-based edible coating in combination with sweet potato starch batter system resulted in a significantly higher fat-uptake reduction (P < .05) and moisture retention. In general, fried fish samples with sweet potato starch batter and the protein-based edible coating had a better texture, color and frying yield than samples with cornstarch batter. Future studies on descriptive sensory evaluation and assessment of storage stability are recommended.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adedeji, A. A., Liu, L., & Ngadi, M. O. (2011). Microstructural evaluation of deep-fat fried chicken nugget batter coating using confocal laser scanning microscopy. Journal of Food Engineering, 102(1), 49–57. doi:10.1016/j.jfoodeng.2010.08.002
- Al-Abdullah, B. M., Angor, M. M., Al-Ismail, K. M., & Ajo, R. Y. (2011). Reducing fat uptake during deep-frying of minced chicken meat-balls by coating them with different materials, either alone or in combination. Italian Journal of Food Science, 23, 3.
- Alakhrash, F., Anyanwu, U., & Tahergorabi, R. (2016). Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT-Food Science and Technology, 66, 41–47. doi:10.1016/j.lwt.2015.10.015
- Albert, S., & Mittal, G. S. (2002). Comparative evaluation of edible coatings to reduce fat uptake in a deep-fried cereal product. Food Research International, 35(5), 445–458. doi:10.1016/S0963-9969(01)00139-9
- Alhazmi, A., Stojanovski, E., McEvoy, M., & Garg, M. L. (2014). The association between dietary patterns and type 2 diabetes: A systematic review and meta‐analysis of cohort studies. Journal of Human Nutrition and Dietetics, 27(3), 251–260. doi:10.1111/jhn.12139
- Ananey-Obiri, D., Matthews, L., Azahrani, M., Ibrahim, S. A., Galanakis, C. M., & Tahergorabi, R. (2018). Application of protein-based edible coatings for fat uptake reduction in deep-fat fried foods with an emphasis on muscle food proteins. Trends in Food Science & Technology, 80, 167–174. doi:10.1016/j.tifs.2018.08.012
- Association of Official Analytical Chemists. (1995). Official methods of analysis (16th ed.). Washington, DC: Author.
- Bakar, J., Moradi, Y., & Man, Y. C. (2010). Fat uptake evaluation in fried fish fillet by using Scanning Electron Microscopy (SEM). Iranian Journal of Fisheries Sciences, 9(2), 327–336.
- Bertoft, E. (2017). Understanding starch structure : recent progress. Agronomy, 7, 56. doi:10.3390/agronomy7030056
- Bourne, M. (2002). Food texture and viscosity: Concept and measurement. San Diego, CA: Elsevier.
- Chen, Z. (2003). Physicochemical properties of sweet potato starches and their applications in noodle products. Laboratory of Food Chemistry - Wageningen University, 1, 1.
- Cortez-Vega, W. R., Pizato, S., de Souza, J. T. A., & Prentice, C. (2014). Using edible coatings from Whitemouth croaker (Micropogonias furnieri) protein isolate and organo-clay nanocomposite for improve the conservation properties of fresh-cut ‘Formosa’papaya. Innovative Food Science & Emerging Technologies, 22, 197–202. doi:10.1016/j.ifset.2013.12.007
- Dogan, S. F., Sahin, S., & Sumnu, G. (2005). Effects of batters containing different protein types on the quality of deep-fat-fried chicken nuggets. European Food Research and Technology, 220(5), 502–508. doi:10.1007/s00217-004-1099-7
- Donfrancesco, C., Noce, C. L., Brignoli, O., Riccardi, G., Ciccarelli, P., Dima, F., … Giampaoli, S. (2008). Italian network for obesity and cardiovascular disease surveillance: A pilot project. BMC Family Practice, 9(1), 53. doi:10.1186/1471-2296-9-53
- García, M., Bifani, V., Campos, C., Martino, M. N., Sobral, P., Flores, S., … Ramírez, C. (2008). Edible coating as an oil barrier or active system. In G. F. Gutiérrez-López, J. Welti-Chanes, & E. Parada-Arias (Eds.), Food engineering: Integrated approaches (pp. 225–241). New York, NY: Springer.
- Gehring, C. K., Gigliotti, J. C., Moritz, J. S., Tou, J. C., & Jaczynski, J. (2011). Functional and nutritional characteristics of proteins and lipids recovered by isoelectric processing of fish by-products and low-value fish: A review. Food Chemistry, 124(2), 422–431. doi:10.1016/j.foodchem.2010.06.078
- Guallar-Castillón, P., Rodríguez-Artalejo, F., Fornés, N. S., Banegas, J. R., Etxezarreta, P. A., Ardanaz, E., … Losada, A. (2007). Intake of fried foods is associated with obesity in the cohort of Spanish adults from the European prospective investigation into cancer and nutrition. The American Journal of Clinical Nutrition, 86(1), 198–205. doi:10.1093/ajcn/86.1.198
- He, S., Franco, C., & Zhang, W. (2015). Fish protein hydrolysates: Application in deep‐fried food and food safety analysis. Journal of food science, 80(1), E108–E115.
- Hoover, R. (2001). Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydrate Polymers, 45(3), 253–267.
- Issa, A. T., Schimmel, K. A., Worku, M., Shahbazi, A., Ibrahim, S. A., & Tahergorabi, R. (2018). Sweet potato starch‐based nanocomposites: Development, characterization, and biodegradability. Starch‐Stärke, 1700273. doi:10.1002/star.201700273
- Mah, E., & Brannan, R. G. (2009). Reduction of oil absorption in deep-fried, battered, and breaded chicken patties using whey protein isolate as a postbreading dip: Effect on flavor, color, and texture. Journal of Food Science, 74(1), 412–417. doi:10.1111/j.1750-3841.2008.00973.x
- Marquez, G. R., Di Pierro, P., Esposito, M., Mariniello, L., & Porta, R. (2014). Application of transglutaminase-crosslinked whey protein/pectin films as water barrier coatings in fried and baked foods. Food and Bioprocess Technology, 7(2), 447–455. doi:10.1007/s11947-012-1045-9
- Mellema, M. (2003). Mechanism and reduction of fat uptake in deep-fat fried foods. Trends in Food Science and Technology, 14(9), 364–373. doi:10.1016/S0924-2244(03)00050-5
- Ouchon, P. B., Aguilera, J. M., & Pyle, D. L. (2003). Structure oil‐absorption relationships during deep‐fat frying. Journal of Food Science, 68(9), 2711–2716. doi:10.1111/jfds.2003.68.issue-9
- Parikh, A. A., & Nelson, D. C. (2013). Fat absorption in commercial french fries depending on oil type and coating. Hospitality Review, 30(2), 1.
- Sánchez-Zapata, E., Viuda-Martos, M., Fernández-LÓpez, J., & Pérez-Alvarez, J. A. (2015). Resistant starch as functional ingredient. Polysaccharides: Bioactivity and Biotechnology, 43(4), 1911–1931. doi:10.1007/978-3-319-16298-0_34
- Schokker, E. P., Singh, H., Pinder, D. N., & Creamer, L. K. (2000). Heat-induced aggregation of β-lactoglobulin AB at pH 2.5 as influenced by ionic strength and protein concentration. International Dairy Journal, 10(4), 233–240. doi:10.1016/S0958-6946(00)00047-9
- Sharaf Eddin, A., & Tahergorabi, R. (2017). Application of a surimi-based coating to improve the quality attributes of shrimp during refrigerated storage. Foods, 6(9), 76. doi:10.3390/foods6080062
- Soriguer, F., Rojo-Martínez, G., Dobarganes, M. C., García Almeida, J. M., Esteva, I., Beltrán, M., … González-Romero, S. (2003). Hypertension is related to the degradation of dietary frying oils. The American Journal of Clinical Nutrition, 78(6), 1092–1097. doi:10.1093/ajcn/78.6.1092
- Tahergorabi, R., Beamer, S. K., Matak, K. E., & Jaczynski, J. (2013). Chemical properties of ω-3 fortified gels made of protein isolate recovered with isoelectric solubilisation/precipitation from whole fish. Food Chemistry, 139(1–4), 777–785. doi:10.1016/j.foodchem.2013.01.077
- Tahergorabi, R., & Jaczynski, J. (2016). Seafood proteins and human health. In S. Raatz & D. Bibus (Eds.), Fish and fish oil in health and disease prevention (pp. 323–330). Cambridge, MA, USA: Academic Press.
- Tongnuanchan, P., Benjakul, S., Prodpran, T., & Songtipya, P. (2011). Characteristics of film based on protein isolate from red tilapia muscle with negligible yellow discoloration. International Journal of Biological Macromolecules, 48(5), 758–767. doi:10.1016/j.ijbiomac.2011.02.017
- Ufheil, G., & Escher, F. (1996). Dynamics of oil uptake during deep-fat frying of potato slices. Lebensmittel-Wissenschaft Und Technologie, 29, 640–644. doi:10.1006/fstl.1996.0097
- Williams, R., & Mittal, G. S. (1999). Water and fat transfer properties of polysaccharide films on fried pastry mix. LWT-Food Science and Technology, 32(7), 440–445. doi:10.1006/fstl.1999.0573
