 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The study investigated the influence of spray- and freeze-drying on the bio-oil powder quality. The powders were characterized by process efficiency, structure, colour, oil and bioactive compounds contents, fatty acid composition and oxidative stability. Cold-pressed bio-oils of evening primrose, borage and blueweed were homogenized with wall materials and water followed by spray-drying at 130°C inlet air temperature or freeze-drying at −56°C. It was shown that lower encapsulation efficiency of freeze-drying process correlated with higher surface oil content. The content of bioactive compounds was more affected by the processes than the fatty acid composition regardless of the method of drying. The powders produced by freeze-drying had a higher tocopherol content than those produced by spray-drying. Both spray- and freeze-drying significantly contributed to the changes of sterols concentration, but the changes were greater for bio-oil powders obtained by spray-drying.
RESUMEN
El presente estudio investigó la influencia del secado por atomización y liofilización en la calidad del polvo de bioaceite. Los polvos se clasificaron de acuerdo con la eficiencia del proceso, la estructura, el color, el contenido de aceites y compuestos bioactivos, la composición de ácidos grasos y la estabilidad oxidativa. Los bioaceites de onagra, borraja y hierba azul prensados en frío se homogeneizaron con materiales de pared y agua, a lo que siguió su secado por atomización a 130°C de temperatura del aire de entrada o por liofilización a −56°C. Se constató que la menor eficiencia de encapsulación atribuible al proceso de liofilización se correlaciona con un mayor contenido de aceite superficial. El contenido de compuestos bioactivos se vio más afectado por los procesos que por la composición de ácidos grasos, independientemente del método de secado utilizado. Los polvos producidos por liofilización tienen mayor contenido de tocoferol que los producidos por atomización. Tanto la atomización como la liofilización contribuyeron significativamente a los cambios registrados en la concentración de esteroles; sin embargo, se detectaron mayores cambios para los polvos de bioaceite obtenidos por atomización.
1. Introduction
One possible way to protect compounds from external factors such as light, high concentration of oxygen, heat, moisture, to prevent evaporation of volatile compounds and mask unpleasant tastes and odours, and thus develop of value-added products is encapsulation. In this technique, one or more compounds are coated or immobilized by one or more materials (Anwar & Kunz, Citation2011). Depending on the core and coating material parameters, expected capsule morphology and particle size as well as the release mechanism desired, different methods and process parameters can be used. Usually the processes are adapted from the pharmaceutical and chemical industries (Celli, Ghanem, & Brooks, Citation2015) and the newest proposed methods are based on the application of supercritical fluid techniques e.g. particles from Gas-Saturated Solutions (PGSS)-drying (Melgosa, Benito-Román, Sanz, de Paz, & Beltrán, Citation2019; Soh & Lee, Citation2019). This technique depends on mixing an aqueous solution with supercritical carbon dioxide upon saturation, and subsequently expanding the gas-saturated solution down to atmospheric pressure through a nozzle, a technique that has been successfully used for encapsulation pure omega-3 fatty acids (Melgosa et al., Citation2019) and fish oil (Karim et al., Citation2017). However, mainly for economic reasons, still the most popular methods of transforming oil into powders are spray- and freeze-drying. The principle of this spray-drying method is conversion a liquid or slurry (e.g. emulsion) into a large number of small droplets which then pass into a rapid stream of hot air. Because of the large surface area of droplets, water evaporates almost immediately and droplets transform into particles. As a result, despite the high temperature of the air fed into the dryer (usually 130°C or higher), the temperature of dried particles is not greater than the temperature of the humid inlet air (Westergaard, Citation2004). Additionally, during spray-drying process, the temperature of the solid does not exceed the corresponding wet bulb temperature since evaporation takes place in a saturated surface (Cal & Sollohub, Citation2010; Dobry et al., Citation2009). Because of these properties, spray drying is recommended for processing thermolabile materials such as oils. Encapsulation of oils can also be achieved through freeze-drying. The freeze-drying process is carried out in three stages: freezing (temperature between −90°C and −40°C), primary drying (sublimation of the frozen free water; temperature is typically below −50°C) and secondary drying (the bound frozen water is eliminated by desorption; temperature rises to above 0°C). It is worth noting that the freezing temperatures in the first and second stages and the absence of air prevents product deterioration caused by oxidation or chemical modification (Anwar & Kunz, Citation2011; Kawasaki, Shimanouchi, & Kimura, Citation2019; Velasco, Holgado, Dobarganes, & Márquez-Ruiz, Citation2009).
Cold-pressed plant bio-oils have limited applicability in the food industry. They are not subjected to technological processes applied in the production of refined oils like degumming, neutralization, bleaching, deodorization and winterization. As a result, they are characterized by high contents of bioactive compounds, which make them desirable for the pharmaceutical industry (El Asbahani et al., Citation2015). Bio-oils obtained from some families, such as Boraginaceae and Onagraceae consist of a variety of fatty acids, including γ-linolenic acid (GLA), linoleic acid (LA), oleic acid, palmitic acid and stearidonic acid which are nutritionally important (Coupland, Citation2008; López-Martínez, Campra-Madrid, & Guil-Guerrero, Citation2004).
Encapsulation increases the application potential of bio-oils in food processing. Oil in the form of capsules, and hence with modified physicochemical properties, is characterized by increased applicability, i.e. the possibility of being applied in powdered and hydrophilic products (Celli et al., Citation2015).
Previously, studies have focused on the encapsulation process but there is a lack of knowledge concerning the degradation of bioactive compounds during encapsulation, especially in bio-oils. Therefore, the aim of this paper was to determine the effect of the encapsulation method on the quality and oxidative stability of encapsulated bio-oils and, in particular, to determine the degradation degree of biologically active compounds (tocopherols and sterols) as well as unsaturated fatty acids.
2. Materials and methods
2.1. Materials
Three kinds of cold-pressed bio-oils were investigated in this study. Seeds of evening primrose (Oenothera biennis), borage (Borago officinalis), and blueweed (Echium vulgare) came from “Szarłat” company (Łomża, Poland). Bio-oils were obtained by pressing the raw material on an IBG Monforts & Reiners, Komet CA59G (Germany) laboratory expeller equipped with a 4 mm diameter nozzle at a temperature below 45°C. Obtained oils were purified by centrifugation at 12,333 × g (type 5810R, Eppendorf AG, Hamburg, Germany).
Maltodextrin (DE 14–22) and guar gum were purchased from “EDPOL Food & Innovation” company (Łomża, Poland). Whey protein concentrate (WPC 80) was purchased from ”Ostrowia” company (Ostrów Mazowiecka, Poland).
2.2. Methods
2.2.1. Preparation of encapsulated bio-oils
The first step of oil encapsulation involved the formation of emulsion of the core material (bio-oils) in the wall material solution. The type of wall materials (maltodextrin, whey protein concentrate and guar gum) was chosen based on results of other researchers (e.g. Carneiro, Tonon, Grosso, and Hubinger (Citation2013) and Karaca, Low, and Nickerson (Citation2013)) and our previous work (Ogrodowska, Tańska, & Brandt, Citation2017). The final composition of emulsion was established on the base of preliminary studies, in which different combinations of oil loading (10–15% of emulsion weight) and proportion of wall components (maltodextrin (15–20% of emulsion weight), whey protein concentrate (0–5% of emulsion weight) and guar gum (0–1% of emulsion weight)) were tested for each type of bio-oil. The emulsion properties (stability, viscosity and particle size) were observed during the tests. It was expected that emulsion will be stable after 24 h storage at room temperature, with viscosity below 1.5 mPa·s and particle size (D4,3) in the range of 5–20 μm. These properties allowed the spray-dryer to work properly without generating large product losses.
Finally, each bio-oil (10.2%, w/w) was blended with maltodextrin (15.4, w/w), whey protein concentrate (3.9%, w/w), guar gum (0.5%, w/w) and water (70%, w/w) using Thermomix equipment (Vorwerk, Germany) operated at 9,000 rpm for 120 s at 40°C. The emulsion was then homogenized at 24 MPa (I step) and 4 MPa (II step) using a high-pressure laboratory valve homogenizer (Panda 2K, GEA Niro Soavi, Parma, Italy).
The spray-dried encapsulated oil was prepared using a pilot-plant spray-dryer (A/S Niro Atomizer, Copenhagen Denmark; spraying mechanism – disc). The drying parameters during the process were controlled to keep 130°C inlet temperature and 90°C outlet temperature. The feed flow rate was 77 mL/min. The freeze-dried encapsulated oil was prepared using a lyophilizer (New Brunswick, USA). The emulsion was included into aluminium pans and frozen at −20°C for 24 h. Then, the freeze-drying process temperature was raised from the initial value of −56°C to a final temperature of 18°C. Freeze-drying was performed at a pressure of 0.12 × 10–4 MPa and drying time reaching 72 h. After the freeze-drying process, the lyophilizate was grinded using a glass rod to transform it into a powder form. The drying conditions were typical for the used equipment (recommended by producers) and previously adjusted during the encapsulation of pumpkin oil (Ogrodowska et al., Citation2017).
The encapsulation procedure for each oil and method was done in triplicate and powders from three repetitions were mixed before analysis.
2.2.2. Effectiveness determination of bio-oil encapsulation process
2.2.2.1. Total and surface oil content in powders
The total content of encapsulated oil was determined after extraction with a chloroform:methanol mixture (2:1, v/v) performed according to the method described in our previous work (Ogrodowska et al., Citation2017). The content of non-encapsulated oil in powder (surface oil) was determined by the washing three times with hexane according to the method described by Liu, Low, and Nickerson (Citation2010).
2.2.2.2. Encapsulation process efficiency
The encapsulation efficiency (EE) was calculated as follows (Gallardo et al., Citation2013):
2.2.2.3. Colour and morphology of powders
The colour of powder was measured using digital image analysis (DIA) according to the method described by Tańska, Rotkiewicz, Kozirok, and Konopka (Citation2005). The results were expressed in the CIEL*a*b* colour model (L* – lightness, a* – greenness/redness, b* – blueness/yellowness).
The morphology of the capsules was investigated using scanning electron microscopy by an SEM Quanta 200 (FEI Company, Hillsboro, OR, USA) as described by Ogrodowska et al. (Citation2017).
2.2.3. Fatty acid composition analysis of crude and encapsulated bio-oils
Fatty acid composition was determined with the gas chromatography technique as described by Czaplicki, Tańska, and Konopka (Citation2016). Briefly, prepared fatty acid methyl esters were analysed by gas chromatography with a GC-MS QP2010 PLUS (Shimadzu, Japan) system. Separation was performed on a BPX70 (25 m × 0.22 mm × 0.25 μm) capillary column (SGE Analytical Science, Victoria, Australia) with helium as the carrier gas at a flow rate of 0.9 mL/min. The column temperature was programmed as follows: a subsequent increase from 150°C to 180°C at the rate of 10°C/min, to 185°C at the rate of 1.5°C/min, to 250°C at the rate of 30°C/min, and then 10 min hold. The interface temperature of GC-MS was set at 240°C. The temperature of the ion source was 240°C. The results were expressed as the percentage of each fatty acid.
2.2.4. Tocopherol content analysis of crude and encapsulated bio-oils
The content of tocopherols was determined using an Agilent Technologies 1200 RP-HPLC apparatus with a fluorescence detector (Santa Clara, CA, USA) equipped with a LiChrospher Si60 column (250 mm × 4 mm × 5 μm; Merck, Darmstadt, Germany) as described by Czaplicki, Ogrodowska, Derewiaka, Tańska, and Zadernowski (Citation2011). A 0.7% isopropanol solution in n-hexane at a 1 mL/min flow rate was used as a mobile phase. The fluorescence detector was set at 296 nm for excitation and 330 nm for emission. Peaks were identified on the basis of retention times determined for α-, β-, γ-, and δ-tocopherol standards (Merck, Darmstadt, Germany) separately. The content of β + γ-tocopherols was calculated based on the external calibration curves which were as follows: α(mg/L) = (Area-5.0012446)/44.9157976 (R2 = 0.99990, range of linearity: 2–16 mg/L) for α-tocopherols; γ(mg/L) = (Area-2.0032309)/56.3575597 (R2 = 0.99999, range of linearity: 1–16 mg/L) for β + γ-tocopherols; δ(mg/L) = (Area-1.5664511)/74.1102628 (R2 = 0.99925, range of linearity: 0.2–2.5 mg/L) for δ-tocopherol.
2.2.5. Sterol content analysis of crude and encapsulated bio-oils
The content of sterols was determined using GC-MS QP2010 PLUS system (Shimadzu, Kyoto, Japan) according to the method described by Ogrodowska et al. (Citation2017). Briefly, silylated compounds were separated on ZB-5MSi (30 m × 0.25 mm × 0.25 μm) capillary column (Phenomenex Inc., Torrance, CA, USA) with helium as a carrier gas (0.9 mL/min). The injector temperature was set at 230°C and the column temperature was programmed as follows: 70°C for 2 min, a subsequent increase to 230°C at the rate of 15°C/min, to 310°C at the rate of 3°C/min, and then 10 min hold. The GC-MS interface and ion source temperatures were set at 240°C and 220°C, the electron energy 70 eV. The total ion current (TIC) mode (100–600 m/z range) was used. Compounds were identified on the basis of their mass spectra compared with mass spectral libraries (NIST08 library, Shimadzu, Kyoto, Japan). A quantitative analysis was carried out with GC-FID using the same parameters of analysis and column as those used in the GC-MS analysis. 5α-cholestane was used as an internal standard.
2.2.6. Oxidative stability analysis of crude and encapsulated bio-oils
The oxidative oil stability analysis was performed with the use of 743 Rancimat (Metrohm, Switzerland) instrument (Metrohm, Herisau, Switzerland) according to the method used by Czaplicki et al. (Citation2016). For this purpose, 2.5 g of oil was heated at 110°C and an air flow rate of 20 L/h was given. The oxidative stability index (OSI) was expressed in hours.
2.3. Statistical analysis
The obtained results of all analysis were analysed statistically using Statistica 12.0 PL software (StatSoft, Kraków, Poland). The differences between the spray- and freeze-dried samples were determined using the analysis of variance (ANOVA) with Tukey test at p ≤ 0.05 significance level.
3. Results and discussion
3.1. Fatty acid composition and bioactive compounds content of crude bio-oils
Fatty acid composition of analysed bio-oils is presented in . While both evening primrose oil and borage oil have high level of linoleic acid, evening primrose oil has almost double the share (79.45%) of that found in borage oil (38.58%). Other fatty acids in evening primrose oil included stearic acid (1.28%), oleic acid (4.88%), palmitic acid (6.03%) and γ-linolenic acid (8.37%). These fatty acids were also found in borage oil (11.01%, 4.05%, 17.70%, 20.09%, respectively). Additionally, negligible amounts of eicosanoic acid (3.94%), erucic acid (3.00%) and nervonic acid (1.65%) were determined in borage oil. Compared to evening primrose and borage oils, blueweed oil was characterised by the lowest percentage of linoleic acid (16.04%). Furthermore, two fatty acids belonging to omega-3 family, α-linolenic and stearidonic acids, were present in the blueweed oil in amount of 33.17% and 11.42%, respectively.
Table 1. Characteristics of oils used to prepare powders.
Tabla 1. Características de los aceites utilizados para preparar polvos.
Due to the high linoleic acid content, evening primrose oil was characterised by the highest PUFA percentage (87.82%) compared to borage oil (58.67%) and blueweed oil (70.48%). Also, SFA and MUFA percentages were the lowest in the evening primrose oil. In turn, borage oil was characterized by the highest percentage of MUFA (26.28%) ().
Literature data show some differences in the fatty acid composition in these bio-oils. Eskin (Citation2008) also reported that the main fatty acids in evening primrose and borage oils are linoleic acid and γ-linolenic acid. However, compare to our study, the linolenic acid percentages were lower (71.6% and 37.9%, respectively), while the γ-linolenic acid percentages were higher (12.6% and 24.6%, respectively). Szterk, Roszko, Sosińska, Derewiaka, and Lewicki (Citation2010) also analysed evening primrose and borage oils and reported higher content linoleic acid (86.0%) and lower content of others fatty acids for evening primrose oil but opposed results they were observed for borage oil where there was a higher content of linoleic (46.3%) and γ-linoleic (23.1%) acids. In turn, evening primrose oil analysed by Montserrat-de la Paz, Fernandez-Arche, Angel-Martin, and Garcia-Gimenez (Citation2014) characterized by 73.88% of linoleic acid and 9.24% γ-linolenic acid. Scrimgeour and Clough (Citation2014) reported that borage oil is an economic source of γ-linolenic acid (GLA). This is due to the highest content of this acid compared to other plant oils. They showed that typical GLA contents of borage oils from seeds cultivated in the UK and New Zealand are in the range of 22–23%; Holland and Poland – 20–22%; Canada – 18–22%; and China – 16–20%. Czaplicki, Zadernowski, and Ogrodowska (Citation2009) analysing oil extracted from blueweed (Echium vulgare L.) oil concluded that α-linolenic acid accounted for 31.75%, γ-linolenic acid for 10.70% and stearidonic acid for 12.61% of the total fatty acid content. Slightly higher percentages of α-linolenic acid (36.12%) and stearidonic acid (12.92%), but lower percentage of γ-linolenic acid (12.18%) in seeds of Echium vulgare found Özcan (Citation2008). These noteworthy differences probably are a result of different cultivation conditions in the crop year or region. Such was indicated by research carried out, among others by Balch, McKenney, and Auld (Citation2003) for evening primrose, by Peiretti, Palmegiano, and Salamano (Citation2004) for borage oil and by Berti et al. (Citation2007) for blueweed oil.
Tocopherols are an important minor component in oils, and they act as antioxidants and slow down the process of oxidation. Therefore, oils with high levels of tocopherols are expected to have greater oxidative stability (Senanayake & Shahidi, Citation2002). The highest tocopherol content (125.81 mg/100 g) in this study was determined in the oil cold-pressed from borage seeds. In this oil, δ-tocopherol (112.65 mg/100 g) accounted for 89% of total tocopherols (). In evening primrose and blueweed oils, the highest share was sum of β- and γ-homologues, and their content amounted to 42.11 mg/100 g and 43.25 mg/100 g in particular oils, respectively. The highest α-tocopherol content (23.54 mg/100 g) was determined in the evening primrose oil, while in the borage oil this homologue was not detected. The total tocopherol content in studied bio-oils was in the range of 48.66 mg/100 g in blueweed oil to 125.81 mg/100 g for borage oil. The literature data also present β + γ-tocopherol as the dominant homologue in evening primrose and blueweed oils and δ-tocopherol in borage oils (Khan & Shahidi, Citation2002; Nogala-Kalucka, Rudzinska, Zadernowski, Siger, & Krzyzostaniak, Citation2010; Szterk et al., Citation2010). In the work of Khan and Shahidi (Citation2002) the tocopherol contents of borage and evening primrose oils were lower compared to our results. In turn, Szterk et al. (Citation2010) found more than double the higher content of tocopherols in evening primrose and borage oils. Limited studies have been published on the tocopherol content in blueweed oil. Özcan (Citation2013) determined similar content of α-tocopherol (5.92 mg/100 g of oil) in oil from Echium italicum seeds obtained in the middle stage of ripening. The author pointed out that oils obtained from seeds in early and late stages of ripening were characterized by a completely different content of this compound, amounted to 9.00 and 1.25 mg/100 g of oil, respectively. Other authors also showed a significant effect of genotype and environment on the tocopherol content (e.g. Marwede, Schierholt, Möllers, & Becker, Citation2004; Shaw, Kakuda, & Rajcan, Citation2016).
Table 2. Surface oil, total oil and encapsulation efficiency [%].
Tabla 2. Aceite superficial, aceite total y eficiencia de encapsulación [%].
The studied bio-oils from evening primrose, borage and blueweed seeds were characterised by a high β-sitosterol content, which amounted to 811.98 mg/100 g, 760.88 mg/100 g, and 737.00 mg/100 g, respectively. Despite of quite similar level of β-sitosterol, the oils were characterised by different content of total sterols. Borage and blueweed oils were characterised by similar level of ∆5-avenasterol and campesterol and significantly higher compared to evening primrose. It is the reason why amount of total sterols of evening primrose oil was the lowest (1444.75 mg/100 g). The content of these bioactive compounds in 100 g of blueweed and borage oils was 3054.88 and 3192.96 mg, respectively. The literature data indicated significant variability of this component in the analysed bio-oils. In studies carried out by Szterk et al. (Citation2010) the β-sitosterol content in commercial crude evening primrose and borage oils was significantly lower and amounted to 645.8 and 80.7 mg/100 g, respectively. Also, Nogala-Kalucka et al. (Citation2010) reported lower amount of β-sitosterol in the same oils (557 and 158 mg/100 g, respectively). The most similar result as in our work for evening primrose oil (795.2 mg/100 g) was obtained by Montserrat-de la Paz et al. (Citation2014). The content of this sterol in blueweed oil may reach the level of 137 mg/100 g of oil (Nogala-Kalucka et al., Citation2010). Despite the fact that in the cited papers, authors also analysed oils from seeds cultivated in Poland, the amounts of individual sterols and their sum were significantly different from our results. It can be explained by the use of different methods for the determination of these compounds and different seeds for oil extraction. The influence of cultivation conditions of oil plants on the composition of lipophylic compounds was emphasized in many studies. For example, Roche, Mouloungui, Cerny, and Merah (Citation2019) highlighted in their study the effect of the sowing date on the profile and content of phytosterols in safflower seeds. Also, results of Giuffrè and LouAdj (Citation2013) confirmed the existing significant differences for sterols in olive oils depending on the cultivar and crop season.
3.2. Surface and total oil content in powders, and encapsulation process efficiency
As presented in , the efficiency of encapsulation by the spray-drying method was higher (78.76–85.22%) compared to encapsulation by the freeze-drying method (53.75–63.02%). In turn, powders produced by freeze-drying were characterised by a high surface oil content (8.46–13.93%). For comparison, the surface oil content in powders produced by spray-drying of emulsion was determined at a much lower level of 3.98–5.56%. The total fat content of all analysed samples ranged from 22.88% for borage freeze-dried encapsulated oil (BO-FDEO) to 30.11% blueweed freeze-dried encapsulated oil (BW-FDEO).
Similar results for lipid content of the powder, depending on the used method of encapsulation, are presented in studies by other authors. In a study carried out by Anwar and Kunz (Citation2011), the surface oil content of fish oil powders produced by freeze-drying was 11.83%, while in powders produced by spray-drying it was 2.62%. In an experiment conducted by Quispe-Condori, Saldaña, and Temelli (Citation2011) produced flaxseed oil powders using zein as coating material and observed that freeze-dried oils showed low encapsulation efficiency (32.68–59.63%) when compared to spray-dried oils (75.42–93.26%). The higher surface oil content and lower encapsulation efficiency obtained in the freeze-dried oil powders than spray-dried oil powders probably result from the difference in the mechanism of dehydration. The dehydration conditions affect the microstructure and integrity of the capsule wall. Silva, Zabot, Bargas, and Meireles (Citation2016) explained that during freezing the formation of ice crystals occurs, which contributes to the rupture of the emulsion droplets, thus releasing oil from the particle to the surface.
3.3. Colour and morphology of bio-oil powders
The results of colour measurement of powders are shown in . All analysed powders were very light (L* close to 100) and had negative a* and positive b* components. The colour of the powders obtained by different methods varied at a statistically significant level (p ≤ 0.05). Samples produced by spray-drying were characterised by higher lightness (L* component ranging from 98.80% to 99.11%), and a lower share of greenness (a* ranging from (−4.46) to (−4.98)) compared to powders obtained by freeze-drying (L* = 95.92–97.05%, a* = (−5.92) – (−6.56)). In contrast, in powders produced by freeze-drying, a colour with a greater share of yellowness was noted (component b* ranging from 11.71 to 13.22), compared to powders produced by spray-drying (b* = 6.37–7.40). The same trends were observed by Caparino et al. (Citation2012) who investigated the influence of different drying methods on the physical properties of mango powder. The differences in the colour of the powders obtained by spray- and freeze-drying may be explained by differences in the size of particles in these powders. As can be seen in , the smallest particles were typical for spray-dried oil powders, whereas the oil powders obtained by freeze-drying were characterized by much larger particles. Similar effect has been shown in our previous study in which effect of encapsulation conditions on pumpkin oil powder quality was tested (Ogrodowska et al., Citation2017). Furthermore, the darker colour of the freeze-dried oil powders may also be the result of the higher surface oil content. Benković and Bauman (Citation2011) reported that the colour of cocoa powder is influenced by the optical effect in which the fat on the solid particles affects the light absorption.
Table 3. Colour of encapsulated oils.
Tabla 3. Color de los aceites encapsulados.
Figure 1. SEM images of encapsulated oils. Evening primrose spray-dried encapsulated oil (1a), evening primrose freeze-dried encapsulated oil (1b), Borage spray-dried encapsulated oil (2a), Borage freeze-dried encapsulated oil (2b), blueweed spray-dried encapsulated oil (3a), blueweed freeze-dried encapsulated oil (3b).
Figura 1. Imágenes SEM de aceites encapsulados. Aceite de onagra encapsulado y secado por atomización (1a), Aceite de onagra encapsulado y liofilizado (1b), Aceite de borraja encapsulado y secado por atomización (2a), Aceite de borraja encapsulado y liofilizado (2b), Aceite de hierba azul encapsulado y secado por atomización (3a), Aceite de hierba azul encapsulado y liofilizado (3b).
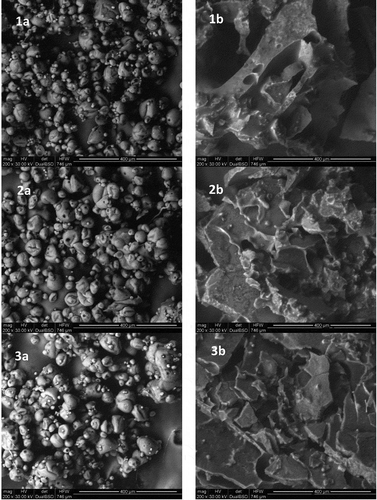
The morphology of encapsulated oils was observed by SEM (). The capsules obtained by spray-drying were characterised by a spherical form with numerous fractures and pores ()–()). This shape is typical of powders whose matrix is composed of, inter alia, maltodextrin, guar gum, and milk protein concentrate, produced by spray-drying. Ferreira, Janaína, Aparecida, Machado, and Hermes (Citation2016), Carneiro et al. (Citation2013), and Anwar and Kunz (Citation2011) who encapsulated palm oil, flaxseed oil and fish oil, respectively, observed similar capsule morphology. It is known that freeze-dried powder generally has an irregular shape and very light, highly porous structure compared to powders obtained using the spray-drying technique (Anwar & Kunz, Citation2011). It is seen that freeze-dried powder has a larger surface area, similar to flakes ()–()). During the freezing stage, when free water in the bulk freezes, the oil droplets push each other. This brings oil droplets in close contact, causing them to flocculate into packed clusters and look like flakes after ice crystals have sublimated (Najafi & Kadkhodaee, Citation2011). These types of structure are typical of powders produced by freeze-drying.
An analysis of samples produced by both spray-drying and freeze-drying revealed no effects of the type of oil on the structure of obtained powders. However, Wagdare, Marcelis, Boom, and van Rijn (Citation2011) observed that the morphology of the capsules depends on the compatibility between the oil that is encapsulated and the material which is used as a core of the capsule. In their experiment, the use of long-chain oils with poor compatibility with the polymer Eudragit, mainly yielded powders with a single-encapsulated oil droplet covered with an Eudragit-rich shell. Furthermore, capsules with many small oil droplets encapsulated in an Eudragit-matrix can be prepared with oil composed with medium-chain fatty acids (which have better compatibility with Eudragit).
3.4. Composition of fatty acids in encapsulated bio-oils
presents the fatty acid profile of encapsulated oils. Highest percentage of linoleic acid was found in the encapsulated evening primrose oil and it was at a higher level in powder produced by freeze-drying (EP-FDEO). It was also dominant in borage oil powder, while blueweed oil powder was characterised by the highest percentage of α-linolenic acid. All analysed powders, irrespective of the type of encapsulated oil, had a greater share of palmitic and oleic acids compared to crude oils (). In addition, the lower percentages of γ-linolenic acid were observed in freeze-dried powders.
Table 4. Fatty acid composition of encapsulated oils [%].
Tabla 4. Composición de ácidos grasos de aceites encapsulados [%].
We observed that encapsulated evening primrose oil obtained by spray-drying was characterized by the highest changes in the fatty acid percentages (20% increase of SFA, 59% increase of MUFA and 5% decrease of PUFA). Opposite relation was found in the samples of encapsulated borage and blueweed oils, where the increase of SFA (15% and 8%, respectively) and MUFA (9% and 11%, respectively) was noted in the case of freeze-dried samples. The losses of PUFAs, regardless of the drying method, were the highest (up to 8%) in the case of borage oil powders ().
This trend in fatty acid composition was also noted in an experiment by Calvo, Castaño, Hernández, and González-Gómez (Citation2011) who encapsulated walnut oil by the freeze-drying method. However, the observed changes were not statistically significant. A modest effect of the processes of spray- and freeze-drying on the fatty acid profile of encapsulated oils was confirmed by studies into the characteristics and stability of palm oil (Ferreira et al., Citation2016) and olive oil (Calvo, Castaño, Lozano, & González-Gómez, Citation2012) powders.
3.5. Content of bioactive compounds in encapsulated bio-oils
According to the data presented in , the process of encapsulation by spray-drying contributes to the degradation of tocopherols to a much greater extent than the process of freeze-drying. In all analysed encapsulated oils, the total content of β- and γ-homologues was lower in spray-dried products. This difference was particularly clear in the samples of encapsulated evening primrose oil, where the content of these tocopherols was lower by approx. 64% in the spray-dried sample (EP-SDEO) compared to the sample obtained by freeze-drying (EP-FDEO). In products containing borage oil, a high δ-tocopherol content was noted which, in the case of powders produced by freeze-drying (BO-FDEO), was 112.38 mg/100 g, while in the oil from powders produced by spray-drying (BO-SDEO), it was lower by 8.70%.
Table 5. Content of tocopherols and sterols in encapsulated oils [mg/100 g of oil].
Tabla 5. Contenido de tocoferoles y esteroles en aceites encapsulados [mg/100 g de aceite].
When comparing the results of analysis of tocopherols for the encapsulated oil with the results obtained for crude oils (), it was noted that for the encapsulated evening primrose oil produced by spray-drying (EP-SDEO), the loss of the total tocopherol content was approx. 60%, while for the same type of oil produced by freeze-drying (EP-FDEO) it was only 4%. In encapsulated borage (BO-SDEO) and blueweed (BW-SDEO) oils produced by spray-drying, losses of tocopherols also occurred, but they were not so great and amounted to 9.5% and 15%, respectively.
The minor contribution of freeze-drying to the degradation of tocopherols found in our study is confirmed by previous studies involving the use of other plant oils (Calvo et al., Citation2011, Citation2012). However, in our previous work (Ogrodowska et al., Citation2017) the freeze-drying method has contributed to larger changes of these compounds in pumpkin oil compared to the spray-drying method. Also, Ng, Jessie, Tan, Long, and Nyam (Citation2013) showed a relative high degradation of tocopherols (up to 18%) in spray-dried kenaf oil. Calvo et al. (Citation2012) analysed the influence of encapsulation on the quality of olive oil encapsulated in different wall compounds. In a protein-based model, degradation of tocopherols was at the level of 2.6%, while in a carbohydrate-based model it was 11.48%.
The process of oil encapsulation contributed to a significant degradation of sterols, which was greater in the case of spray-drying (). As a result of spray-drying, only from 8.44% (EP-SDEO) to 10.6% (BW-SDEO) of β-sitosterol was extractable. Evening primrose and borage oils, encapsulated by freeze-drying (EP-FDEO and BO-FDEO), were characterized by a much higher content of campesterol. In all powders made by freeze-drying a much higher content of Δ7-avenasterol was also observed. Additionally, as a result of spray-drying, for samples EP-SDEO and BO-SDEO about 40% of loss of total sterols was observed. This is confirmed by other studies. About 40% of the loss of sterols in kenaf oil due to encapsulation with spray-drying process was found by Ng et al. (Citation2013). Many authors suggested factors such as temperature and aeration responsible for sterols degradation. Lin et al. (Citation2017) reported that increased heating rates and times resulted in an increase in the oxidation rate of plant sterols. In their experiment, the highest oxidation rate for sitosterol was found for plant oils (rapeseed and sunflower) under the most severe heating condition (210°C for 16 min). Also, Rudzińska, Przybylski, and Wąsowicz (Citation2009) established that a diverse group of compounds is formed during thermo-oxidative degradation of sterols, and their amount and type are affected by temperature and time. However, sterols may also form bonds with the wall components and the use of standard extraction methods does not allow for the complete separation of these compounds (Toivo, Phillips, Lampi, & Piironen, Citation2001).
3.6. Oxidative stability of encapsulated bio-oils in relation to crude bio-oils
Apart from the differentiation in terms of chemical composition, the tested oils also differed in oxidative stability (). Borage oil was the least susceptible to oxidation, compared to other analysed plant oils (OSI = 4.87 h). The lowest oxidative stability was in blueweed oil (1.12 h). The obtained results are consistent with the data reported by Czaplicki et al. (Citation2016). In their experiment, the induction time for evening primrose oil was 3.97 h, while for borage oil it was 3.91 h. The lowest stability of blueweed oil may be associated with its high share of polyunsaturated fatty acids (α-linolenic, γ-linolenic and stearidonic acid), which are characterised by a high oxidation rate. Choe and Min (Citation2006) reported that the rate of linoleic acid oxidation is 10–40 times higher than that of oleic acid, and the rate of linolenic acid oxidation is 2–4 times faster than that of linoleic acid. This finding is compatible with the work of Yüksel and Yeşilçubuk (Citation2012), who studied of hazelnut oil (source of linoleic acid) and commercial oil mixture containing Echium oil and DHA algal oil (source of stearidonic and linolenic acids). They showed that induction time determined by Rancimat test for these oils was 21.8 h and 1.3 h, respectively.
Figure 2. Oxidative stability index of crude and encapsulated oils.
Figura 2. Índice de estabilidad oxidativa de aceites crudos y encapsulados.
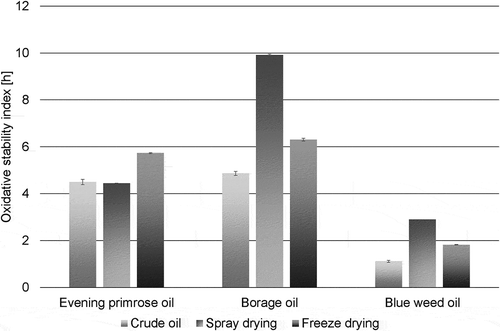
The influence of encapsulation process on the bio-oils oxidative stability was generally positive. The only exception was evening primrose oil powder obtained by spray-drying, for which no changes were found compared to the crude oil. The evening primrose oil powder produced by freeze-drying was characterized by higher oxidative stability (5.74 h) (). A different trend was noted for borage oil and blueweed oil powders obtained by spray-drying which oxidative stability values were more than twice as high as crude oils and amounted to 9.92 h and 2.90 h, respectively. Calvo et al. (Citation2011), while characterising encapsulated walnut oil, also observed an adverse effect of the freeze-drying process on oxidative stability. In their experiment, the oxidative stability index of walnut oil was 8.49 h, while in encapsulated products it ranged from 5.18 h to 6.91 h. The results in the experiment conducted by El Ghannam, El Nemr, Hassan, and Dyab (Citation2015) showed that freeze-dried powder had oxidation values only slightly higher than the spray-dried powder.
4. Conclusions
The effect of two drying methods used in encapsulation process of three bio-oils on process efficiency and main lipophilic compounds has been assessed in this work. It was found that the production of bio-oil powders by spray-drying was more effective and obtained products characterized by a much lower content of surface oil compared to powders produced by freeze-drying. The process of encapsulation, regardless of drying method, contributes to lower changes in the fatty acid composition in the encapsulated bio-oils than in the content of bioactive compounds. However, the drying method had a significant impact on the content of bioactive compounds and oxidative stability of bio-oil powders. The evening primrose oil encapsulated by freeze-drying was much more resistant against oxidation and richer in tocopherols and sterols than oil encapsulated by spray-drying. And despite of lower process efficiency, freeze-drying would be more recommended for this bio-oil. In turn, the borage and blueweed oil powders should be produced by spray-drying. Thanks to this drying method higher process efficiency and oxidative stability, and only slightly lower content of bioactive compounds was achieved than using the freeze-drying.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Anwar, S. H., & Kunz, B. (2011). The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. Journal of Food Engineering, 105, 367–378. doi:10.1016/j.jfoodeng.2011.02.047
- Balch, S. A., McKenney, C. B., & Auld, D. L. (2003). Evaluation of gamma-linolenic acid composition of evening primrose (Oenothera) species native to Texas. HortScience, 38, 595–598. doi:10.21273/HORTSCI.38.4.595
- Benković, M., & Bauman, I. (2011). Agglomeration of cocoa powder mixtures–Influence of process conditions on physical properties of the agglomerates. Journal on Processing and Energy in Agriculture, 15, 46–53.
- Berti, M., Johnson, B. L., Dash, S., Fischer, S., Wilckens, R., & Hevia, F. (2007). Echium: A source of stearidonic acid adapted to the northern great plains in the US. In J. Janick & A. Whipkey (Eds.), Issues in new crops and new uses (pp. 120–125). Alexandria, VA: ASHS Press.
- Cal, K., & Sollohub, K. (2010). Spray drying technique. I: Hardware and process parameters. Journal of Pharmaceutical Sciences, 99, 575–586. doi:10.1002/jps.21886
- Calvo, P., Castaño, Á. L., Hernández, M. T., & González-Gómez, D. (2011). Effects of microcapsule constitution on the quality of microencapsulated walnut oil. European Journal of Lipid Science and Technology, 113, 1273–1280. doi:10.1002/ejlt.201100039
- Calvo, P., Castaño, Á. L., Lozano, M., & González-Gómez, D. (2012). Influence of the microencapsulation on the quality parameters and shelf-life of extra-virgin olive oil encapsulated in the presence of BHT and different capsule wall components. Food Research International, 45, 256–261. doi:10.1016/j.foodres.2011.10.036
- Caparino, O. A., Tang, J., Nindo, C. I., Sablani, S. S., Powers, J. R., & Fellman, J. K. (2012). Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. Journal of Food Engineering, 111, 135–148. doi:10.1016/j.jfoodeng.2012.01.010
- Carneiro, H. C. F., Tonon, R. V., Grosso, C. R. F., & Hubinger, M. D. (2013). Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. Journal of Food Engineering, 115, 443–451. doi:10.1016/j.jfoodeng.2012.03.033
- Celli, G. B., Ghanem, A., & Brooks, M. S.-L. (2015). Bioactive encapsulated powders for functional foods – A review of methods and current limitations. Food and Bioprocess Technology, 8, 1825–1837. doi:10.1007/s11947-015-1559-z
- Choe, E., & Min, D. B. (2006). Comprehensive reviews in food science and food safety mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety, 5, 169–186. doi:10.1111/crfs.2006.5.issue-4
- Coupland, K. (2008). Stearidonic acid: A plant produced omega-3 PUFA and a potential alternative for marine oil fatty acids. Lipid Technology, 20, 152–154. doi:10.1002/lite.v20:7
- Czaplicki, S., Ogrodowska, D., Derewiaka, D., Tańska, M., & Zadernowski, R. (2011). Bioactive compounds in unsaponifiable fraction of oils from unconventional sources. European Journal of Lipid Science and Technology, 113, 1456–1464. doi:10.1002/ejlt.201000410
- Czaplicki, S., Tańska, M., & Konopka, I. (2016). Sea-buckthorn oil in vegetable oils stabilisation. Italian Journal of Food Science, 28, 412–425.
- Czaplicki, S., Zadernowski, R., & Ogrodowska, D. (2009). Triacylglycerols from viper bugloss (Echium vulgare L.) seed bio-oil. European Journal of Lipid Science and Technology, 111, 1266–1269. doi:10.1002/(ISSN)1438-9312
- Dobry, D. E., Settell, D. M., Baumann, J. M., Ray, R. J., Graham, L. J., & Beyerinck, R. A. (2009). A model-based methodology for spray-drying process development. Journal of Pharmaceutical Innovation, 4, 133–142. doi:10.1007/s12247-009-9064-4
- El Asbahani, A., Miladi, K., Badri, W., Sala, M., Addi, E. H. A., Casabianca, H., … Elaissari, A. (2015). Essential oils: From extraction to encapsulation. International Journal of Pharmaceutics, 483, 220–243. doi:10.1016/j.ijpharm.2014.12.069
- El Ghannam, M., El Nemr, T., Hassan, A., & Dyab, N. (2015). Encapsulation efficiency, microstructure and oxidation stability of fish oil encapsulated powder made by using whey protein concentrate. Alexandria Science Exchange Journal, 36, 236–248.
- Eskin, N. A. M. (2008). Borage and evening primrose oil. European Journal of Lipid Science and Technology, 110, 651–654. doi:10.1002/ejlt.v110:7
- Ferreira, C. D., Janaína, E., Aparecida, B., Machado, S., & Hermes, V. S. (2016). Physicochemical characterization and oxidative stability of microencapsulated crude palm oil by spray drying. Food and Bioprocess Technology, 9, 124–136. doi:10.1007/s11947-015-1603-z
- Gallardo, G., Guida, L., Martinez, V., López, M. C., Bernhardt, D., Blasco, R., … Hermida, L. G. (2013). Microencapsulation of linseed oil by spray drying for functional food application. Food Research International, 52, 473–482. doi:10.1016/j.foodres.2013.01.020
- Giuffrè, A. M., & LouAdj, L. (2013). Influence of crop season and cultivar on sterol composition of monovarietal olive oils in Reggio Calabria (Italy). Czech Journal of Food Sciences, 31, 256–263. doi:10.17221/CJFS
- Karaca, C. A., Low, N., & Nickerson, M. (2013). Encapsulation of flaxseed oil using a benchtop spray dryer for legume protein – Maltodextrin microcapsule preparation. Journal of Agricultural and Food Chemistry, 61, 5148–5155. doi:10.1021/jf400787j
- Karim, F. T., Ghafoor, K., Ferdosh, S., Al-Juhaimi, F., Ali, E., Yunus, K. B., … Sarker, M. Z. I. (2017). Microencapsulation of fish oil using supercritical antisolvent process. Journal of Food and Drug Analysis, 25, 654–666. doi:10.1016/j.jfda.2016.11.017
- Kawasaki, H., Shimanouchi, T., & Kimura, Y. (2019). Recent development of optimization of lyophilization process. Journal of Chemistry, 2019, 1–14. Article ID 9502856 doi: 10.1155/2019/9502856
- Khan, M. A., & Shahidi, F. (2002). Photooxidative stability of stripped and non-stripped borage and evening primrose oils and their emulsions in water. Food Chemistry, 79, 47–53. doi:10.1016/S0308-8146(02)00176-0
- Lin, Y., Knol, D., Valk, I., van Andel, V., Friedrichs, S., Lütjohann, D., … Trautwein, E. A. (2017). Thermal stability of plant sterols and formation of their oxidation products in vegetable oils and margarines upon controlled heating. Chemistry and Physics of Lipids, 207, 99–107. doi:10.1016/j.chemphyslip.2017.01.007
- Liu, S., Low, N. H., & Nickerson, M. T. (2010). Entrapment of flaxseed oil within gelatin-gum arabic capsules. Journal of American Oil Chemists’ Society, 87, 809–815. doi:10.1007/s11746-010-1560-7
- López-Martínez, J. C., Campra-Madrid, P., & Guil-Guerrero, J. L. (2004). γ-Linolenic acid enrichment from Borago officinalis and Echium fastuosum seed oils and fatty acids by low temperature crystallization. Journal of Bioscience and Bioengineering, 97, 294–298. doi:10.1016/S1389-1723(04)70208-X
- Marwede, V., Schierholt, A., Möllers, C., & Becker, H. C. (2004). Genotype × environment interactions and heritability of tocopherol contents in canola. Crop Science, 44, 728–731. doi:10.2135/cropsci2004.7280
- Melgosa, R., Benito-Román, Ó., Sanz, M. T., de Paz, E., & Beltrán, S. (2019). Omega–3 encapsulation by PGSS-drying and conventional drying methods. Particle characterization and oxidative stability. Food Chemistry, 270, 138–148. doi:10.1016/j.foodchem.2018.07.082
- Montserrat-de la Paz, S., Fernandez-Arche, M. A., Angel-Martin, M., & Garcia-Gimenez, M. D. (2014). Phytochemical characterization of potential nutraceutical ingredients from evening primrose oil (Oenothera biennis L.). Phytochemistry Letters, 8, 158–162. doi:10.1016/j.phytol.2013.08.008
- Najafi, M. N., & Kadkhodaee, R. (2011). Effect of drying process and wall material on the properties of encapsulated cardamom oil. Food Biophysics, 6, 68–76. doi:10.1007/s11483-010-9176-x
- Ng, S. K., Jessie, L. Y. L., Tan, C. P., Long, K., & Nyam, K. L. (2013). Effect of accelerated storage on microencapsulated kenaf seed oil. Journal of the American Oil Chemists’ Society, 90, 1023–1029. doi:10.1007/s11746-013-2249-5
- Nogala-Kalucka, M., Rudzinska, M., Zadernowski, R., Siger, A., & Krzyzostaniak, I. (2010). Phytochemical content and antioxidant properties of seeds of unconventional oil plants. Journal of the American Oil Chemists’ Society, 87, 1481–1487. doi:10.1007/s11746-010-1640-8
- Ogrodowska, D., Tańska, M., & Brandt, W. (2017). The Influence of drying process conditions on the physical properties, bioactive compounds and stability of encapsulated pumpkin seed oil. Food and Bioprocess Technology, 10, 1265–1280. doi:10.1007/s11947-017-1898-z
- Özcan, T. (2008). Analysis of the total oil and fatty acid composition of seeds of some Boraginaceae taxa from Turkey. Plant Systematics and Evolution, 274, 143–153. doi:10.1007/s00606-008-0039-6
- Özcan, T. (2013). Molecular (RAPDs and Fatty acid) and micromorphological variations of Echium italicum L. populations from Turkey. Plant Systematics and Evolution, 299, 631–641. doi:10.1007/s00606-012-0749-7
- Peiretti, P. G., Palmegiano, G. B., & Salamano, G. (2004). Quality and fatty acid content of borage (Borago officinalis L.) during the growth cycle. Italian Journal of Food Science, 16, 177–184.
- Quispe-Condori, S., Saldaña, M. D. A., & Temelli, F. (2011). Microencapsulation of flax oil with zein using spray and freeze drying. LWT – Food Science and Technology, 44, 1880–1887. doi:10.1016/j.lwt.2011.01.005
- Roche, J., Mouloungui, Z., Cerny, M., & Merah, O. (2019). Effect of sowing dates on fatty acids and phytosterols patterns of Carthamus tinctorius L. Applied Sciences, 9(2839), 1–10. doi:10.3390/app9142839
- Rudzińska, M., Przybylski, R., & Wąsowicz, E. (2009). Products formed during thermo‐oxidative degradation of phytosterols. Journal of the American Oil Chemists’ Society, 86, 651–662. doi:10.1007/s11746-009-1397-0
- Scrimgeour, C., & Clough, P. (2014). Authentication of borage oil. Lipid Technology, 26, 230–233. doi:10.1002/lite.201400059
- Senanayake, S. P. J. N., & Shahidi, F. (2002). Chemical and stability characteristics of structured lipids from borage (Borago officinalis L.) and evening primrose (Oenothera biennis L.) oils. Journal of Food Science, 67, 2038–2045. doi:10.1111/jfds.2002.67.issue-6
- Shaw, E. J., Kakuda, Y., & Rajcan, I. (2016). Effect of genotype, environment, and genotype × environment interaction on tocopherol accumulation in soybean seed. Crop Science, 56, 40–50. doi:10.2135/cropsci2015.02.0069
- Silva, E. K., Zabot, G. L., Bargas, M. A., & Meireles, M. A. A. (2016). Microencapsulation of lipophilic bioactive compounds using prebiotic carbohydrates: Effect of the degree of inulin polymerization. Carbohydrate Polymers, 152, 775–783. doi:10.1016/j.carbpol.2016.07.066
- Soh, S. H., & Lee, L. Y. (2019). Microencapsulation and nanoencapsulation using supercritical fluid (SCF) techniques. Pharmaceutics, 11, 21. doi:10.3390/pharmaceutics11010021
- Szterk, A., Roszko, M., Sosińska, E., Derewiaka, D., & Lewicki, P. P. (2010). Chemical composition and oxidative stability of selected plant oils. Journal of the American Oil Chemists’ Society, 87, 637–645. doi:10.1007/s11746-009-1539-4
- Tańska, M., Rotkiewicz, D., Kozirok, W., & Konopka, I. (2005). Measurement of the geometrical features and surface color of rapeseeds using digital image analysis. Food Research International, 38, 741–750. doi:10.1016/j.foodres.2005.01.008
- Toivo, J., Phillips, K., Lampi, A. M., & Piironen, V. (2001). Determination of sterols in foods: Recovery of free, esterified, and glycosidic sterols. Journal of Food Composition and Analysis, 14, 631–643. doi:10.1006/jfca.2001.1019
- Velasco, J., Holgado, F., Dobarganes, C., & Márquez-Ruiz, G. (2009). Influence of relative humidity on oxidation of the free and encapsulated oil fractions in freeze-dried microencapsulated oils. Food Research International, 42, 1492–1500. doi:10.1016/j.foodres.2009.08.007
- Wagdare, N. A., Marcelis, A. T. M., Boom, R. M., & van Rijn, C. J. M. (2011). Microcapsules with a pH responsive polymer: Influence of the encapsulated oil on the capsule morphology. Colloids and Surfaces B: Biointerfaces, 88, 175–180. doi:10.1016/j.colsurfb.2011.06.028
- Westergaard, V. (2004). Milk powder technology evaporation and spray drying (5th ed.). Copenhagen, Denmark: GEA Niro A/S.
- Yüksel, A., & Yeşilçubuk, N. Ş. (2012). Enzymatic production of human milk fat analogues containing stearidonic acid and optimization of reactions by response surface methodology. LWT – Food Science and Technology, 46, 210–216. doi:10.1016/j.lwt.2011.10.004
