ABSTRACT
Fresh products demand natural preservatives without solvent traces. Calcium-based extraction of bioactive compounds from eggplant peels (EP), could be used as an alternative solvent-free natural antimicrobial food preservative agent. In this study, we extracted bioactive compounds from EP using different calcium salts (CaSO4, CaCO3, and CaCl2) and evaluated the total phenolic and flavonoid contents, antioxidant activity, and bactericidal activity against Salmonella Typhimurium. The EP extracts obtained using 1% CaCO3 exhibited the highest total phenolic content and pronounced antioxidant activity. All EP extracts decreased Salmonella concentration after 10 and 12 h. In infected-lettuce leaves, the 1% CaCO3 EP extract inhibited bacterial growth at similar levels as a commercial disinfectant. The main compound identified in this EP extract was the chlorogenic acid. The extracts from EP obtained using calcium salts, represent a natural novel alternative preservative agent for the food industry, with antioxidant properties and potentially positive effects on human health.
RESUMEN
Los productos frescos exigen conservadores naturales sin residuos de solventes. La extracción a base de calcio de compuestos bioactivos de las cáscaras de berenjena (CB), podría utilizarse como un agente conservador de alimentos y antimicrobiano natural libre de solventes. En este estudio extrajimos compuestos bioactivos de las CB utilizando diferentes sales de calcio (CaSO4, CaCO3 y CaCl2) y evaluamos el contenido de fenólicos totales y flavonoides, actividad antioxidante y capacidad bactericida contra Salmonella Typhimurium en hojas de lechuga. Los extractos de CB obtenidos con CaCO3 al 1% exhibieron el mayor contenido de fenólicos totales y una mejor actividad antioxidante. Todos los extractos de CB disminuyeron la concentración de Salmonella después de 10 y 12 h. En hojas de lechuga infectadas, el extracto de CaCO3 al 1% inhibió el crecimiento bacteriano a niveles similares a un desinfectante comercial. El compuesto principal identificado en este extracto de CB fue el ácido clorogénico. Los extractos de CB obtenidos con sales de calcio, representan una alternativa novedosa para la industria alimentaria como un conservador natural inocuo con propiedades antioxidantes que podrían beneficiar la salud humana.
1. Introduction
Fruits and vegetables are components of a healthy diet, and therefore, they are in high demand worldwide. These products are frequently exposed to biological contamination, becoming a source of transmission of pathogens such as Escherichia coli O157:H7, Salmonella enterica, Listeria monocytogenes and Shigella that cause foodborne diseases (Ruiz, Colello, Padola, & Etcheverría, Citation2017).
To control the proliferation of microorganisms in food and prevent infection, interest in the use of natural antimicrobial agents has increased, in an effort to minimize the consumption of synthetic or solvent-extracted preservatives. Agroindustrial waste is considered a potential low-cost preservative option; the food industry generates high amounts of waste, representing a source of pollution and a threat to nearby ecosystems (FAO, Citation2013). However, some agroindustrial residues have preservative characteristics, mainly attributed to their phenolic compounds, which are one of the most abundant secondary metabolites found in plants (Lattanzio, Citation2013). The nutraceutical industry has focused on the optimization of the extraction processes of these compounds from different sources, including guava leaves (Liu, Wang, Lu, & Chiang, Citation2014), grape seeds (Dang, Zhang, & Xiu, Citation2014), and eggplant (Gil-Chavez, Contreras-Angulo, Valdez-Torres, Gonzalez-Aguilar, & Heredia, Citation2015) among others. Extracts rich in phenolic compounds have been of great interest because they are natural antioxidants with antimicrobial effects, essential properties to consider in the replacement of synthetic additives. Additionally, these compounds have therapeutic and dietary applications (Frevel, Pipingas, Grigsby, Frampton, & Gilchrist, Citation2012).
Eggplant production is an important crop; its production ranked in the top six worldwide (FAO, Citation2019). The state of Sinaloa produces the highest quantity of eggplants in Mexico (SIAP-SAGARPA, Citation2019), with large amounts of peel wasted during marmalade production. Interestingly, semi-purified extracts of this peel have demonstrated a strong antioxidant activity (Braga et al., Citation2016; Salerno et al., Citation2014). However, the extraction process of bioactive compounds uses organic solvents, and after their application, traces could remain in the food, jeopardizing human health. Therefore, the extraction of these compounds without the use of organic solvents, such as through food-grade solutions of calcium salts, is of great interest in the preparation of harmless extracts that could be used as natural fresh produce preservatives.
In this study, therefore, we extracted and identified the bioactive compounds in eggplant peels (EP) extracts by a solvent-free process using different calcium salts, and evaluated their antioxidant and bactericidal activity against Salmonella enterica serovar Typhimurium.
2. Materials and methods
2.1. Sample preparation
EP (Solanum melongena, Italian cultivar) were collected from production processes where the pulp was used. The skins were disinfected with 2% sodium hypochlorite for 5 min. Subsequently, they were rinsed with sterile water to remove chlorine residues and stored at −20°C until use.
2.2. Extraction of bioactive compounds
To obtain the extracts, 10 g of EP was homogenized for 30 s in a blender (medium speed) using 20 mL of different food-grade solutions of calcium salts such as calcium sulfate (CaSO4), calcium carbonate (CaCO3), and calcium chloride (CaCl2), in concentrations between 1-3%. Then, the mixtures were heat-treated at 95°C for 30 min, with shaking every 5 min. Samples were subsequently homogenized for 3 h at 30°C, before incubation 16 h at 4°C. The mixtures were centrifuged (4000 rpm/15 min) and supernatants were collected, filtered through a 0.22 µm sterile membrane (Millipore Sigma™ Millex™ Sterile Syringe Filters), and stored at 4°C for the quantification of total phenolic compounds, flavonoids, and antioxidant activity.
2.3. Quantification of total phenolic compounds and flavonoids
The content of total phenolic compounds (TPC) in the EP extracts, henceforth referred to as EP extracts, was determined using the method described by Singleton, Orthofer, and Lamuela-Raventós (Citation1999). Two hundred microliters of the EP extract were oxidized with 1800 μL of the Folin-Ciocalteu reagent for 5 min. Then, 500 μL of 7% sodium carbonate was added, and the mixture was incubated at room temperature, in the dark, for 90 min. The absorbance at 750 nm was measured using a cell spectrophotometer (SP-3000nano, Optima). A calibration curve was performed using gallic acid as a standard (25–300 ppm), with the resultant equation as follows: y = 0.0047× +0.06 (r2 = 0.992). The TPC was expressed as mg of gallic acid equivalents (mg GAE)/100 g of sample (wb; wet basis).
The total flavonoid (TF) content was quantified by mixing 200 μL of the EP extract with 800 μL of distilled water and 60 μL of 5% NaNO2, and incubating for 5 min at room temperature. Then, 120 μL of 10% AlCl3 was added, followed by addition of 400 μL of 1M NaOH. Finally, after 30 min of incubation protected from light, the absorbance was measured at 510 nm. A calibration curve was generated using catechin as standard (25–300 ppm), and the resultant equation was as follows: y = 0.001× + 0.0861 (r2 = 0.989) was used. The TF content is expressed as mg of catechin equivalent (mg CE)/100 g of sample (wb) (B. J. Xu & Chang, Citation2007).
2.4. Determination of antioxidant activity
2.4.1. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) method
A solution of DPPH (150 μM) dissolved in methanol was prepared and maintained in the dark at −20°C. Then, 145 μL of the EP extract was added to 1320 μL of the DPPH solution and incubated for 40 min at room temperature, protected from light (Brand-Williams, Cuvelier, & Berset, Citation1995). After incubation, the absorbance was measured at 517 nm. A calibration curve using trolox as a standard (25–100 μM), and the resultant equation was as follows: y = −0.0005× + 0.972 (r2 = 0.987) was used. The results were expressed as μmol of trolox equivalent (μmol TE)/100 g of sample (wb). The percentage of inhibition of DPPH oxidation was calculated with the following formula: DPPH_inhibition(%) = [(Acontrol-Asample)/Acontrol]×100 (Matuszewska, Jaszek, Stefaniuk, Ciszewski, & Matuszewski, Citation2018).
2.4.2. The 2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) method
Inhibition of the ABTS radical was determined according to the method described by Re et al. (Citation1999). The ABTS radical was obtained after the reaction of ABTS (7 mM) with potassium persulfate (2.45 mM), mixed in equal parts and incubated at room temperature in the dark for 16 h. Once the ABTS radical was formed, it was diluted in a phosphate-buffered solution (PBS) until the absorbance value at 735 nm was approximately 0.70 ± 0.02. Then, 1980 μL of the ABTS radical dilution was mixed with 20 μL of the EP extract and the mixture incubated for 15 min in the dark, before the absorbance was subsequently measured at 735 nm. A calibration curve was obtained using trolox as standard (25–100 μM), with the equation y = −0.0004× +0.690 (r2 = 0.982). The results of the ABTS assays are expressed as μmol of trolox equivalents (μmol TE)/100 g of sample (wb). The percentage of inhibition of ABTS oxidation is calculated with the following formula: ABTS∙+_inhibition(%) = [(Acontrol-Asample)/Acontrol] ×100 (Matuszewska et al., Citation2018).
2.5. Evaluation of anti-Salmonella activity
The disk diffusion method was used to evaluate the inhibitory activity of EP extracts against Salmonella enterica Typhimurium (ATCC 14028). bacteria were grown in trypticase soy broth (TSB) (MCD Lab, Tlalnepantla, Mexico) at 37°C for 24 h. The inoculum was adjusted to a concentration of 1 × 108 colony forming units (CFU)/mL using the 0.5 McFarland scale. Salmonella was diffused through the Mueller-Hinton agar by smear. Sterile disks of 6.5 mm were placed, and 20 μL of the EP extracts were added to the disks and incubated at 35°C for 24 h. The antimicrobial activity was confirmed by the formation of transparent halos (zone of growth inhibition) around the disks (Wanger, Citation2007). Controls included disks treated with the EP extracts without bacteria and treated with a commercial disinfectant (Microdyn®). The results were reported as mm of inhibition.
2.6. Assay for kinetics of inhibition of S. Typhimurium in liquid medium
Salmonella was inoculated in TSB and incubated at 37°C for 24 h. The culture was adjusted to a concentration of 1 × 108 CFU/mL using the 0.5 McFarland scale. Culture tubes containing 1880 μL of brain heart infusion broth (BHI) (MCD Lab, Tlalnepantla, Mexico) were inoculated with 20 μL of the bacterial suspension and 100 μL of EP extract. Controls included tubes containing 1980 μL of TSB and 20 μL of bacterial suspension; tubes with 1900 μL of TSB and 100 μL of each EP extract, and tubes with a commercial disinfectant (Microdyn®) (1880 μL of TSB, 20 μL of the bacterial suspension and 100 μL of the commercial disinfectant). All treatments were incubated at 35°C for 12 h. Absorbance at 600 nm was measured every 2 h.
2.7. Evaluation of S. Typhimurium inhibition in lettuce leaves
For the inoculum, the bacteria were inoculated and incubated as previously described in the anti-Salmonella activity assay. Romaine lettuce leaves (Lactuca sativa L. var. longifolia) were washed and disinfected with a solution of 2% sodium hypochlorite for 5 min and washed with sterilized water. After disinfection, lettuce leaves were cut in 3.0 × 3.0 cm squares using blades disinfected with 95% ethanol. Three squares of lettuce leaves were immersed for 1 min in a solution containing 3 × 108 CFU/mL of S. Typhimurium. The infected lettuce leaf squares were treated with 1 mL of EP extract and incubated at 4, 10, and 25°C for 24 h. As controls, Salmonella-infected lettuce leaf squares treated with PBS or with a commercial disinfectant (Microdyn®) were included. After incubation, infected leaf squares were homogenized in sterile peptone water (3M Health Care, St. Paul, MN, USA) and serial dilutions were performed to determine the bacterial count and calculate the percentage of its reduction.
2.8. Identification of phenolic compounds using UPLC-MS spectrometry analysis
Phenolic compounds were identified according to the procedure described by Quintero-Soto et al. (Citation2018). Briefly, the 1% CaCO3 EP extract was resuspended in high-performance liquid chromatography (HPLC) grade methanol and injected into an ultra-performance liquid chromatography with diode array detection (UPLC-DAD) system (ACCELA, Thermo Scientific, USA); the separation was made using a C18 column (3 µm, 50 × 2.1 mm) (Fortis Technologies Ltd., Cheshire, UK). The gradients used were 1% (v/v) formic acid (A) and acetonitrile (B), and a linear gradient from 0.5 to 60% of B in 40 min at 0.2 mL/min. The UPLC was connected to a mass spectrometer (MS) with an electrospray ionization source (ESI) (LTQ XL, Thermo Scientific, USA).
2.9. Statistical design
A one-way factorial design was used. The comparison of means ± SD among groups was performed using Tukey´s test for multiple comparisons. Each experiment was replicated three times. Differences with p ≤ 0.05 were considered significant at 95% confidence interval. Statistical analysis was performed using Minitab 16.0 (State College, PA).
3. Results and discussion
3.1. Total phenolic content
The total phenolic contents assessed in the different extracts are shown in . The EP extracts showed a clear variation in their phenolic contents, depending on the concentration of salt used in the extraction solution. In general, the total phenolic content of all EP extracts ranged between 48 and 124 mg GAE/100 g sample (wb). The solutions of 2% CaSO4, and 1% and 2% CaCO3 were the most efficient in the extraction of phenolic compounds (p ≤ 0.05), while the EP extract obtained with the 3% CaCl2 solution exhibited the lowest phenolic content. These results are in accordance with those of other studies that determined the total phenolic content in different extracts obtained with ethanol from green, white, and purple eggplants, with values of between 22–234, 44–90, 50–56, and 22–73 mg GAE/100 g sample (wb), respectively (Kaur, Nagal, Nishad, Kumar, & Sarika, Citation2014). High phenolic concentrations have also been observed in extracts of EP obtained with 50% ethanol and microwave irradiation (Salerno et al., Citation2014), and in methanol-extracts of DR2B and DR2C EP (Di Sotto et al., Citation2018). However, in this study, saline solutions were used to incorporate the EP extracts directly into the food without the need for evaporation.
Figure 1. Total phenolic (a) and flavonoid (b) content of EP extracts obtained using different calcium salts. Data represent mean values ± SD. Different letters represent significant differences among treatments (p ≤ 0.05).
Figura 1. Contenido total de fenólicos (a) y flavonoides (b) en extractos de CB obtenidos con diferentes sales de calcio. Los datos representan valores promedio ± DE. Las diferentes letras representan diferencias significativas entre los tratamientos (p ≤ 0.05).
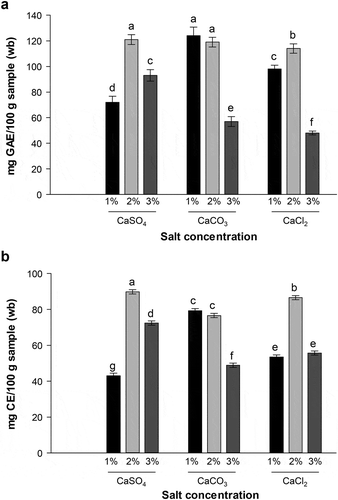
The total flavonoids content varied between 43–89 mg CE/100 g sample (wb) (). The highest concentration (p ≤ 0.05) was extracted using the 2% CaSO4 solution, while the lowest was obtained with 1% CaSO4. However, significantly higher values were also observed in EP extracts obtained with 2% CaCl2, and 1% and 2% CaCO3, compared to 1% CaSO4-extracts. Ninfali, Mea, Giorgini, Rocchi, and Bacchiocca (Citation2005) determined the content of total flavonoids with the AlCl3 test, of an eggplant extract (cultivar “Violeta Lunga”), using acetone as the extraction solvent and obtained a value of 25 mg CE/100 g sample (wb). The variation in the flavonoid content may be due to the solvent used for extraction, as well as the eggplant genotype studied. In this study, total flavonoids and total phenolics were obtained from EP subjected to hydrolysis; thus, the release of more compounds as a result of the break of the cellular structure of the EP could have been facilitated. According to Acosta-Estrada, Gutiérrez-Uribe, and Serna-Saldívar (Citation2014) and Santos-Zea, Villela-Castrejón, and Gutiérrez-Uribe (Citation2018), phenolic compounds in fruits and vegetables are found in a free form, and soluble-conjugated to proteins or polysaccharides, and less than 24% are in their bound form, where treatments such as hydrolysis are ideal for their release.
3.2. Antioxidant activity
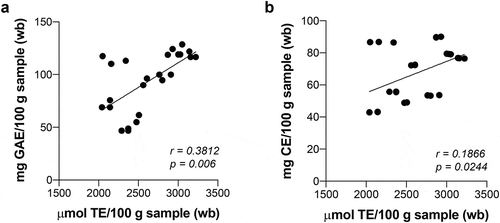
Similar patterns between the DPPH and ABTS methods were observed in the EP extracts () with scavenging activities ranging between 2092–3328 μmol TE/100 g (wb) and 4200–5689 μmol TE/100 g (wb), respectively. The EP extract obtained using the 1% CaCO3 solution showed the highest DPPH-scavenging activity (89%; p ≤ 0.05) followed by the 2% CaCO3-extract (68%), while the EP extract obtained using the solution of 1% CaSO4 presented the lowest activity (25%; p ≤ 0.05, ). The ABTS scavenging assay confirmed these results with the highest antioxidant activity exhibited by the EP extracts obtained from the 1% CaCO3 solution (93%; p ≤ 0.05) (). This high antioxidant activity observed in the 1% CaCO3-extract could be related to the high phenolic content or type of compounds extracted (), but not entirely to flavonoids, because the 2% CaSO4-extract, with the highest concentration of flavonoids, did not produce the highest antioxidant activity. Some researchers have reported the antioxidant activity by the ABTS assay in eggplant using aqueous extracts, and found the activity ranged from 267 to 825 μmol TE/100 g (wb) (Okmen et al., Citation2009).
Figure 2. Antioxidant activity of EP extracts determined through the DPPH (a) and ABTS (b) assays. Right: percentage of inhibition of DPPH or ABTS oxidation. Data represent mean values ± SD. Different letters represent significant differences among treatments (p ≤ 0.05).
Figura 2. Actividad antioxidante en extractos de CB determinada por los métodos de DPPH (a) y ABTS (b). Derecha: porcentage de inhibición de la oxidación de DPPH o ABTS. Los datos representan valores promedio ± DE. Las diferentes letras representan diferencias significativas entre los tratamientos (p ≤ 0.05).
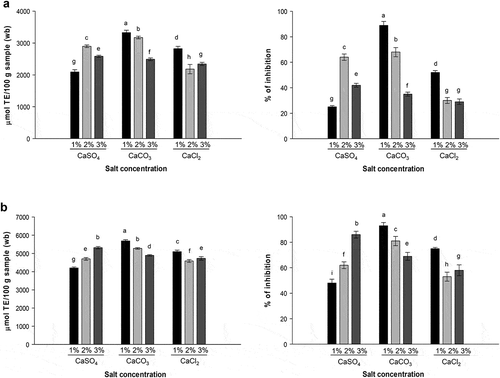
Figure 3. Correlation between antioxidant activity and total phenolic content (a) and flavonoids (b) of the EP extracts obtained using different calcium salts.
Figura 3. Correlación entre la actividad antioxidante y el contenido fenólico total (a) y los flavonoides (b) de los extractos de CB obtenidos usando diferentes sales de calcio.
3.3. Anti-Salmonella activity
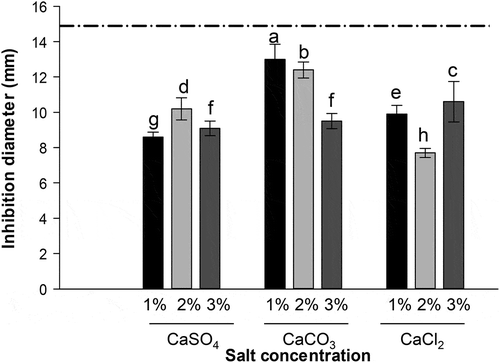
All EP extracts showed a zone of growth inhibition greater than 7.7 mm (). S. Typhimurium exhibited greater resistance to the EP extracts obtained with the 2% CaCl2 solution, while the bacteria presented high sensitivity to the EP extracts obtained with the 1% CaCO3 solution, followed by the 2% CaCO3 solution. The control treated with the commercial disinfectant showed the highest inhibitory action against Salmonella.
Figure 4. S. Typhimurium antimicrobial activity of extracts obtained from EP using different calcium salts, determined by the disc diffusion method. The line indicates the control treated with a commercial disinfectant. Data represent mean values ± SD. Different letters represent significant differences among treatments (p ≤ 0.05).
Figura 4. Actividad antimicrobiana de extractos obtenidos de CB utilizando diferentes sales de calcio contra S. Typhimurium determinadas por el método de difusión en disco. La línea indica el control tratado con el desinfectante commercial. Los datos representan valores promedio ± DE. Las diferentes letras representan diferencias significativas entre los tratamientos (p ≤ 0.05).
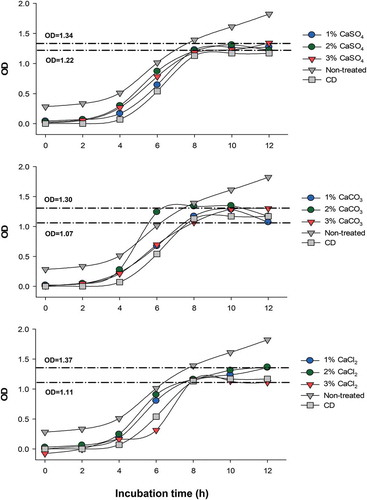
S. Typhimurium growth kinetics were decreased by all EP extracts after 10 h and 12 h as compared to the non-treated control (). After 12 h of incubation, the 1% CaCO3 and 3% CaCl2-extracts decreased bacterial growth (~40% reduction), while the 1% and 2% CaCl2, and 3% CaSO4-extracts showed lower inhibition. The EP extracts obtained with the 3% CaCO3 and 1% CaSO4 solutions displayed similar effectiveness (~30% reduction) against Salmonella growth rate. No significant differences were observed between the 2% CaSO4 and 3% CaCl2 EP extracts as compared to the commercial disinfectant (p > 0.05); while the 1% CaCO3 EP extract exhibited significant higher bacterial inhibition (p ≤ 0.05).
Figure 5. Bioactivity of extracts obtained from eggplant peel using different calcium salts on the growth kinetics of S. Typhimurium. Lines indicate the minimal and maximal optical densities (OD). CD, commercial disinfectant.
Figura 5. Bioactividad de los extractos obtenidos de cáscara de berenjena utilizando diferentes sales de calcio en la cinética de crecimiento de S. Typhimurium. Las líneas indican las densidades ópticas mínimas y máximas (OD). CD, desinfectante commercial.
The mechanism of action of bioactive compounds such as phenolics on microorganisms is not clear. Although studies have found these compounds could interact with enzymes of the microorganism by inactivating them, they can also react with components of cell membranes or alter the function of their genetic material. The type of microorganism and the structure of the cell wall plays an important role in the resistance and sensitivity to an antimicrobial agent (Borges, Ferreira, Saavedra, & Simões, Citation2013). Also, some phenolic acid compounds have antimicrobial activities related to changes in bacterial membrane properties such as charge, permeability, and physicochemical alterations, resulting in pore formation and the release of essential intracellular components (Borges et al., Citation2013).
3.4. S. Typhimurium inhibition in lettuce leaves
Leafy vegetables, such as lettuce, are responsible for non-typhoidal Salmonella outbreaks presented in North America, Australia, Finland, and England (Brandl & Amundson, Citation2008), and is linked to the contamination of fresh and minimally processed vegetables (Hanning, Nutt, & Ricke, Citation2009). For this reason, we tested the effectiveness of the 1% CaCO3 EP extract in controlling S. Typhimurium on contaminated lettuce leaves. Salmonella-infected lettuce leaves stored at 4, 10, and 25°C showed a significant decrease in bacterial growth when they were treated with the 1% CaCO3 EP extract or the commercial disinfectant (p ≤ 0.001, ). However, at 4°C, the commercial disinfectant induced a more significant inhibition than the 1% CaCO3 EP extract (p ≤ 0.001) with a reduction of 68% (0.5 Log reduction) compared to 50% (0.3 Log reduction) of the 1% CaCO3 EP extract. On the contrary, at 10°C, the 1% CaCO3 EP extract presented a significantly higher activity than the commercial disinfectant (p ≤ 0.002), with inhibition of bacterial growth up to 79% (0.7 Log reduction). No significant differences were observed between the commercial disinfectant and the 1% CaCO3 EP extract at 25°C (p > 0.05). Bio-sanitization has been reported for different essential oils against E. coli O157:H7 (Mouatcho, Tzortzakis, Soundy, & Sivakumar, Citation2017); these authors found that a mixture of essential oils from thyme and tea tree had the greatest inhibitory effect against the bacteria after five days of incubation at 10°C. In our study, the 1% CaCO3 EP extracts were able to inhibit the growth of S. Typhimurium after 24 h of incubation at all temperatures studied. For all this, obtaining eggplant extracts with saline solutions represents an alternative to be used as a natural antimicrobial agent against S. Typhimurium in fresh lettuce leaves.
Table 1. Effect of phenolic compounds obtained from eggplant peels extracted with a solution of 1% CaCO3 on the growth of S. Typhimurium in lettuce leaves.
Tabla 1. Efecto de compuestos fenólicos obtenidos de cáscaras de berenjena extraídas con una solución de CaCO3 al 1% sobre el crecimiento de S. Typhimurium en hojas de lechuga.
3.5. Identification of phenolic compounds
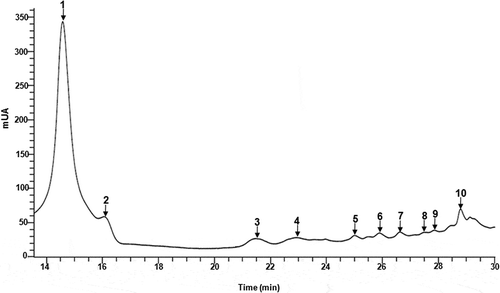
The phenolic compounds identified in the 1% CaCO3 EP extract are shown in . The chlorogenic acid was the main compound identified, followed by 4-caffeoylquinic acid and cyanidin-3-O-acetylglucoside (). Other compounds identified were the caffeoyl rutinoside, delphinidin 3-O-rutinoside-5-O-glucoside, and petunidin-3-O-glucoside. Some authors have identified different compound profiles in EP using various extraction solvents including an acidified aqueous solution [chlorogenic acid, delphinidin-3-rutinoside, and nasunin (Braga et al., Citation2016)], and an acidified aqueous methanol solution [delphinidin-3-rutinoside, and delphinidin-3-glucoside (Li et al., Citation2012) and some isomers of caffeoylquinic dehydrodimer, and caffeoylshikimic acid (García-Salas, Gómez-Caravaca, Morales-Soto, Segura-Carretero, & Fernández-Gutiérrez, Citation2014)].
Table 2. Identification of phenolic compounds in EP extracts obtained using a solution of 1% CaCO3.
Tabla 2. Identificación de compuestos fenólicos de extractos de CB obtenidos con una solución de CaCO3 al 1%.
Figure 6. UPLC chromatograms of phenolic compounds in EP extract obtained using 1% CaCO3.
Figura 6. Cromatogramas de UPLC de compuestos fenólicos de extractos de CB obtenidos con CaCO3 al 1%.
The presence of chlorogenic acid in the 1% CaCO3 EP extract could be related to the inhibitory effect observed against Salmonella. This acid has been demonstrated to be able to disrupt the bacterial cell membrane (Li, Wang, Xu, Zhang, & Xia, Citation2014), and anthocyanins present in the peel of fruits such as pomegranates (Wafa et al., Citation2017) and blueberries (Lacombe, Wu, White, Tadepalli, & Andre, Citation2012; Llivisaca et al., Citation2018) have shown antimicrobial capacity against Salmonella (Ma et al., Citation2019).
4. Conclusions
The use of synthetic and solvent-extracted antimicrobial agents as preservatives for fresh products is limited due to their toxicity. This work presented an alternative solvent-free process, using calcium salts, that was able to generate an innocuous extract, rich in phenolics (mainly chlorogenic acids) with high antioxidant and anti-Salmonella activities, from an under-used agroindustrial waste product, namely eggplant peel. EP extracts could be used as natural antimicrobial agents capable of being incorporated into fresh produce such as lettuce leaves. The application of this extraction process to obtain natural disinfectants from plants appears to be a viable novel alternative to the production of current preservatives in the food industry.
Disclosure statement
The authors reported no potential conflict of interest.
Additional information
Funding
References
- Acosta-Estrada, B. A., Gutiérrez-Uribe, J. A., & Serna-Saldívar, S. O. (2014). Bound phenolics in foods, a review. Food Chemistry, 152, 46–55. doi:10.1016/J.FOODCHEM.2013.11.093
- Borges, A., Ferreira, C., Saavedra, M. J., & Simões, M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microbial Drug Resistance, 19(4), 256–265. doi:10.1089/mdr.2012.0244
- Braga, P. C., Lo Scalzo, R., Dal Sasso, M., Lattuada, N., Greco, V., & Fibiani, M. (2016). Characterization and antioxidant activity of semi-purified extracts and pure delphinidin-glycosides from eggplant peel (Solanum melongena L.). Journal of Functional Foods, 20, 411–421. doi:10.1016/j.jff.2015.10.032
- Brandl, M. T., & Amundson, R. (2008). Leaf age as a risk factor in contamination of lettuce with Escherichia coli O157: H7and Salmonella enterica. Applied and Environmental Microbiology, 74(8), 2298–2306. doi:10.1128/AEM.02459-07
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28(1), 25–30. doi:10.1016/S0023-6438(95)80008-5
- Dang, -Y.-Y., Zhang, H., & Xiu, Z.-L. (2014). Microwave-assisted aqueous two-phase extraction of phenolics from grape (Vitis vinifera) seed. Journal of Chemical Technology & Biotechnology, 89(10), 1576–1581. doi:10.1002/jctb.4241
- Delgado-Vargas, F., Sicairos-Medina, L. Y., Luna-Mandujan, A. G., López-Angulo, G., Salazar-Salas, N. Y., Vega-García, M. O., … López-Valenzuela, J. Á. (2018). Phenolic profiles, antioxidant and antimutagenic activities of Solanum lycopersicum var. cerasiforme accessions from Mexico. CyTA - Journal of Food, 16(1), 715–722. doi:10.1080/19476337.2018.1481146
- Di Sotto, A., Di Giacomo, S., Amatore, D., Locatelli, M., Vitalone, A., Toniolo, C., … Nencioni, L. (2018). A polyphenol rich extract from Solanum melongena L. DR2 peel exhibits antioxidant properties and anti-herpes simplex virus Type 1 activity In Vitro. Molecules, 23(8), 2066. doi:10.3390/molecules23082066
- Downey, M. O., & Rochfort, S. (2008). Simultaneous separation by reversed-phase high-performance liquid chromatography and mass spectral identification of anthocyanins and flavonols in Shiraz grape skin. Journal of Chromatography A, 1201(1), 43–47. doi:10.1016/j.chroma.2008.06.002
- FAO. (2013). Food wastage footprint: Impacts on natural resources - Summary report. Retrieved from http://www.fao.org/docrep/018/i3347e/i3347e.pdf
- FAO. (2019). FAOSTAT. Retrieved from http://www.fao.org/faostat/en/#data/QC/
- Frevel, M. A. E., Pipingas, A., Grigsby, W. J., Frampton, C. M., & Gilchrist, N. L. (2012). Production, composition and toxicology studies of Enzogenol® Pinus radiata bark extract. Food and Chemical Toxicology, 50(12), 4316–4324. doi:10.1016/j.fct.2012.08.051
- García-Salas, P., Gómez-Caravaca, A. M., Morales-Soto, A., Segura-Carretero, A., & Fernández-Gutiérrez, A. (2014). Identification and quantification of phenolic compounds in diverse cultivars of eggplant grown in different seasons by high-performance liquid chromatography coupled to diode array detector and electrospray-quadrupole-time of flight-mass spectrometry. Food Research International, 57, 114–122. doi:10.1016/j.foodres.2014.01.032
- Gil-Chavez, G. J., Contreras-Angulo, L., Valdez-Torres, J. B., Gonzalez-Aguilar, G. A., & Heredia, J. B. (2015). Optimization of the process for recovering phenolic antioxidant compounds from low-quality eggplant (Solanum melongena L.) pulp by modified supercritical carbon dioxide extraction. Separation Science and Technology, 50(6), 841–850. doi:10.1080/01496395.2014.960049
- Gómez-Romero, M., Segura-Carretero, A., & Fernández-Gutiérrez, A. (2010). Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry, 71(16), 1848–1864. doi:10.1016/j.phytochem.2010.08.002
- Hanning, I. B., Nutt, J. D., & Ricke, S. C. (2009). Salmonellosis outbreaks in the United States due to fresh produce: Sources and potential intervention measures. Foodborne Pathogens and Disease, 6(6), 635–648. doi:10.1089/fpd.2008.0232
- Kaur, C., Nagal, S., Nishad, J., Kumar, R., & Sarika, S. (2014). Evaluating eggplant (Solanum melongena L) genotypes for bioactive properties: A chemometric approach. Food Research International, 60, 205–211. doi:10.1016/j.foodres.2013.09.049
- Lacombe, A., Wu, V. C. H., White, J., Tadepalli, S., & Andre, E. E. (2012). The antimicrobial properties of the lowbush blueberry (Vaccinium angustifolium) fractional components against foodborne pathogens and the conservation of probiotic Lactobacillus rhamnosus. Food Microbiology, 30(1), 124–131. doi:10.1016/j.fm.2011.10.006
- Lattanzio, V. (2013). Phenolic compounds: Introduction. In Natural products (pp. 1543–1580). Berlin, Heidelberg: Springer Berlin Heidelberg. doi:10.1007/978-3-642-22144-6_57
- Li, G., Wang, X., Xu, Y., Zhang, B., & Xia, X. (2014). Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. European Food Research and Technology, 238(4), 589–596. doi:10.1007/s00217-013-2140-5
- Li, H., Deng, Z., Zhu, H., Hu, C., Liu, R., Young, J. C., & Tsao, R. (2012). Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Research International, 46(1), 250–259. doi:10.1016/j.foodres.2011.12.014
- Liu, C.-W., Wang, Y.-C., Lu, H.-C., & Chiang, W.-D. (2014). Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process Biochemistry, 49(10), 1601–1605. doi:10.1016/j.procbio.2014.06.009
- Llivisaca, S., Manzano, P., Ruales, J., Flores, J., Mendoza, J., Peralta, E., & Cevallos-Cevallos, J. M. (2018). Chemical, antimicrobial, and molecular characterization of mortiño (Vaccinium floribundum Kunth) fruits and leaves. Food Science & Nutrition, 6(4), 934–942. doi:10.1002/fsn3.638
- Ma, Y., Ding, S., Fei, Y., Liu, G., Jang, H., & Fang, J. (2019). Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control, 106(June), 106712. doi:10.1016/j.foodcont.2019.106712
- Matuszewska, A., Jaszek, M., Stefaniuk, D., Ciszewski, T., & Matuszewski, Ł. (2018). Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PloS One, 13(6), e0197044. doi:10.1371/journal.pone.0197044
- Mouatcho, J. C., Tzortzakis, N., Soundy, P., & Sivakumar, D. (2017). Bio-sanitation treatment using essential oils against E. coli O157: H7on fresh lettuce. New Zealand Journal of Crop and Horticultural Science, 45(3), 165–174. doi:10.1080/01140671.2016.1269813
- Ninfali, P., Mea, G., Giorgini, S., Rocchi, M., & Bacchiocca, M. (2005). Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. The British Journal of Nutrition, 93(2), 257–266. doi:10.1079/BJN20041327
- Okmen, B., Sigva, H. O., Mutlu, S., Doganlar, S., Yemenicioglu, A., & Frary, A. (2009). Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum Melongena L.) cultivars. International Journal of Food Properties, 12(3), 616–624. doi:10.1080/10942910801992942
- Quintero-Soto, M. F., Saracho-Peña, A. G., Chavez-Ontiveros, J., Garzon-Tiznado, J. A., Pineda-Hidalgo, K. V., Delgado-Vargas, F., & Lopez-Valenzuela, J. A. (2018). Phenolic profiles and their contribution to the antioxidant activity of selected chickpea genotypes from Mexico and ICRISAT collections. Plant Foods for Human Nutrition, 73(2), 122–129. doi:10.1007/s11130-018-0661-6
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26(9–10), 1231–1237. doi:10.1016/S0891-5849(98)00315-3
- Ruiz, M. J., Colello, R., Padola, N. L., & Etcheverría, A. I. (2017). Efecto inhibitorio de Lactobacillus spp. sobre bacterias implicadas en enfermedades transmitidas por alimentos. Revista Argentina De Microbiología, 49(2), 174–177. doi:10.1016/j.ram.2016.10.005
- Salerno, L., Modica, M. N., Pittalà, V., Romeo, G., Siracusa, M. A., Di Giacomo, C., … Acquaviva, R. (2014). Antioxidant activity and phenolic content of microwave-assisted Solanum melongena extracts. The Scientific World Journal, 2014, 1–6. doi:10.1155/2014/719486
- Santos-Zea, L., Villela-Castrejón, J., & Gutiérrez-Uribe, J. A. (2018). Bound phenolics in foods. Bioactive molecules in food (pp. 1–18). doi:10.1007/978-3-319-54528-8_13-1
- SIAP-SAGARPA. (2019). National eggplant production. Retrieved from http://infosiap.siap.gob.mx:8080/agricola_siap_gobmx/AvanceNacionalCultivo.do
- Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. (1999). [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152–178. doi:10.1016/S0076-6879(99)99017-1
- Su, X., Griffin, J., Xu, J., Ouyang, P., Zhao, Z., & Wang, W. (2019). Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon, 5(6), e01964. doi:10.1016/j.heliyon.2019.e01964
- Wafa, B. A., Makni, M., Ammar, S., Khannous, L., Hassana, A. B., Bouaziz, M., … Gdoura, R. (2017). Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. International Journal of Food Microbiology, 241, 123–131. doi:10.1016/j.ijfoodmicro.2016.10.007
- Wanger, A. (2007). Disk diffusion test and gradient methodologies. In R. Schwalbe, L. Steele-Moore, & A. C. Goodwin (Eds.), Antimicrobial susceptibility testing protocols. CRC Press. doi:10.1201/9781420014495.ch3
- Xu, B. J., & Chang, S. K. C. (2007). A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. Journal of Food Science, 72(2), S159–S166. doi:10.1111/j.1750-3841.2006.00260.x
- Xu, W., Luo, G., Yu, F., Jia, Q., Zheng, Y., Bi, X., & Lei, J. (2018). Characterization of anthocyanins in the hybrid progenies derived from Iris dichotoma and I. domestica by HPLC-DAD-ESI/MS analysis. Phytochemistry, 150, 60–74. doi:10.1016/j.phytochem.2018.03.003
